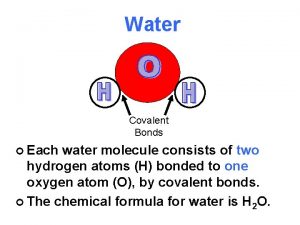
REVIEW Water Structure A water molecule consists of













- Slides: 25

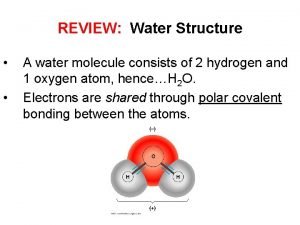
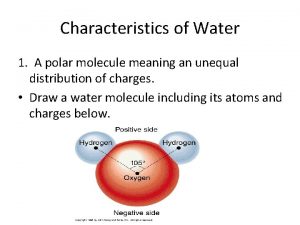
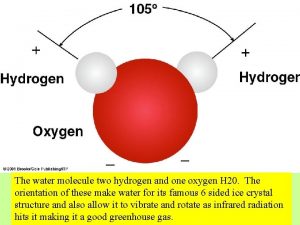
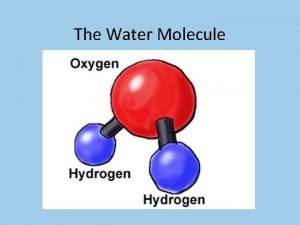
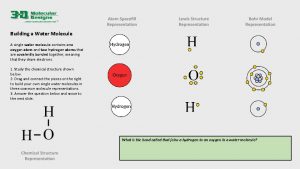
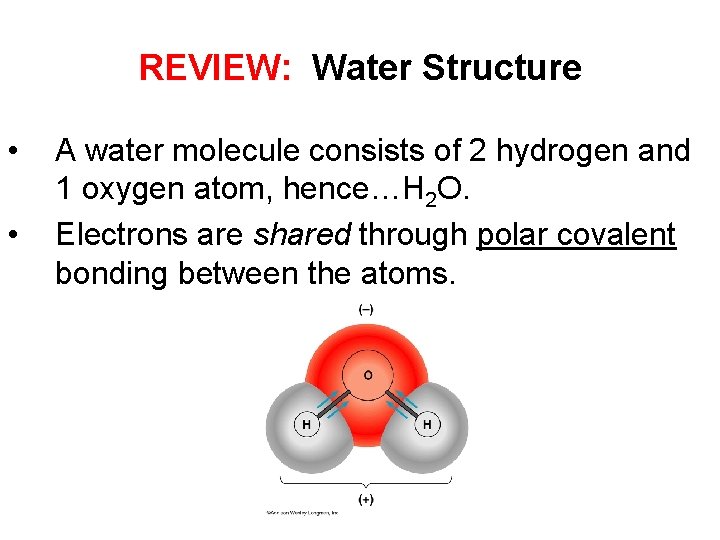
REVIEW: Water Structure • • A water molecule consists of 2 hydrogen and 1 oxygen atom, hence…H 2 O. Electrons are shared through polar covalent bonding between the atoms.

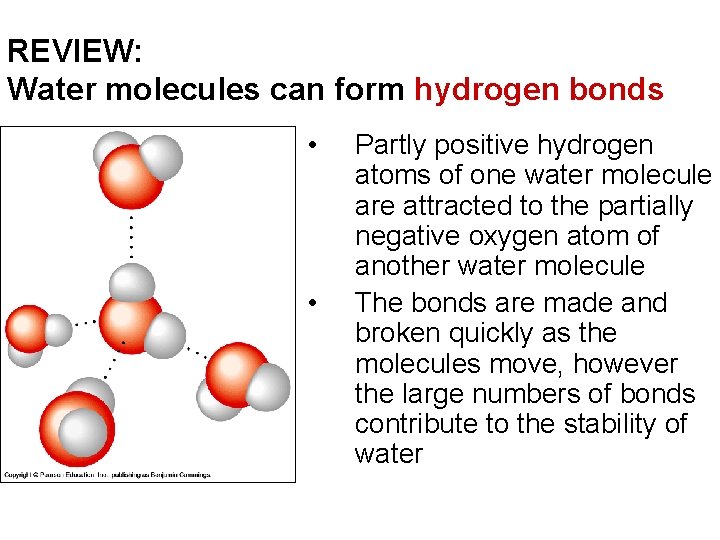
REVIEW: Water molecules can form hydrogen bonds • • Partly positive hydrogen atoms of one water molecule are attracted to the partially negative oxygen atom of another water molecule The bonds are made and broken quickly as the molecules move, however the large numbers of bonds contribute to the stability of water

Properties of Water

#1

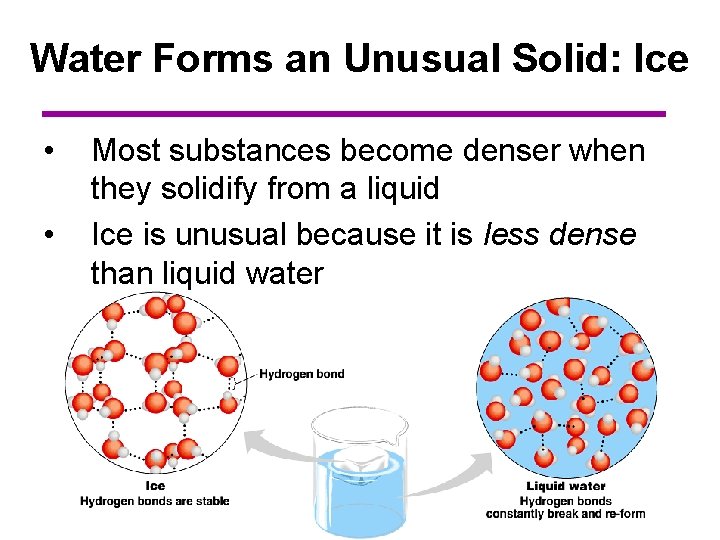
Water Forms an Unusual Solid: Ice • • Most substances become denser when they solidify from a liquid Ice is unusual because it is less dense than liquid water

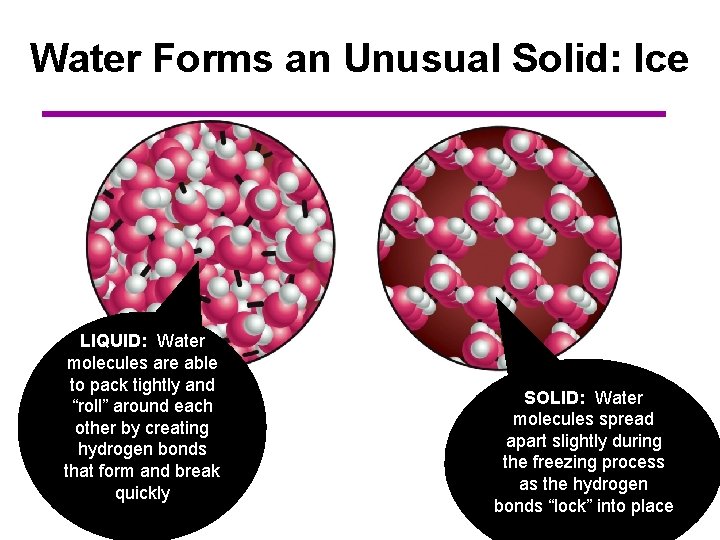
Water Forms an Unusual Solid: Ice LIQUID: Water molecules are able to pack tightly and “roll” around each other by creating hydrogen bonds that form and break quickly SOLID: Water molecules spread apart slightly during the freezing process as the hydrogen bonds “lock” into place

Water Forms an Unusual Solid: Ice • • As a result, ice floats in liquid water Ponds and lakes freeze from the top down and never freeze completely to the bottom – Many plants and fish therefore are not frozen

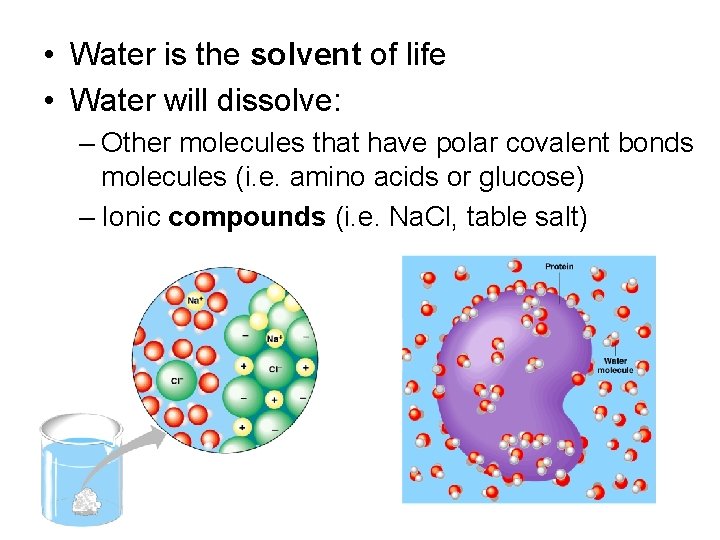
#2 Water Dissolves Many Molecules making it an excellent “SOLVENT”

• Water is the solvent of life • Water will dissolve: – Other molecules that have polar covalent bonds molecules (i. e. amino acids or glucose) – Ionic compounds (i. e. Na. Cl, table salt)

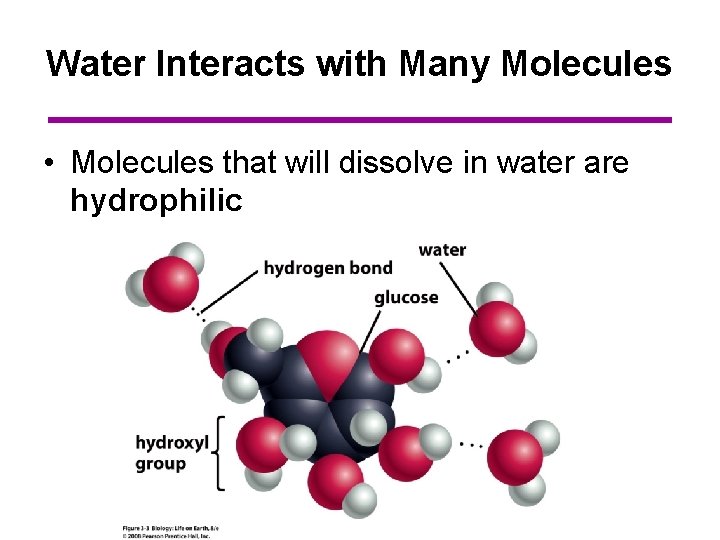
Water Interacts with Many Molecules • Molecules that will dissolve in water are hydrophilic

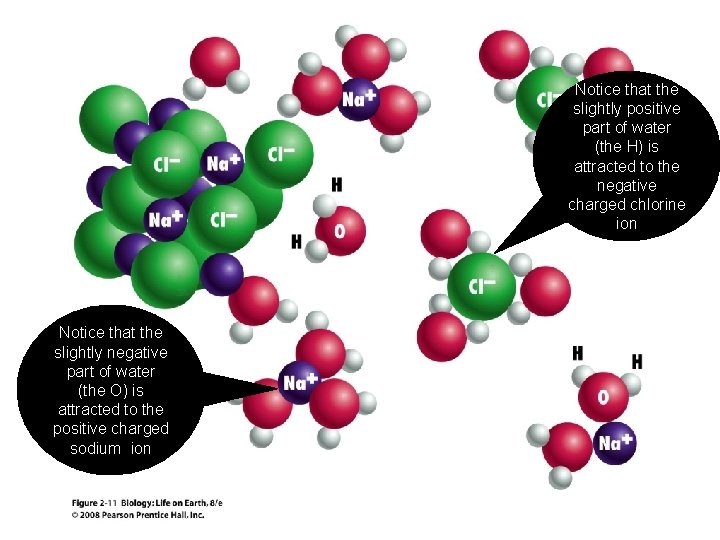
Notice that the slightly positive part of water (the H) is attracted to the negative charged chlorine ion Notice that the slightly negative part of water (the O) is attracted to the positive charged sodium ion

Water Interacts with Many Molecules • Water-insoluble molecules are hydrophobic – Water molecules are not attracted to uncharged and nonpolar molecules like fats and oil


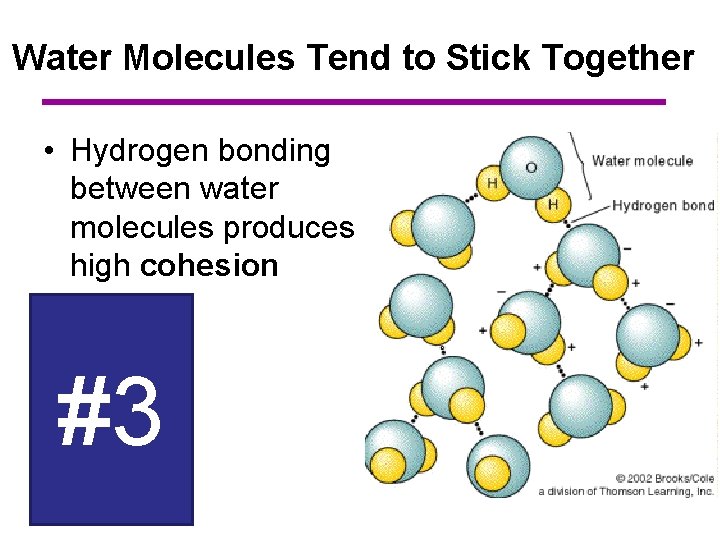
Water Molecules Tend to Stick Together • Hydrogen bonding between water molecules produces high cohesion #3

That’s a long chain of water! That’s a big tree! Water cohesion explains how water molecules can form a chain in delivering water from the roots to the top of a tree Notice the O (slightly -) is attracted to the H (slightly +) of A DIFFERENT water molecule

The water is not falling off the penny because it is sticking to other water molecules = COHESION

• Cohesion of water molecules along a surface produces surface tension Surface tension results from the great attraction of water molecules to each other (due to cohesion). The net effect is an inward force at its surface that causes water to behave as if its surface were covered with a stretched elastic membrane. Because of the relatively high attraction of water molecules for each other, water has a high surface tension compared to that of most other liquids. Fishing spiders and water striders rely on surface tension to move across the surface of ponds

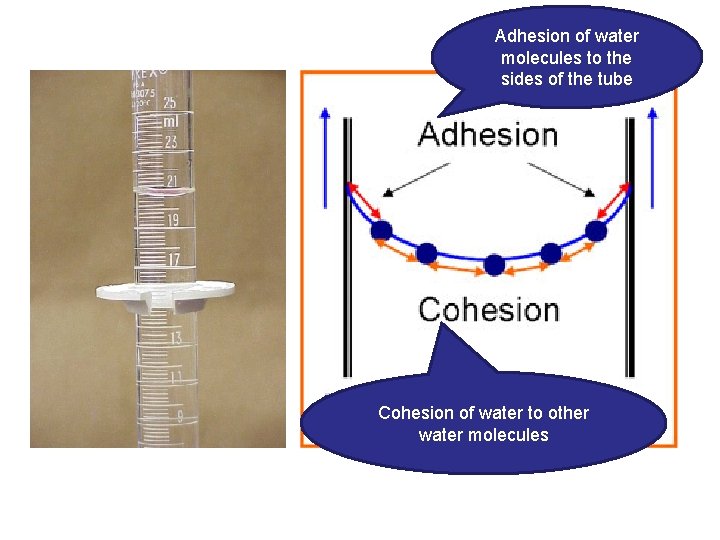
Adhesion #4 • Water molecules sticking to other surfaces • The other surfaces are polar or ionic Adhesion of the water to the spider silk

Adhesion of water molecules to the sides of the tube Cohesion of water to other water molecules

• Adhesion helps water climb up the thin tubes of plants to the leaves • Click link for an overview of “capillary action”

Water Stabilizes Temperature #5 • Compared to other molecules, it takes a lot of energy to change the temperature of water – It requires 1 calorie of energy to raise the temperature of 1 g of water 1 o. C (the specific heat of water) • So water heats up or cools down very slowly – This provides for a stable internal environment and habitat

Water Stabilizes Temperature • Because the human body is mostly water, a sunbather can absorb a lot of heat energy without sending her/his body temperature soaring

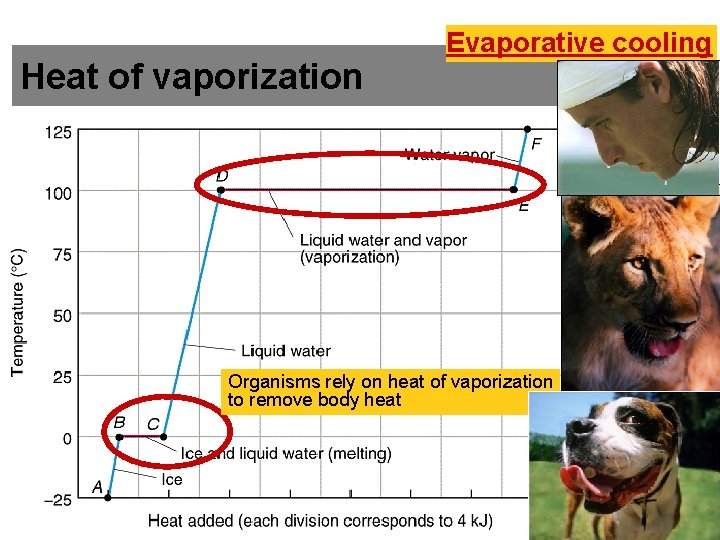
Water Stabilizes Temperature #6 • Water requires a lot of energy to turn from liquid into a gas (heat of vaporization) – Water requires a high input of energy to break the hydrogen bonds to turn it from a liquid to a gas. – Evaporating water uses up heat from its surroundings, cooling the nearby environment (as occurs during sweating) – Here’s a good link to explain evaporative cooling

Heat of vaporization Evaporative cooling Organisms rely on heat of vaporization to remove body heat

Transparency Because water is transparent, light penetrates tissue and aquatic environments, important for photosynthesis.
 Water molecules
Water molecules Water and water and water water
Water and water and water water Water molecule evaporation
Water molecule evaporation Polar molecule meaning
Polar molecule meaning Water molecule
Water molecule Is water covalent
Is water covalent Polar covalent bond in water molecule
Polar covalent bond in water molecule Water molecule
Water molecule Water molecule
Water molecule What is a polar molecule
What is a polar molecule Why is water a polar molecule
Why is water a polar molecule Draw the structure of a molecule with 7 bonding domains
Draw the structure of a molecule with 7 bonding domains Drawing lewis structures practice
Drawing lewis structures practice Structures are laid-back and undefined
Structures are laid-back and undefined Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Uncontrollable spending ap gov
Uncontrollable spending ap gov Nader amin-salehi
Nader amin-salehi Inclusion criteria examples
Inclusion criteria examples Narrative review vs systematic review
Narrative review vs systematic review Enzymes are composed of what organic molecule
Enzymes are composed of what organic molecule Is h2o a polar molecule
Is h2o a polar molecule Chemical formula
Chemical formula Molecule organelle cell
Molecule organelle cell Dna labeled diagram
Dna labeled diagram Bond vs molecular polarity
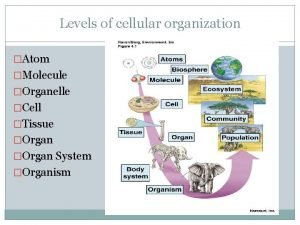
Bond vs molecular polarity Atom molecule organelle cell tissue
Atom molecule organelle cell tissue