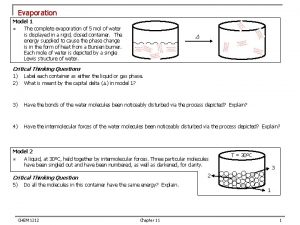
Evaporation H 2 Og molecules water vapor H






- Slides: 32

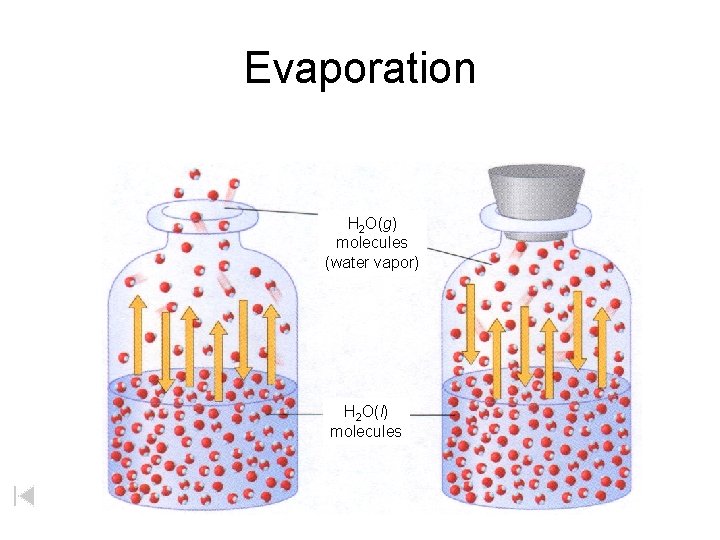
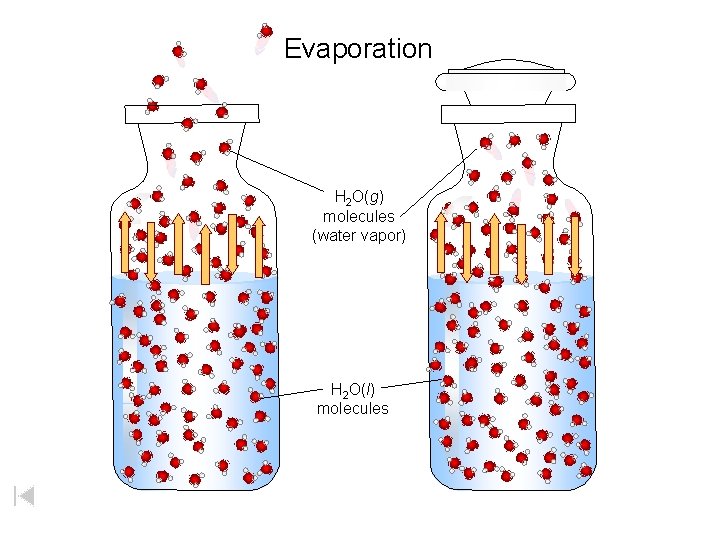
Evaporation H 2 O(g) molecules (water vapor) H 2 O(l) molecules

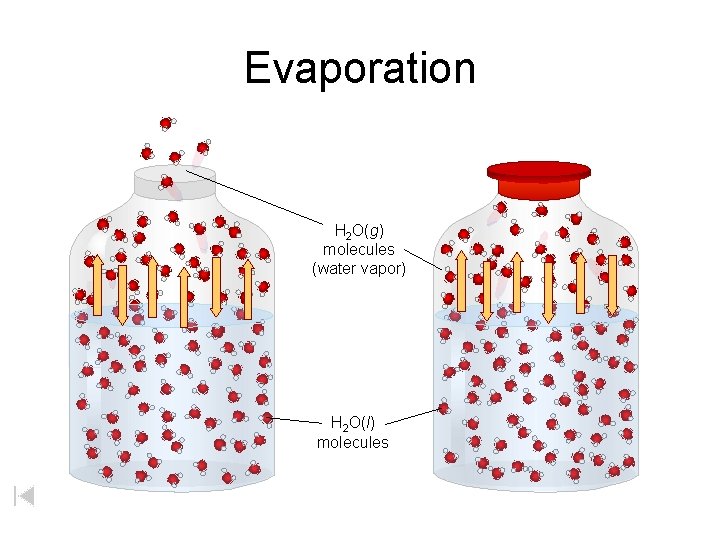
Evaporation H 2 O(g) molecules (water vapor) H 2 O(l) molecules

Evaporation H 2 O(g) molecules (water vapor) H 2 O(l) molecules

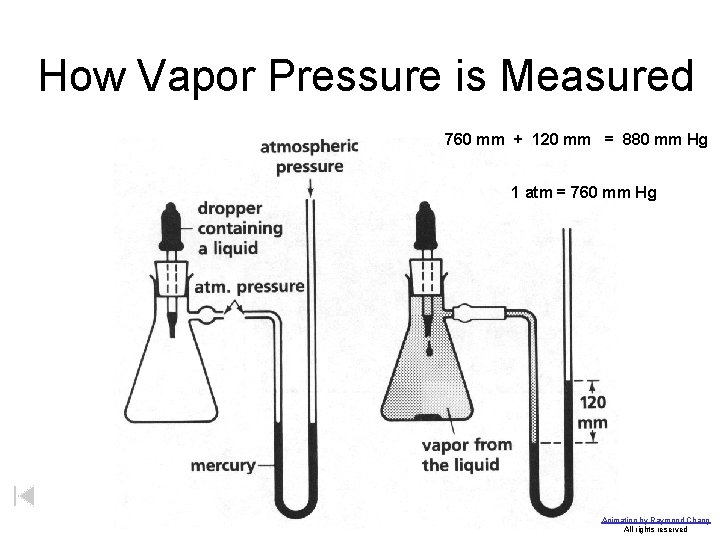
How Vapor Pressure is Measured 760 mm + 120 mm = 880 mm Hg 1 atm = 760 mm Hg Animation by Raymond Chang All rights reserved

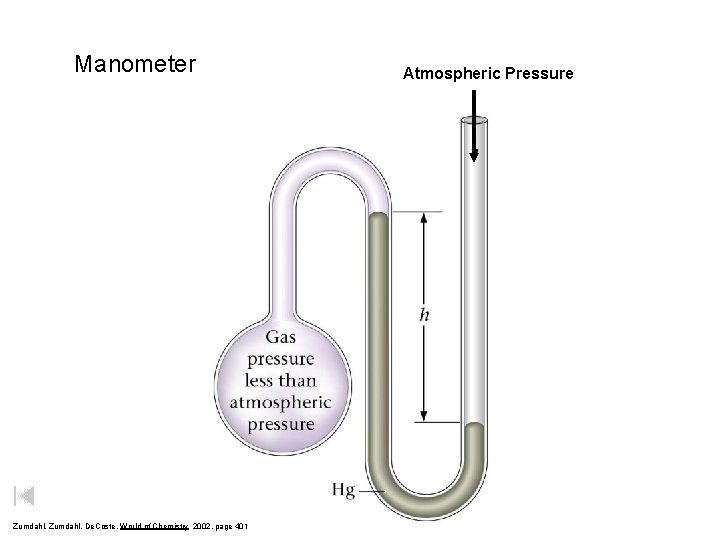
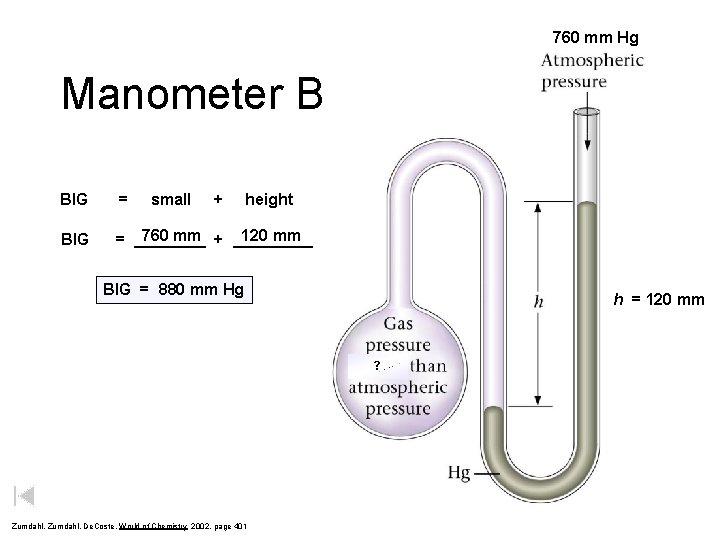
Manometer Zumdahl, De. Coste, World of Chemistry 2002, page 401 Atmospheric Pressure

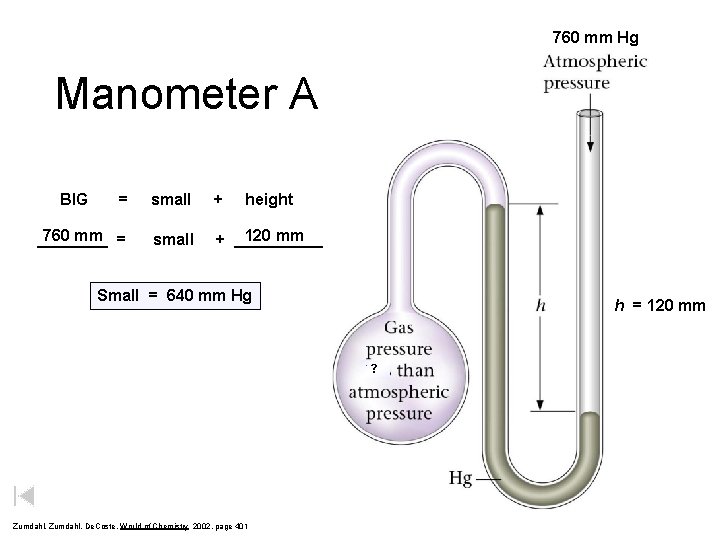
760 mm Hg Manometer A BIG = small + height 760 mm = ____ small 120 mm + _____ Small = 640 mm Hg h = 120 mm ? Zumdahl, De. Coste, World of Chemistry 2002, page 401

760 mm Hg Manometer B BIG = small + height BIG 760 mm + _____ 120 mm = ____ BIG = 880 mm Hg h = 120 mm ? Zumdahl, De. Coste, World of Chemistry 2002, page 401

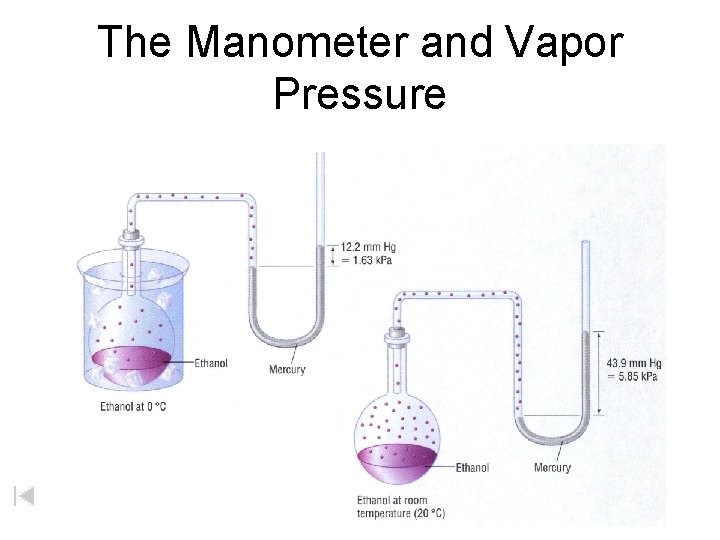
The Manometer and Vapor Pressure

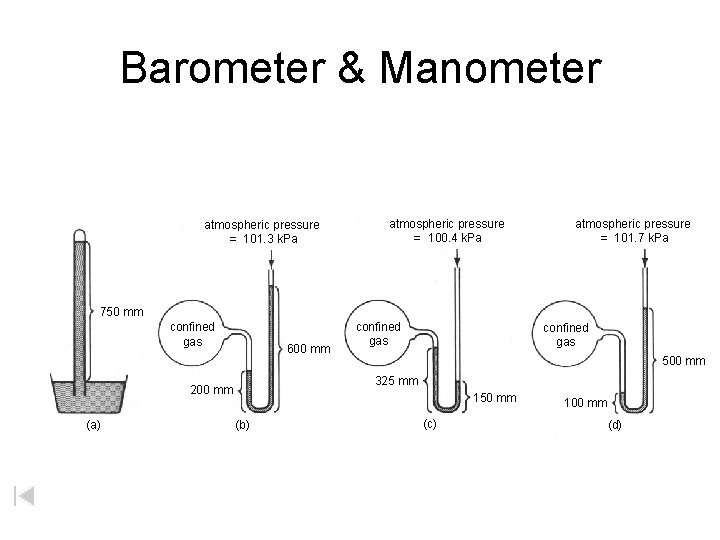
Barometer & Manometer atmospheric pressure = 101. 3 k. Pa atmospheric pressure = 100. 4 k. Pa atmospheric pressure = 101. 7 k. Pa 750 mm confined gas 600 mm confined gas 500 mm 325 mm 200 mm (a) confined gas 150 mm (b) (c) 100 mm (d)

Pressure and Temperature STP (Standard Temperature and Pressure) standard temperature 0 o. C 273 K standard pressure 1 atm 101. 3 k. Pa 760 mm Hg Equations / Conversion Factors: K = o. C + 273 o. C = K – 273 1 atm = 101. 3 k. Pa = 760 mm Hg

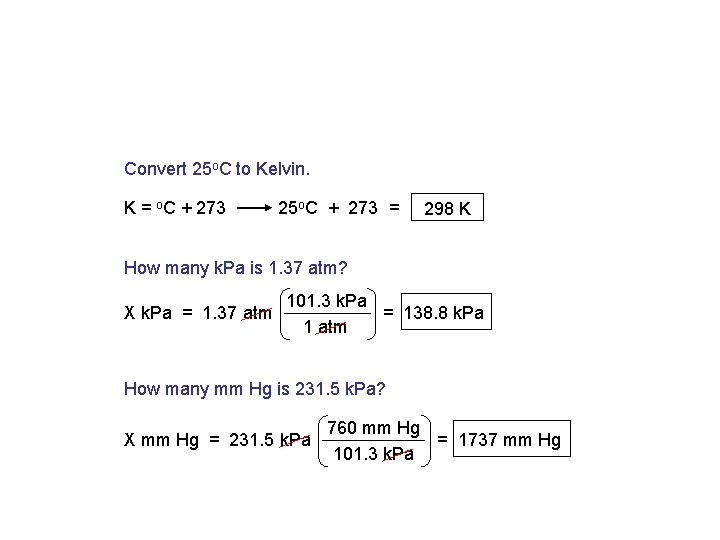
Convert 25 o. C to Kelvin. K = o. C + 273 25 o. C + 273 = 298 K How many k. Pa is 1. 37 atm? X k. Pa = 1. 37 atm 101. 3 k. Pa = 138. 8 k. Pa 1 atm How many mm Hg is 231. 5 k. Pa? X mm Hg = 231. 5 k. Pa 760 mm Hg = 1737 mm Hg 101. 3 k. Pa

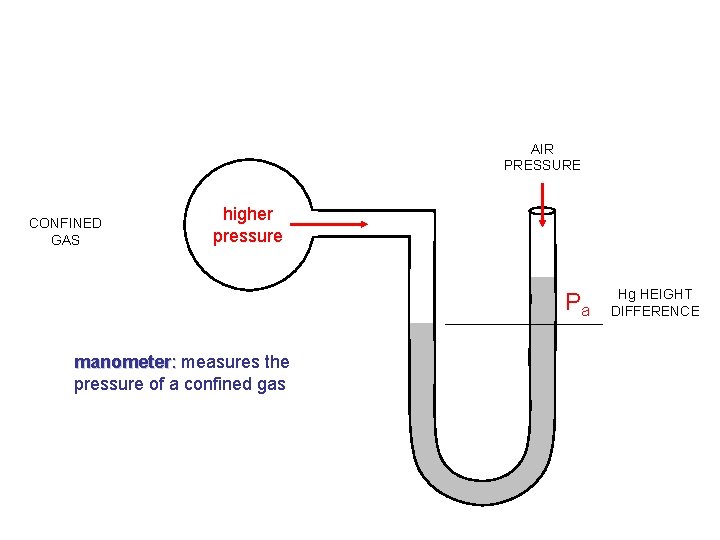
AIR PRESSURE CONFINED GAS higher pressure Pa manometer: measures the pressure of a confined gas Hg HEIGHT DIFFERENCE

small 96. 5 k. Pa Atmospheric pressure is 96. 5 k. Pa; mercury height difference is 233 mm. Find confined gas pressure, in atm. BIG 1. 26 X atm SMALL + HEIGHT = BIG 233 mm Hg 96. 5 k. Pa + 233 mm Hg = X atm 96. 5 k. Pa 1 atm 101. 3 k. Pa + 233 mm Hg 0. 953 atm + 0. 307 atm = X atm X = 1. 26 atm 1 atm = X atm 760 mm Hg

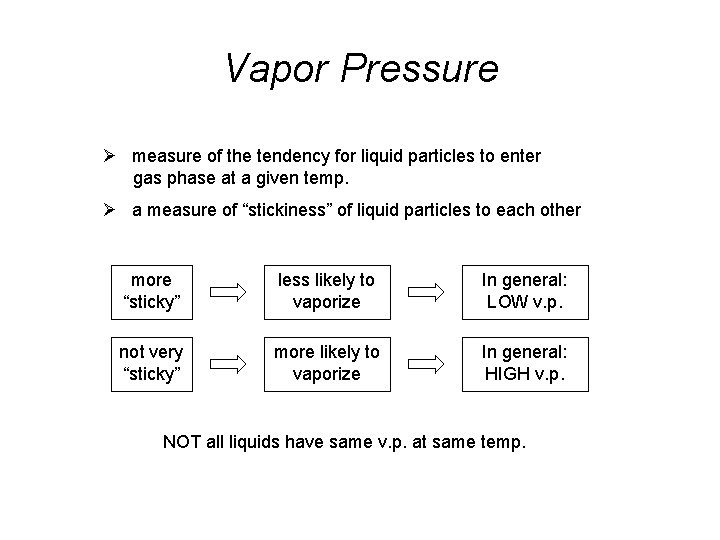
Vapor Pressure Ø measure of the tendency for liquid particles to enter gas phase at a given temp. Ø a measure of “stickiness” of liquid particles to each other more “sticky” less likely to vaporize In general: LOW v. p. not very “sticky” more likely to vaporize In general: HIGH v. p. NOT all liquids have same v. p. at same temp.

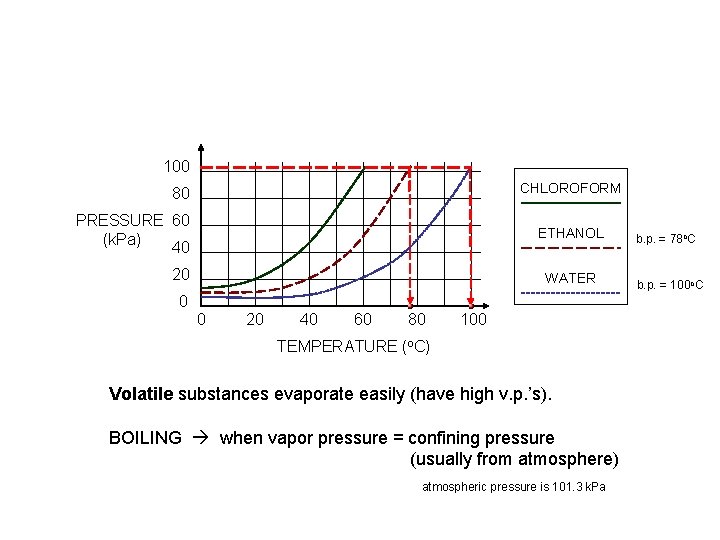
100 CHLOROFORM 80 PRESSURE 60 (k. Pa) 40 ETHANOL 20 WATER 0 0 20 40 60 80 100 TEMPERATURE (o. C) Volatile substances evaporate easily (have high v. p. ’s). BOILING when vapor pressure = confining pressure (usually from atmosphere) atmospheric pressure is 101. 3 k. Pa b. p. = 78 o. C b. p. = 100 o. C

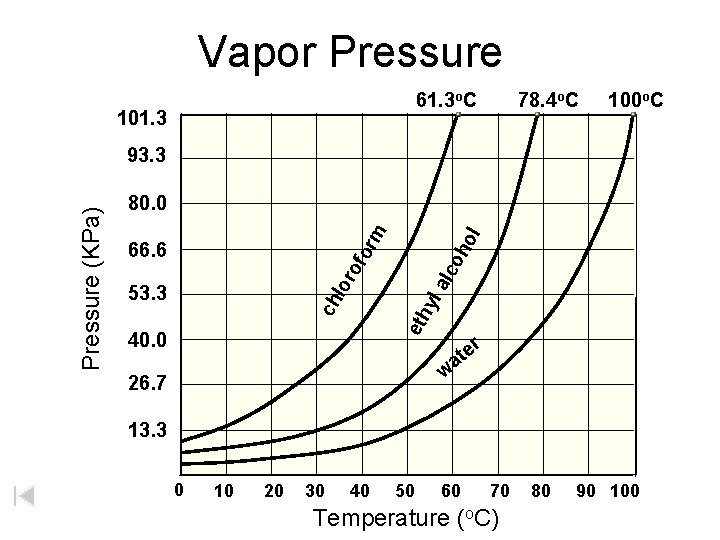
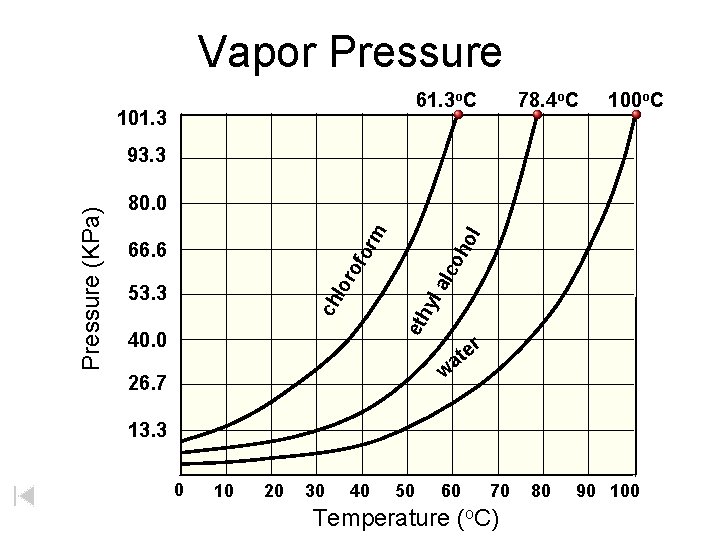
Vapor Pressure 61. 3 o. C 101. 3 78. 4 o. C 100 o. C 53. 3 eth yl lor alc of 66. 6 oh ol or m 80. 0 ch Pressure (KPa) 93. 3 40. 0 e at r w 26. 7 13. 3 0 10 20 30 40 50 60 70 Temperature (o. C) 80 90 100

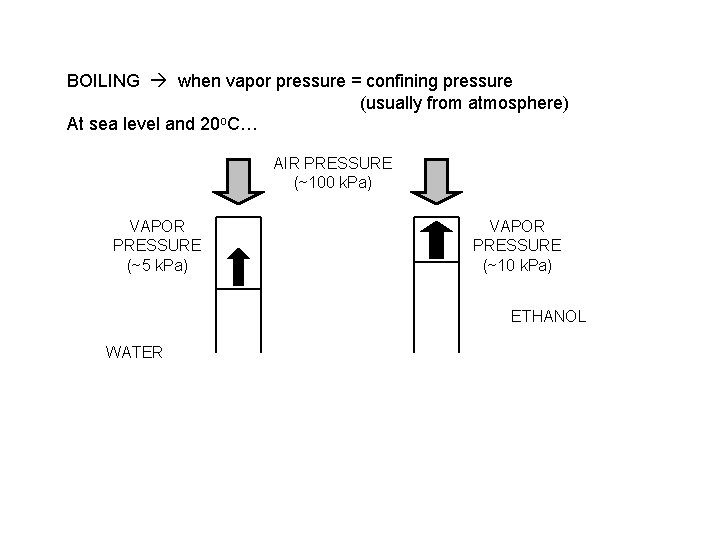
BOILING when vapor pressure = confining pressure (usually from atmosphere) At sea level and 20 o. C… AIR PRESSURE (~100 k. Pa) VAPOR PRESSURE (~5 k. Pa) VAPOR PRESSURE (~10 k. Pa) ETHANOL WATER

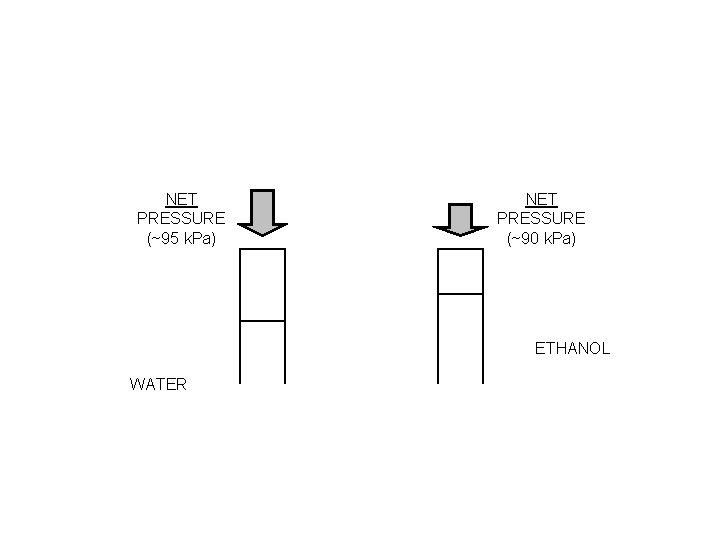
NET PRESSURE (~95 k. Pa) NET PRESSURE (~90 k. Pa) ETHANOL WATER

Water Molecules in Liquid and Steam

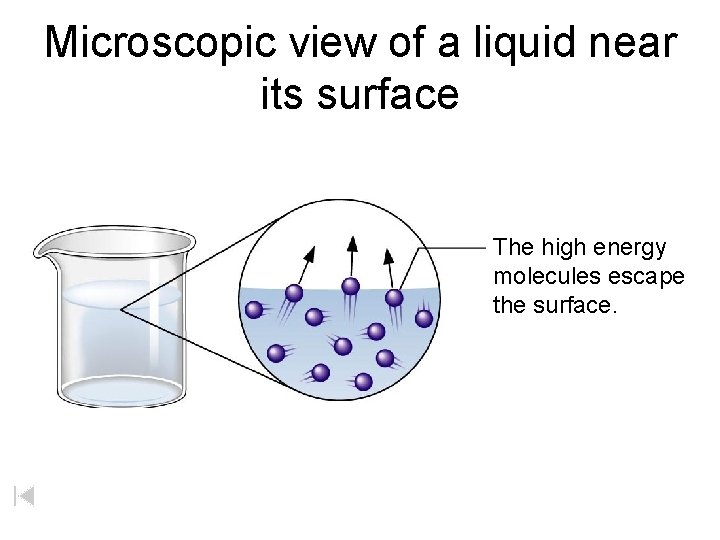
Microscopic view of a liquid near its surface The high energy molecules escape the surface.

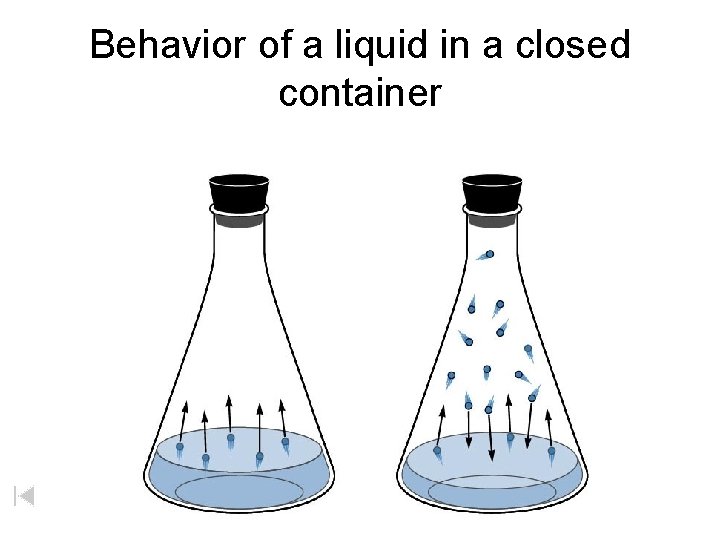
Behavior of a liquid in a closed container

Water rapidly boiling on a stove

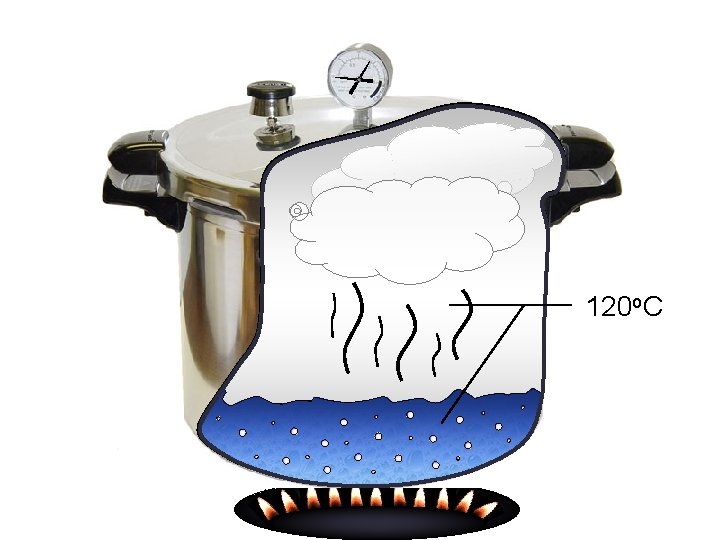
Pressure Cooker Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.

120 o. C

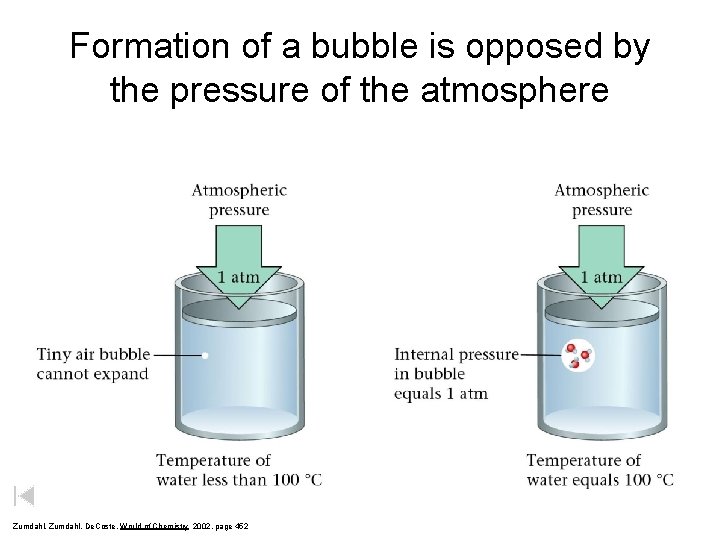
Formation of a bubble is opposed by the pressure of the atmosphere Zumdahl, De. Coste, World of Chemistry 2002, page 452

Vapor Pressure 61. 3 o. C 101. 3 78. 4 o. C 100 o. C 53. 3 eth yl lor alc of 66. 6 oh ol or m 80. 0 ch Pressure (KPa) 93. 3 40. 0 e at r w 26. 7 13. 3 0 10 20 30 40 50 60 70 Temperature (o. C) 80 90 100

Boiling Point and Pressure

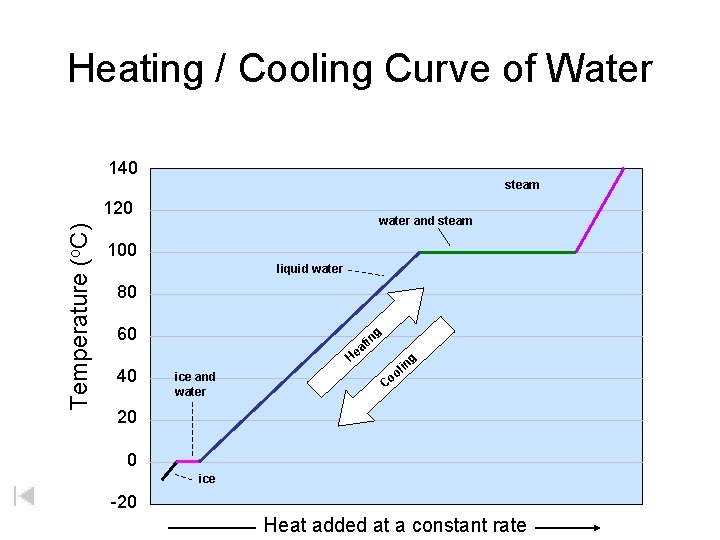
Heating / Cooling Curve of Water 140 steam Temperature (o. C) 120 water and steam 100 liquid water 80 60 tin a e g H 40 ice and water in ol g Co 20 0 ice -20 Heat added at a constant rate

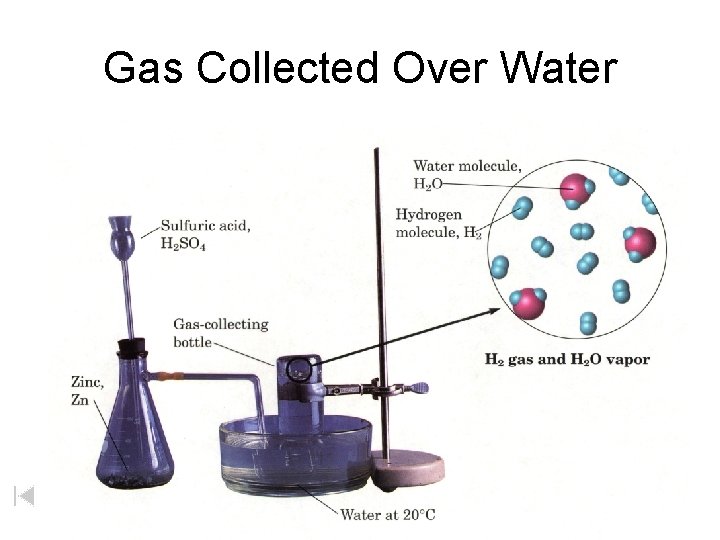
Gas Collected Over Water

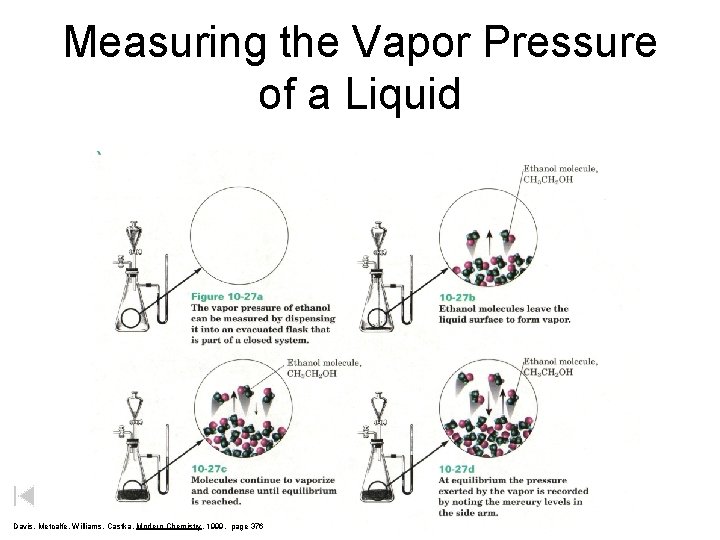
Measuring the Vapor Pressure of a Liquid Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 376

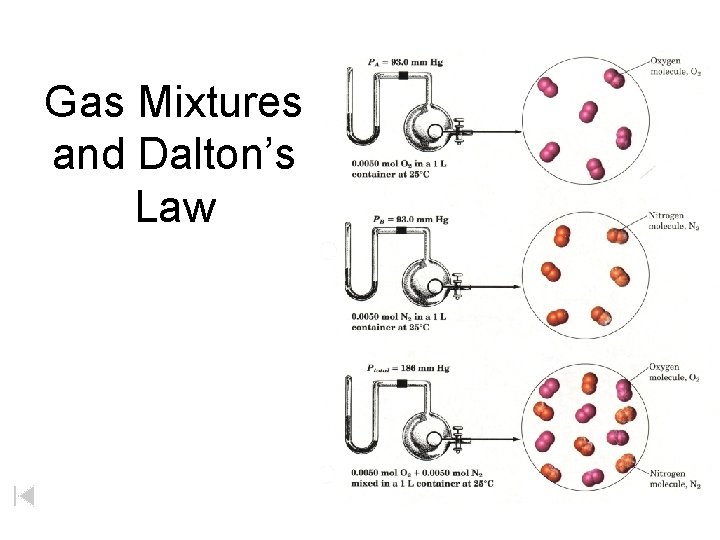
Gas Mixtures and Dalton’s Law

Gases Dissolved in Liquids
 Caldeira flamotubular
Caldeira flamotubular Water and water and water water
Water and water and water water Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Water
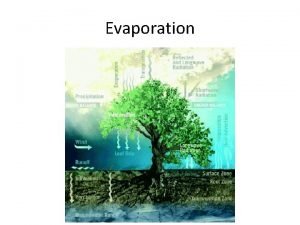
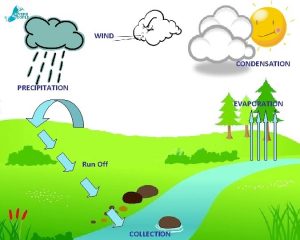
Water Transpiration is the evaporation of water from
Transpiration is the evaporation of water from Evaporation water cycle
Evaporation water cycle The vapor pressure of pure water at 110 c is 1070 torr
The vapor pressure of pure water at 110 c is 1070 torr Clausius clapeyron formula
Clausius clapeyron formula How to calculate vapor pressure of water
How to calculate vapor pressure of water Chemical equation for water vapor
Chemical equation for water vapor Cumulus clouds in oral pathology
Cumulus clouds in oral pathology This precipitation gathers into
This precipitation gathers into Water vapor in the atmosphere percentage
Water vapor in the atmosphere percentage To beat rapidly to incorporate air and increase volume
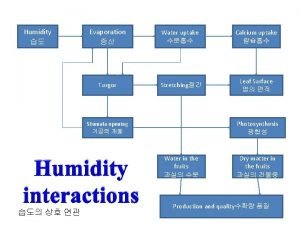
To beat rapidly to incorporate air and increase volume Stomatal transpiration diagram
Stomatal transpiration diagram Water vapor mixing ratio
Water vapor mixing ratio The capacity of air to hold water vapor
The capacity of air to hold water vapor Cohesion bond
Cohesion bond The attractive force between water molecules
The attractive force between water molecules Bond between water molecules
Bond between water molecules A solid compound that contains water molecules
A solid compound that contains water molecules Properties of water
Properties of water Are water molecules polar
Are water molecules polar Evaporation
Evaporation Prep 1
Prep 1 Evaporation
Evaporation Kinetic particle theory o level questions
Kinetic particle theory o level questions Evaporation separating mixtures examples
Evaporation separating mixtures examples Melting freezing boiling evaporation condensation
Melting freezing boiling evaporation condensation Why is separating mixtures important
Why is separating mixtures important Humidity evaporation
Humidity evaporation Picture of evaporation
Picture of evaporation Does evaporation
Does evaporation