Chemistry Chapter 5 THE PERIODIC LAW Mendeleevs Periodic










- Slides: 35

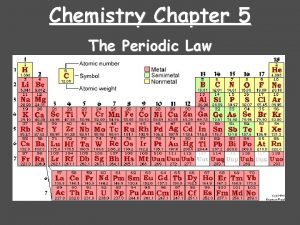
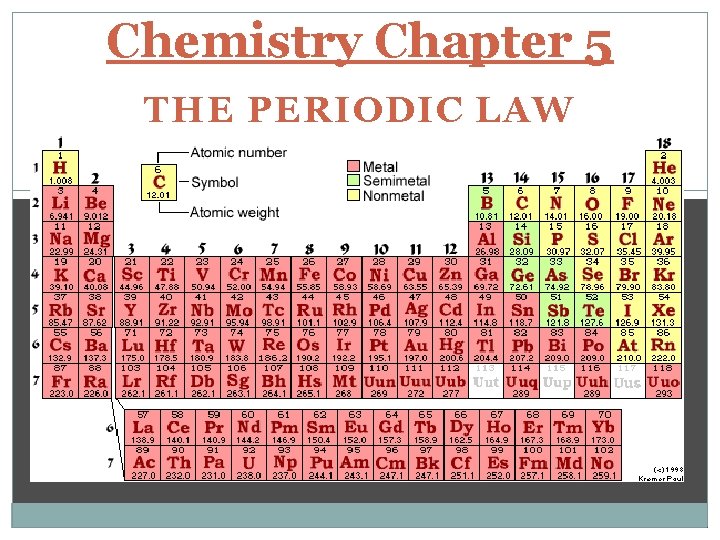
Chemistry Chapter 5 THE PERIODIC LAW

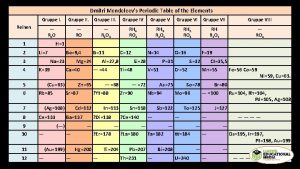
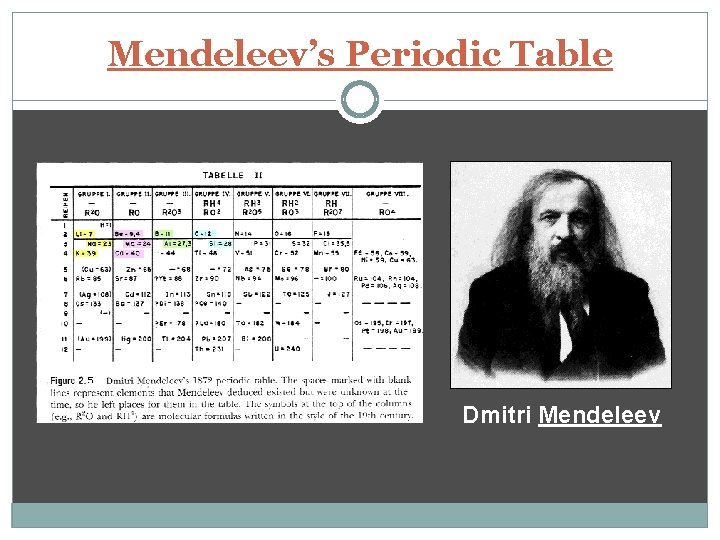
Mendeleev’s Periodic Table Dmitri Mendeleev

Mendeleev – organized periodic table Tried to organize periodic table according to properties Vertical columns in atomic mass order Made some exceptions to place elements in rows with similar properties (Tellurium and Iodine) Horizontal rows have similar chemical properties Gaps for “yet to be discovered” elements Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavior?

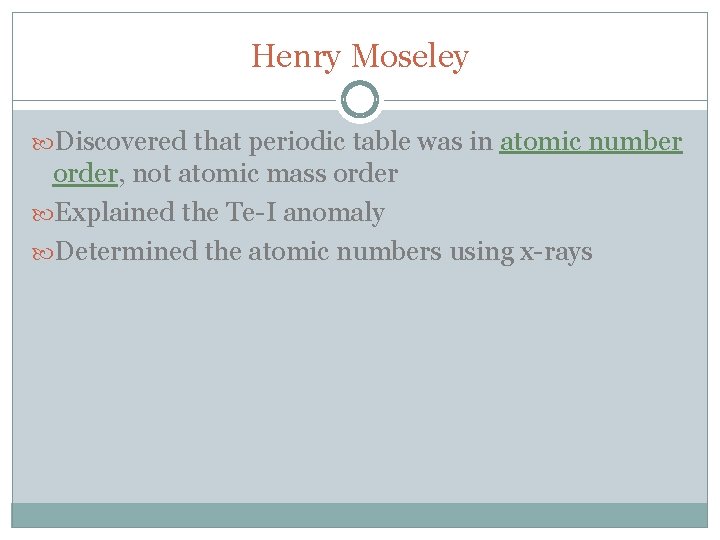
Henry Moseley Discovered that periodic table was in atomic number order, not atomic mass order Explained the Te-I anomaly Determined the atomic numbers using x-rays

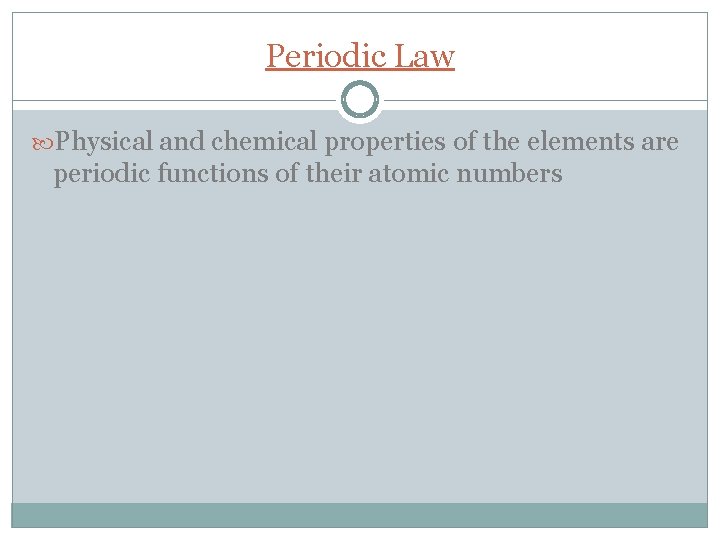
Periodic Law Physical and chemical properties of the elements are periodic functions of their atomic numbers

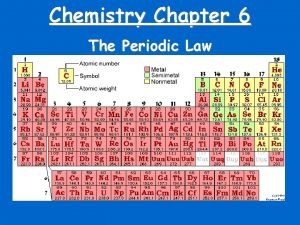
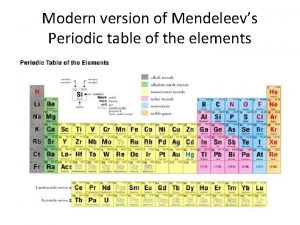
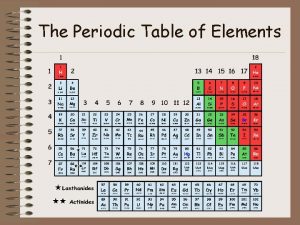
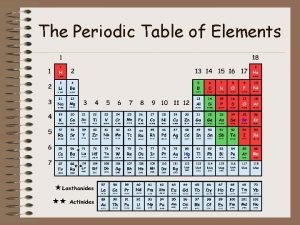
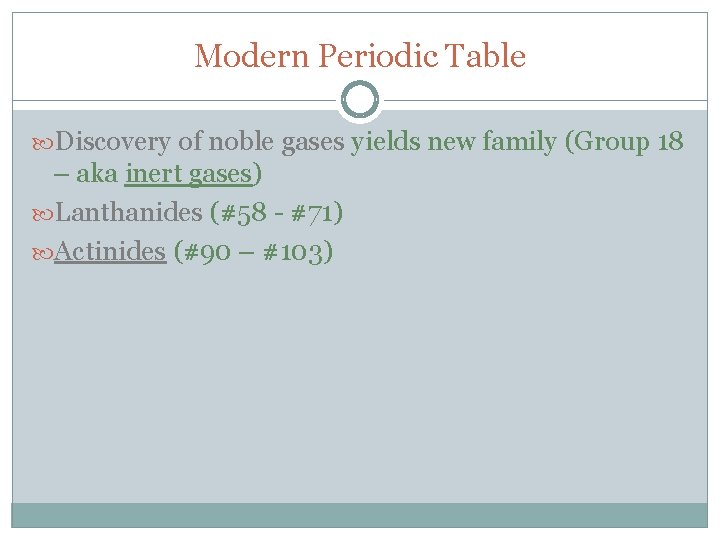
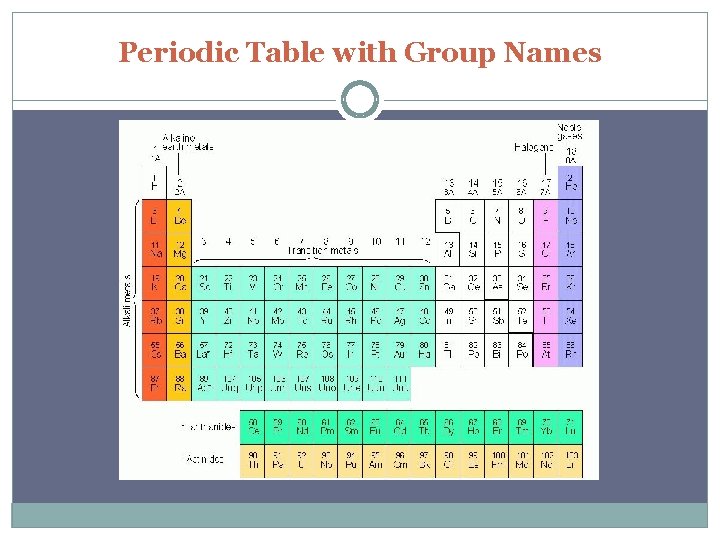
Modern Periodic Table Discovery of noble gases yields new family (Group 18 – aka inert gases) Lanthanides (#58 - #71) Actinides (#90 – #103)

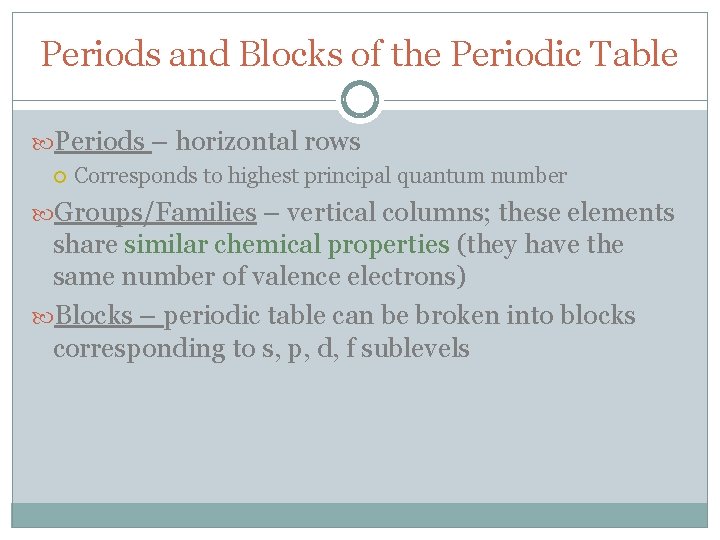
Periods and Blocks of the Periodic Table Periods – horizontal rows Corresponds to highest principal quantum number Groups/Families – vertical columns; these elements share similar chemical properties (they have the same number of valence electrons) Blocks – periodic table can be broken into blocks corresponding to s, p, d, f sublevels

Orbital filling table

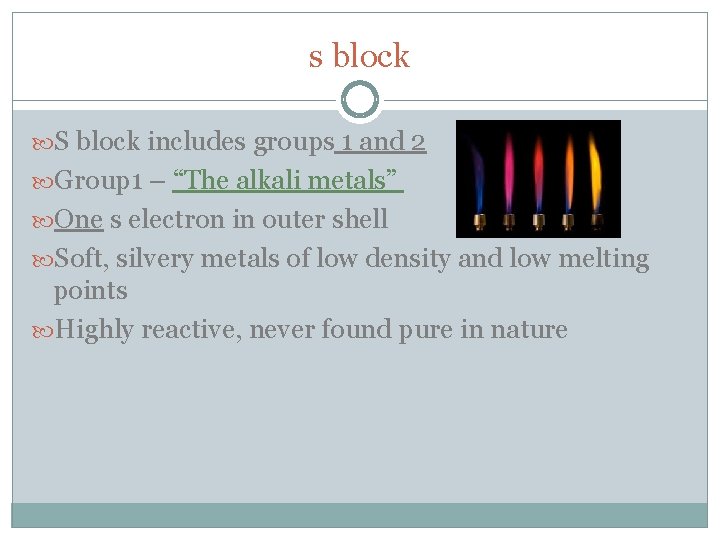
s block S block includes groups 1 and 2 Group 1 – “The alkali metals” One s electron in outer shell Soft, silvery metals of low density and low melting points Highly reactive, never found pure in nature

s block Group 2 – “Alkaline Earth Metals” 2 s electrons in outer shell Denser, harder, stronger, less reactive than Group 1 Too reactive to be found pure in nature

Periodic Table with Group Names

The Properties of a Group: the Alkali Metals Easily lose valence electron (Reducing agents) React violently with water React with halogens to form salts

d block Groups 3 -12 Metals with typical metallic properties Referred to as transition metals Group number = sum of outermost s and d electrons

p block Groups 13 -18 Properties vary greatly – metals, metalloids, and nonmetals Group 17 – halogens are most reactive of non metals Group 18 – noble gases are NOT reactive

f block Lanthanides – shiny metals similar to group 2 Actinides – all are radioactive; plutonium – lawrencium are man-made

Properties of Metals q Metals: q good conductors of heat and electricity q. Malleable q. Ductile q Have high tensile strength q luster

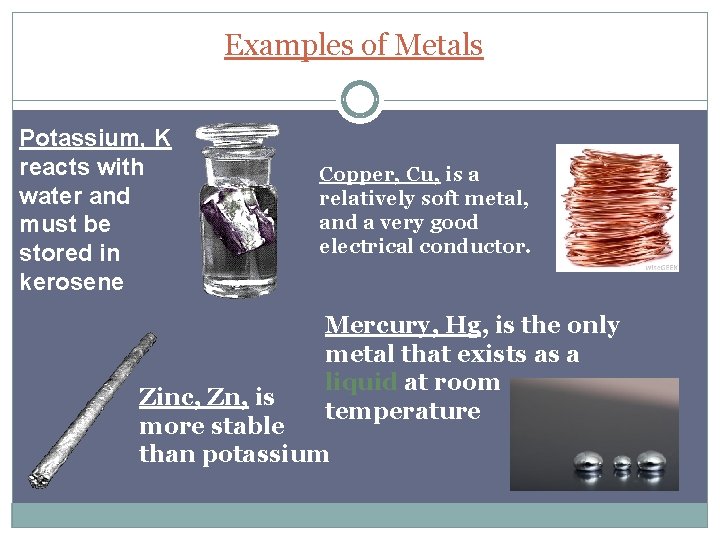
Examples of Metals Potassium, K reacts with water and must be stored in kerosene Copper, Cu, is a relatively soft metal, and a very good electrical conductor. Mercury, Hg, is the only metal that exists as a liquid at room temperature Zinc, Zn, is more stable than potassium

Properties of Nonmetals Carbon, the graphite in “pencil lead” is a great example of a nonmetallic element. q Nonmetals are: q poor conductors of heat and electricity q brittle q. Many are gases at room temperature

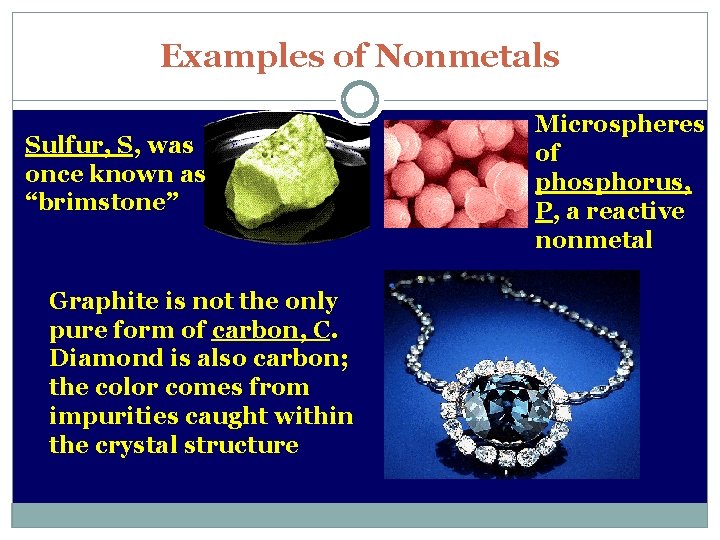
Examples of Nonmetals Sulfur, S, was once known as “brimstone” Graphite is not the only pure form of carbon, C. Diamond is also carbon; the color comes from impurities caught within the crystal structure Microspheres of phosphorus, P, a reactive nonmetal

Properties of Metalloids straddle the border between metals and nonmetals on the periodic table. v They have properties of both metals and nonmetals. v. Metalloids are more brittle than metals, less brittle than most nonmetallic solids v Metalloids are semiconductors of electricity v. Many used in computer parts v Some metalloids possess metallic luster

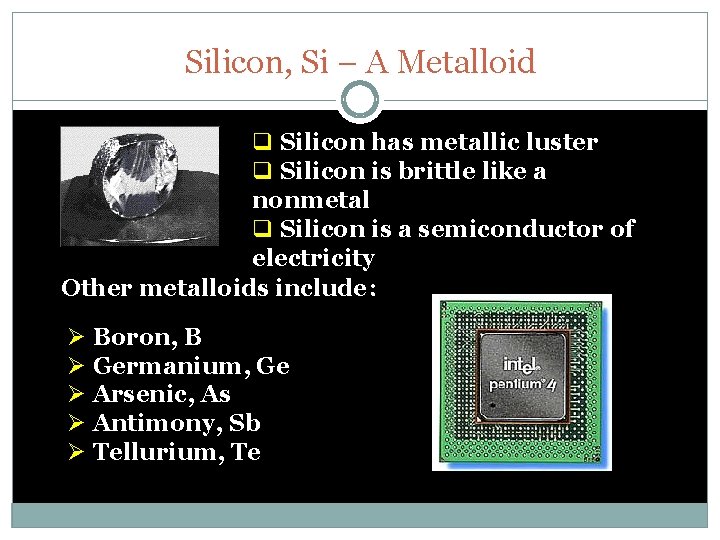
Silicon, Si – A Metalloid q Silicon has metallic luster q Silicon is brittle like a nonmetal q Silicon is a semiconductor of electricity Other metalloids include: Ø Boron, B Ø Germanium, Ge Ø Arsenic, As Ø Antimony, Sb Ø Tellurium, Te

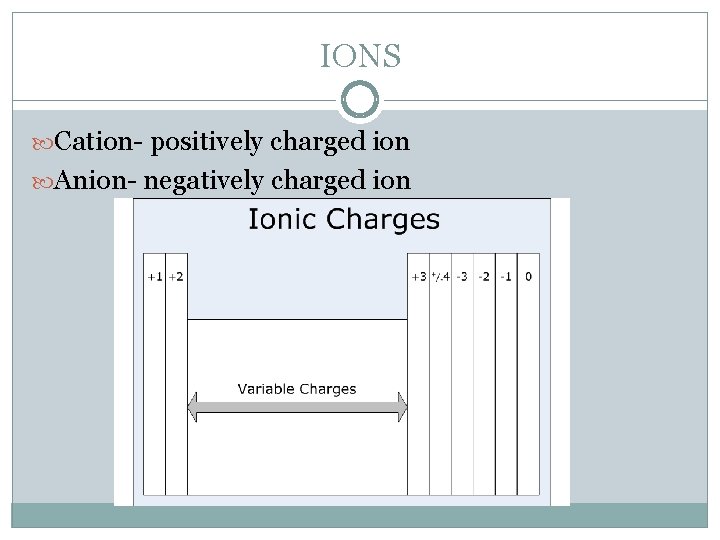
IONS Cation- positively charged ion Anion- negatively charged ion

IONS Group 1 1 valence electron (+1) Will lose 1 electron Group 2 2 valence electrons (+2) Will lose 2 electrons Group 13 3 valence electrons (+3) Will lose 3 electrons

D block IONS Cannot predict Memorize: Ag+ Cd 2+ Zn 2+

IONS Group 14 4 valence e- (4+ or 4 -) Will lose 4 or gain 4 electrons Group 15 5 valence e Gains 3 electrons (3 -) Group 16 6 valence e Gains 2 electrons (2 -) Group 17 7 valence e Gains 1 electron (1 -) Group 18 No ions

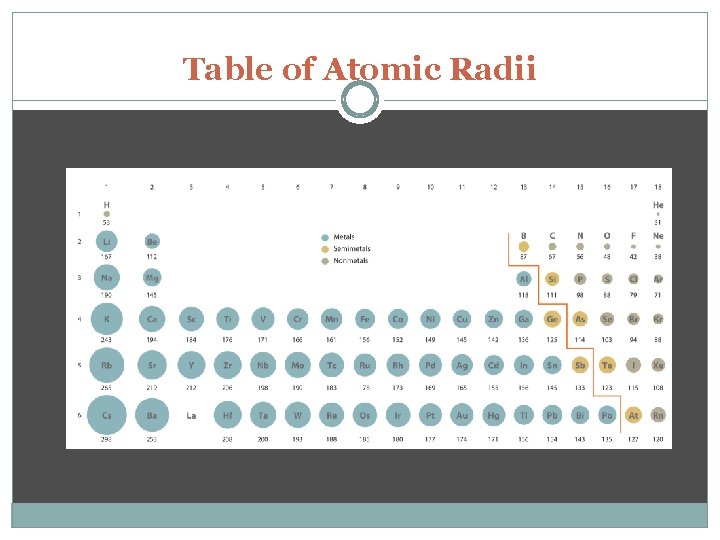
Determination of Atomic Radius: Half the diameter of an atom Periodic Trends in Atomic Radius decreases across a period Increased effective nuclear charge due to increased number of protons and electrons Radius increases down a group Addition of principal quantum levels

Table of Atomic Radii

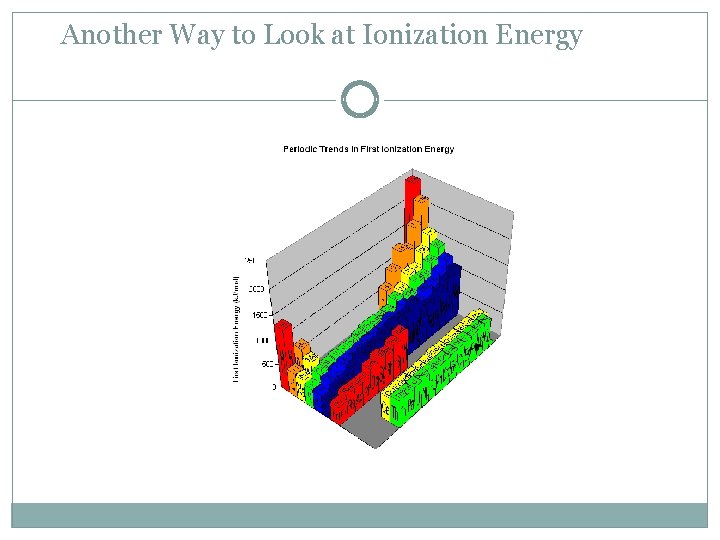
Ionization Energy - the energy required to remove an electron from an atom Increases for successive electrons taken from the same atom Tends to increase across a period Increased number of protons and electrons leads to greater attraction Tends to decrease down a group Outer electrons are farther from the nucleus

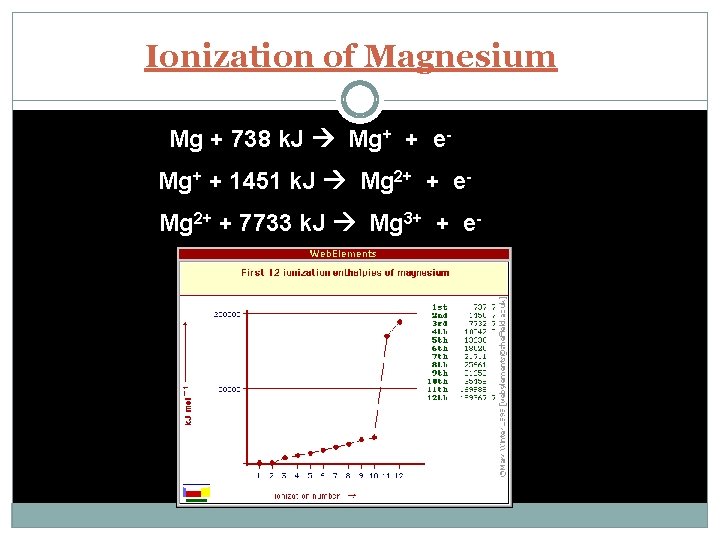
Ionization of Magnesium Mg + 738 k. J Mg+ + e. Mg+ + 1451 k. J Mg 2+ + e. Mg 2+ + 7733 k. J Mg 3+ + e-

Another Way to Look at Ionization Energy

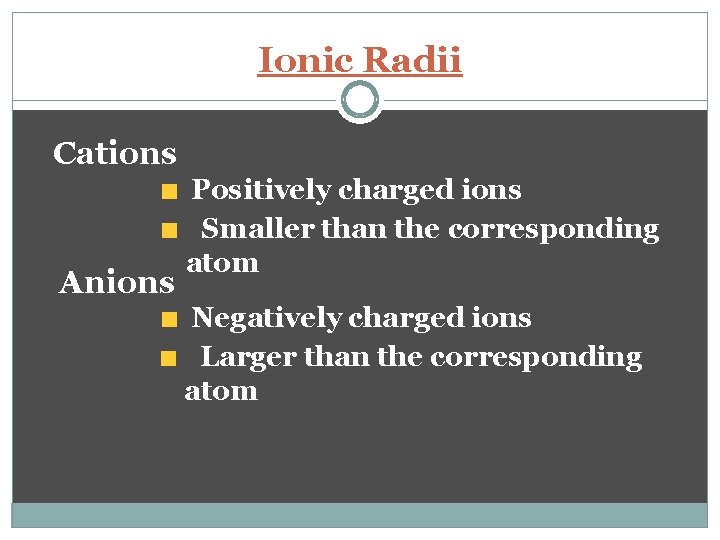
Ionic Radii Cations Anions Positively charged ions Smaller than the corresponding atom Negatively charged ions Larger than the corresponding atom


Electronegativity A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period * more positive charges in the nucleus to attract more elctrons Electronegativities tend to decrease down a group or remain the same * additional energy levels result in less attraction to the nucleus


 Evidence supporting mendeleev's table
Evidence supporting mendeleev's table Do metals have high ionization energy
Do metals have high ionization energy 6 the periodic table
6 the periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law V=k/p
V=k/p P=k/v
P=k/v Chapter 5 the periodic law
Chapter 5 the periodic law Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Valence electrons cheat sheet
Valence electrons cheat sheet Atomic size vs atomic radius
Atomic size vs atomic radius Alien periodic table periodic trends answers
Alien periodic table periodic trends answers Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau