Chemistry 163 General Chemistry 3 Chapter 13 Fundamental




















![Initially, Qc = Kc Increasing the [CH 4] results in the denominator of the Initially, Qc = Kc Increasing the [CH 4] results in the denominator of the](https://slidetodoc.com/presentation_image_h/bad7ef2f8342814dd820b6f92521ba1a/image-46.jpg)











- Slides: 92

Chemistry& 163 General Chemistry 3

Chapter 13: Fundamental Equilibrium Concepts 13. 1 Chemical Equilibria 13. 2 Equilibrium Constants 13. 3 Shifting Equilibria: Le Châtelier’s Principle 13. 4 Equilibrium Calculations 16. 4 Free Energy

Chapter 13: Fundamental Equilibrium Concepts 13. 1 Chemical Equilibria

Chemical kinetics describes how fast a product is produced. Reactants → Products Speed of reaction? = kinetics problem Chemical equilibrium properties describe how much product can be produced. Reactants → Products How much product made? = equilibrium problem

Previously, we have used stoichiometry calculations to determine the amounts of products. A B moles of A = moles of B In these problems we are assuming the reverse reaction is not occurring.

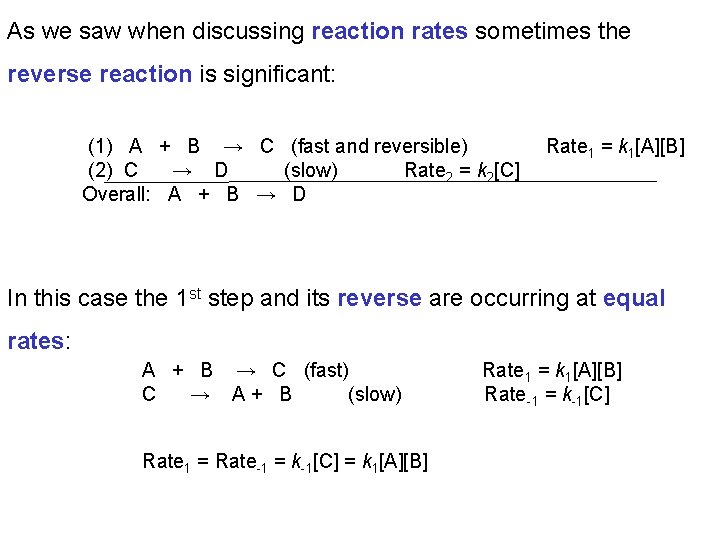
As we saw when discussing reaction rates sometimes the reverse reaction is significant: (1) A + B → C (fast and reversible) (2) C → D (slow) Rate 2 = k 2[C] Overall: A + B → D Rate 1 = k 1[A][B] In this case the 1 st step and its reverse are occurring at equal rates: A + B → C (fast) C → A+ B (slow) Rate 1 = Rate-1 = k-1[C] = k 1[A][B] Rate 1 = k 1[A][B] Rate -1 = k-1[C]

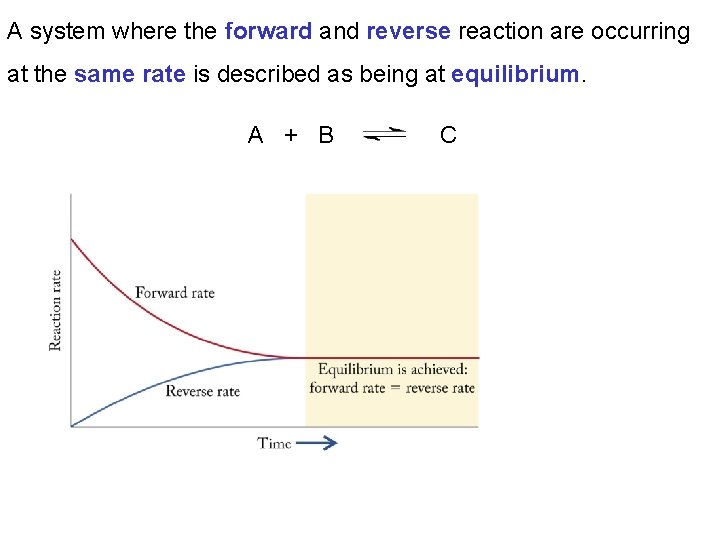
A system where the forward and reverse reaction are occurring at the same rate is described as being at equilibrium. A + B C

When a system is at equilibrium the amounts of products and reactants is constant. Previously we had used the idea of a full car park to visualize this idea “The vapor pressure of liquid is the partial pressure of a liquid when it is in equilibrium with its vapor”

Chapter 13: Fundamental Equilibrium Concepts 13. 1 Chemical Equilibria When a system has obtained a state of dynamic equilibrium the rate of the forward reaction equals the rate of the reverse reaction. From this time on if no change is made to the system the amounts of products and reactants will remain unchanged.

Chapter 13: Fundamental Equilibrium Concepts 13. 2 Equilibrium Constants

Some reactions achieve equilibrium after nearly all reactants have been converted to products. H 2 O + CO H 2 + CO 2 In this kind of system we say the equilibrium “lies to the right”

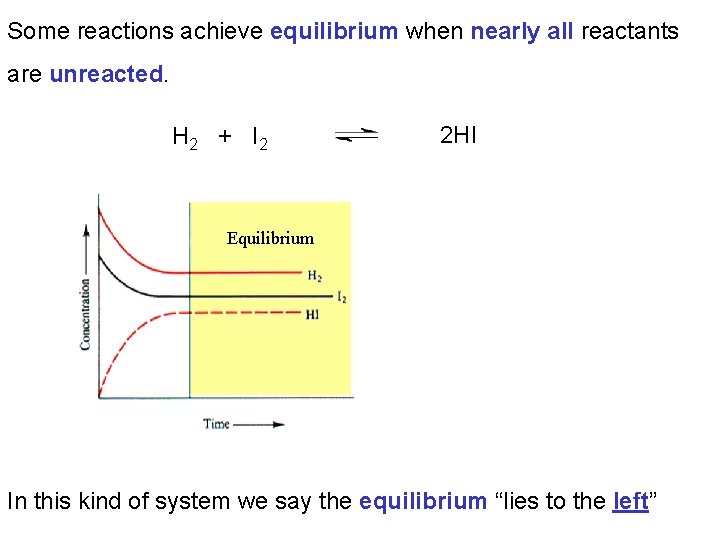
Some reactions achieve equilibrium when nearly all reactants are unreacted. H 2 + I 2 2 HI Equilibrium In this kind of system we say the equilibrium “lies to the left”

For a reversible reaction it is observed that: “at constant temperature the same equilibrium state is achieved regardless of the initial amounts of reactants and products” This is referred to as the “Law of Mass Action” What is meant by the same equilibrium state?

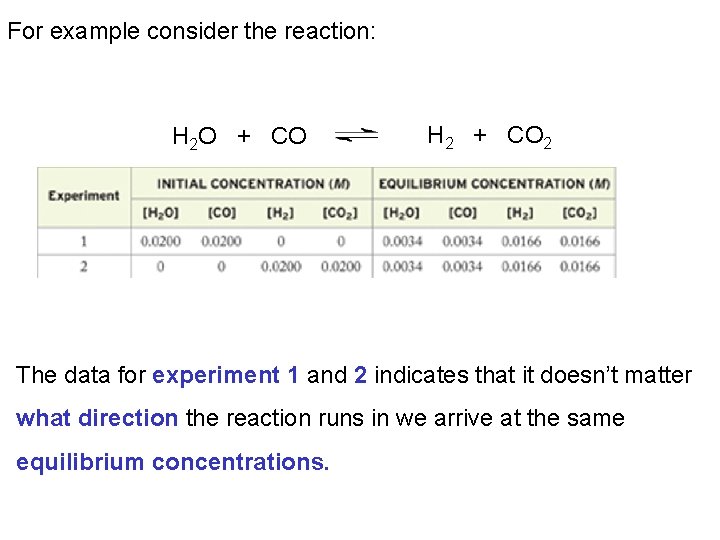
For example consider the reaction: H 2 O + CO H 2 + CO 2 The data for experiment 1 and 2 indicates that it doesn’t matter what direction the reaction runs in we arrive at the same equilibrium concentrations.

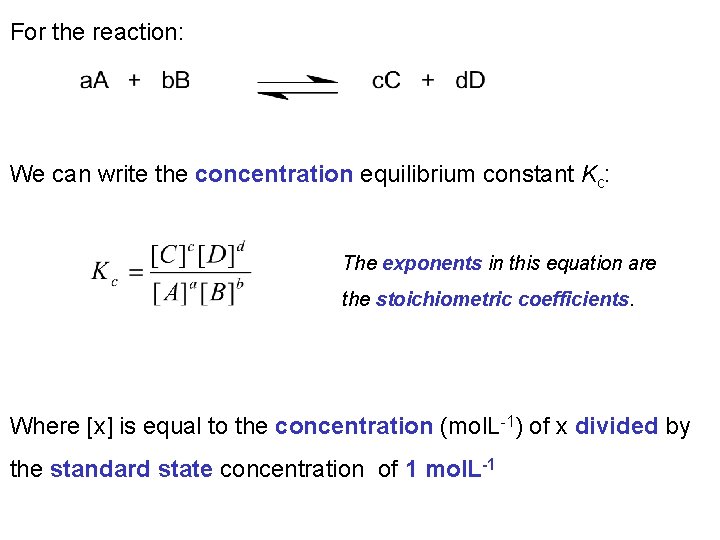
For the reaction: We can write the concentration equilibrium constant Kc: The exponents in this equation are the stoichiometric coefficients. Where [x] is equal to the concentration (mol. L-1) of x divided by the standard state concentration of 1 mol. L-1

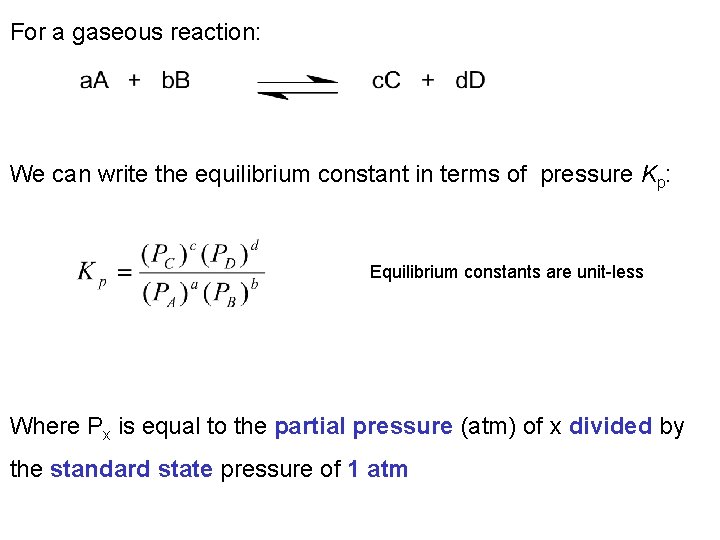
For a gaseous reaction: We can write the equilibrium constant in terms of pressure Kp: Equilibrium constants are unit-less Where Px is equal to the partial pressure (atm) of x divided by the standard state pressure of 1 atm

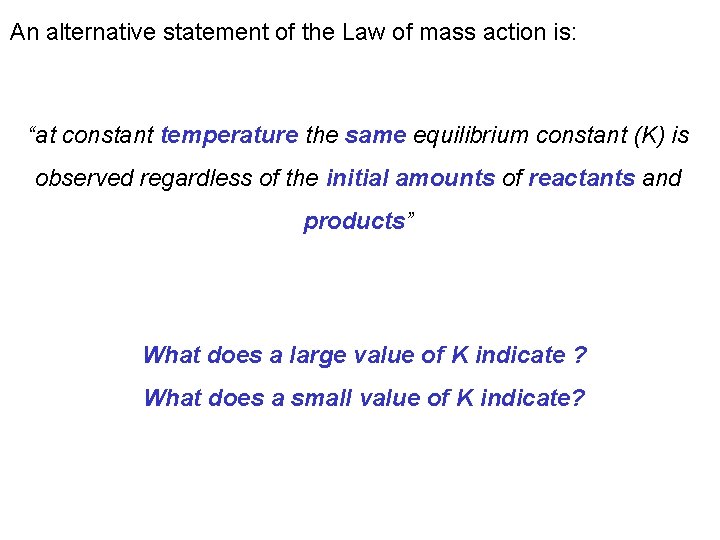
An alternative statement of the Law of mass action is: “at constant temperature the same equilibrium constant (K) is observed regardless of the initial amounts of reactants and products” What does a large value of K indicate ? What does a small value of K indicate?

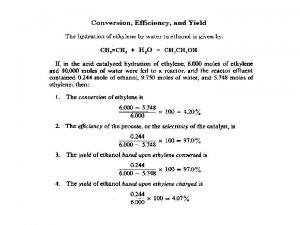
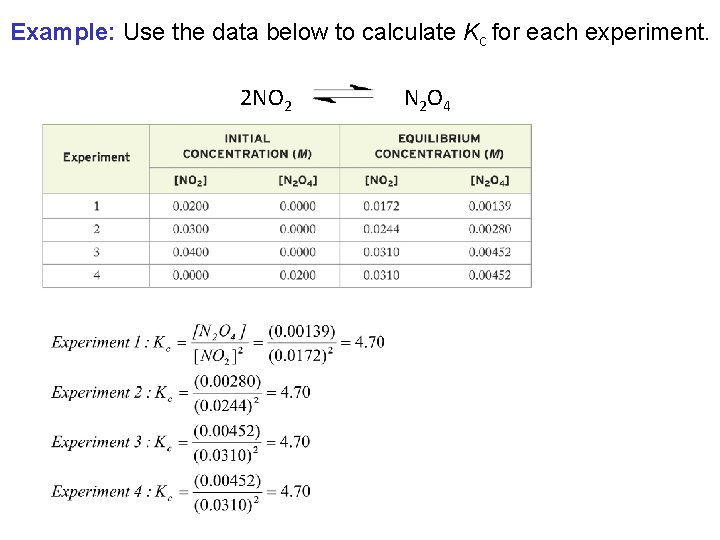
Example: Use the data below to calculate Kc for each experiment. 2 NO 2 N 2 O 4

In most cases: However, if you know one you can calculate the other. Consider the reaction: In this reaction 3 moles of gas reacts to produce 2 moles of gas.

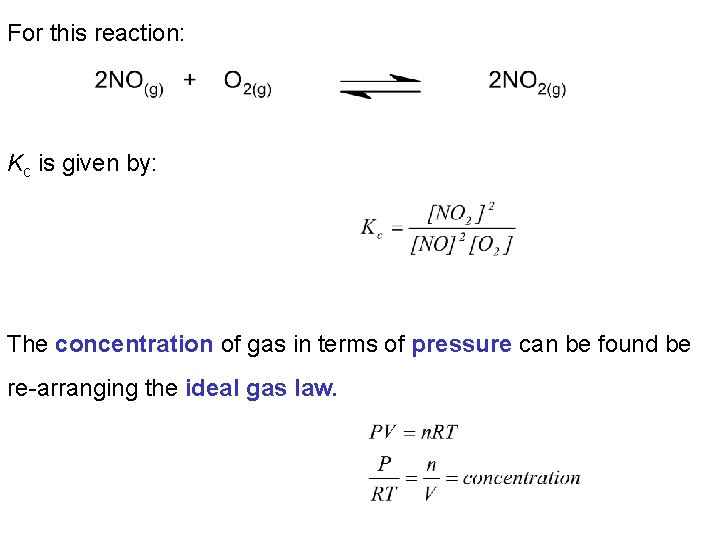
For this reaction: Kc is given by: The concentration of gas in terms of pressure can be found be re-arranging the ideal gas law.

We can now rewrite Kc in terms of pressure:

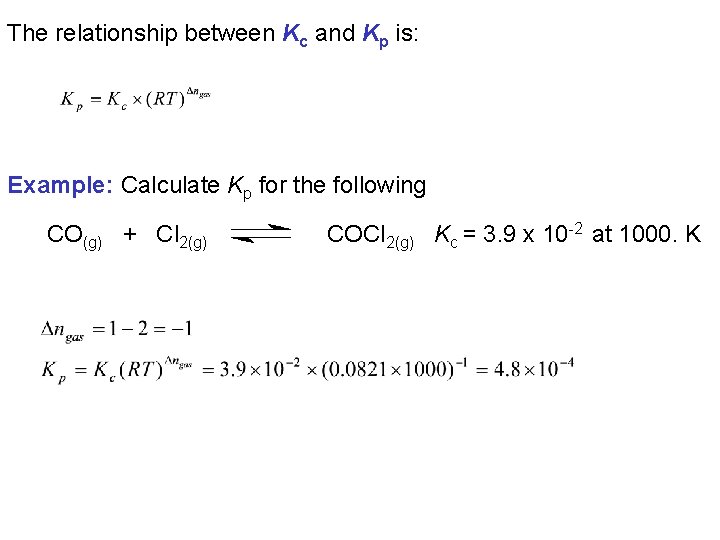
The relationship between Kc and Kp is: Example: Calculate Kp for the following CO(g) + Cl 2(g) COCl 2(g) Kc = 3. 9 x 10 -2 at 1000. K

Your turn! Calculate Kp for the following S 2(g) + C(s) CS 2 Kc = 28. 5 at 500. K H 2(g) + I 2(g) 2 HI(g) Kc = 49 at 730. K Kp = 6. 9 x 10 -1 Kp = 49 Note: if Δngas = 0 then Kc = Kp

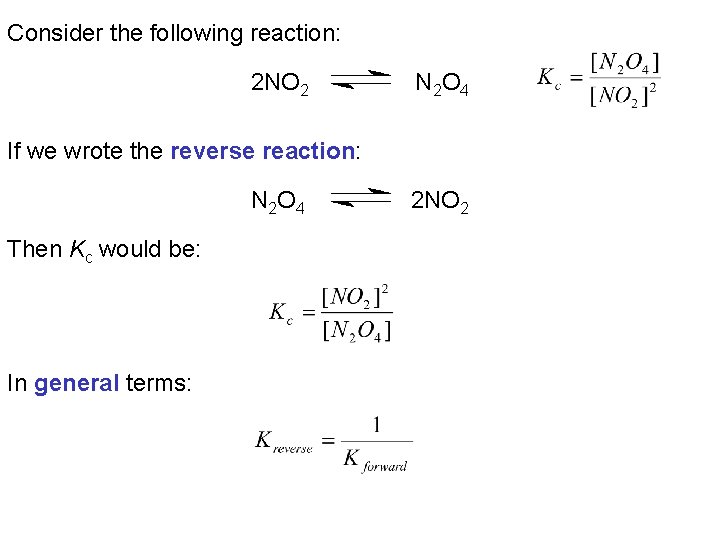
Consider the following reaction: 2 NO 2 N 2 O 4 If we wrote the reverse reaction: N 2 O 4 Then Kc would be: In general terms: 2 NO 2

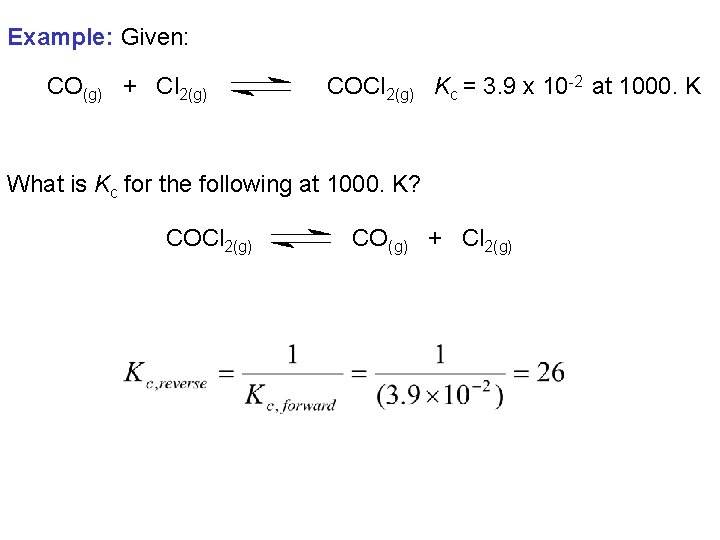
Example: Given: CO(g) + Cl 2(g) COCl 2(g) Kc = 3. 9 x 10 -2 at 1000. K What is Kc for the following at 1000. K? COCl 2(g) CO(g) + Cl 2(g)

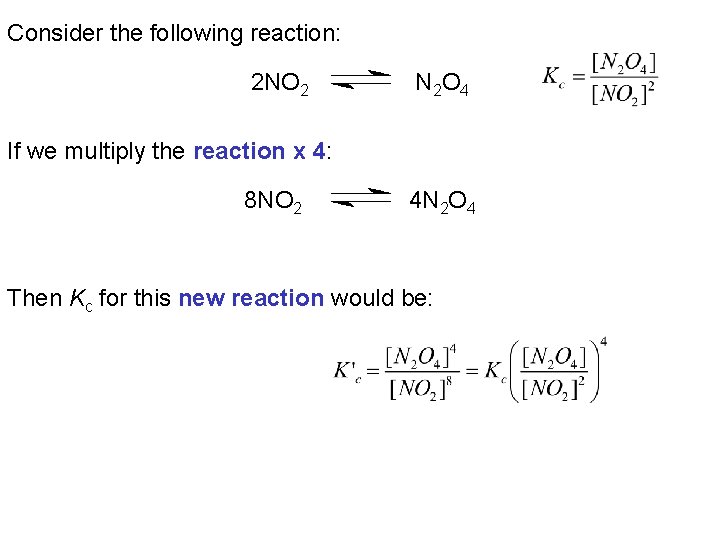
Consider the following reaction: 2 NO 2 N 2 O 4 If we multiply the reaction x 4: 8 NO 2 4 N 2 O 4 Then Kc for this new reaction would be:

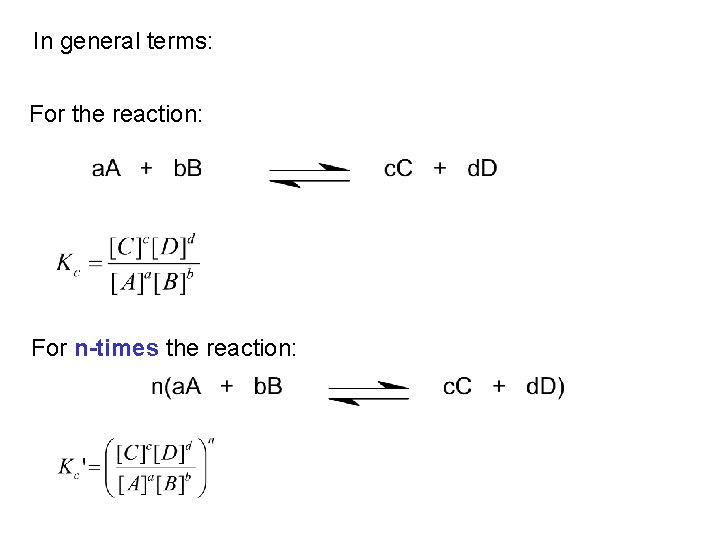
In general terms: For the reaction: For n-times the reaction:

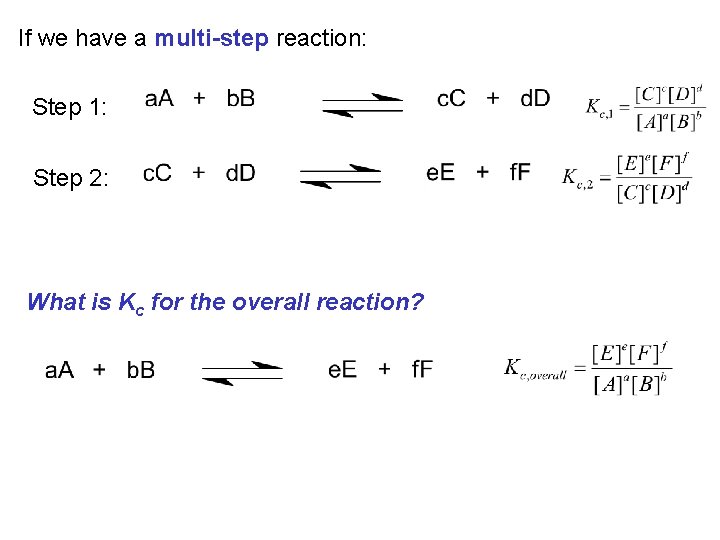
If we have a multi-step reaction: Step 1: Step 2: What is Kc for the overall reaction?

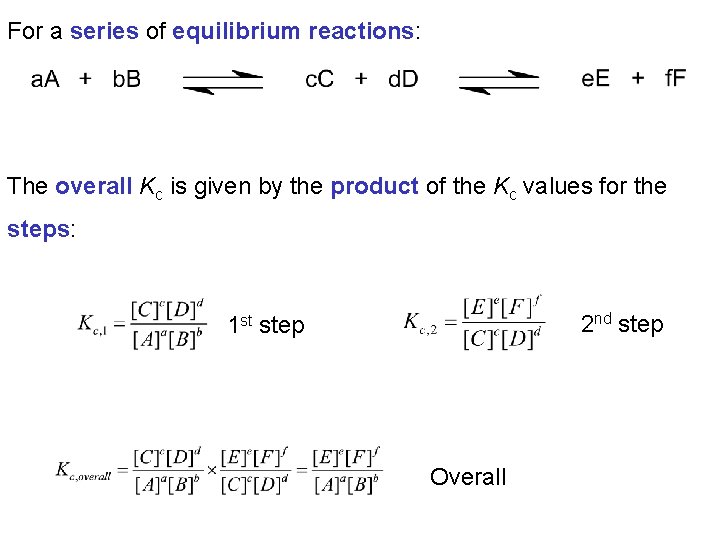
For a series of equilibrium reactions: The overall Kc is given by the product of the Kc values for the steps: 2 nd step 1 st step Overall

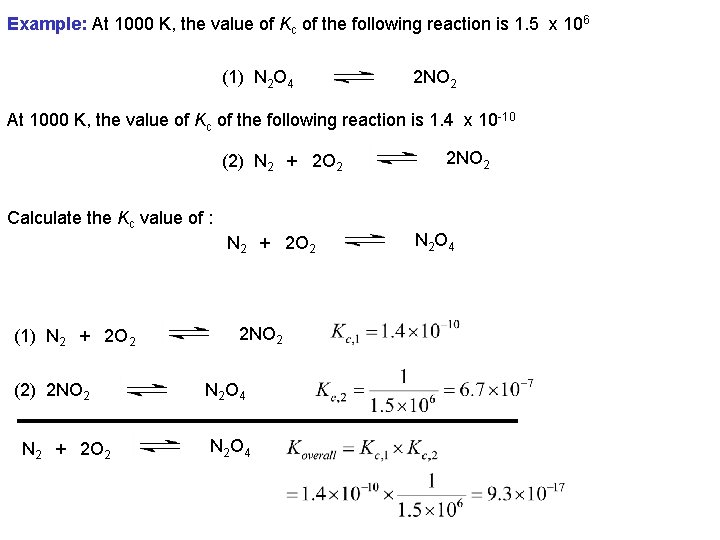
Example: At 1000 K, the value of Kc of the following reaction is 1. 5 x 106 (1) N 2 O 4 2 NO 2 At 1000 K, the value of Kc of the following reaction is 1. 4 x 10 -10 (2) N 2 + 2 O 2 Calculate the Kc value of : N 2 + 2 O 2 (1) N 2 + 2 O 2 (2) 2 NO 2 N 2 + 2 O 2 2 NO 2 N 2 O 4

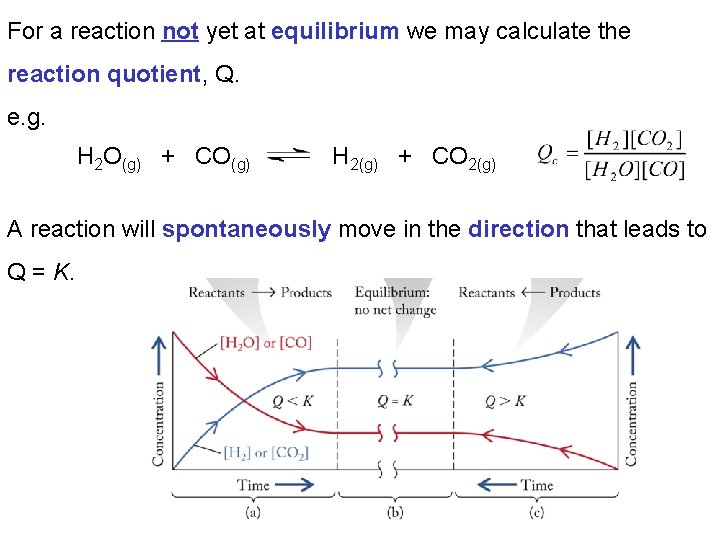
For a reaction not yet at equilibrium we may calculate the reaction quotient, Q. e. g. H 2 O(g) + CO(g) H 2(g) + CO 2(g) A reaction will spontaneously move in the direction that leads to Q = K.

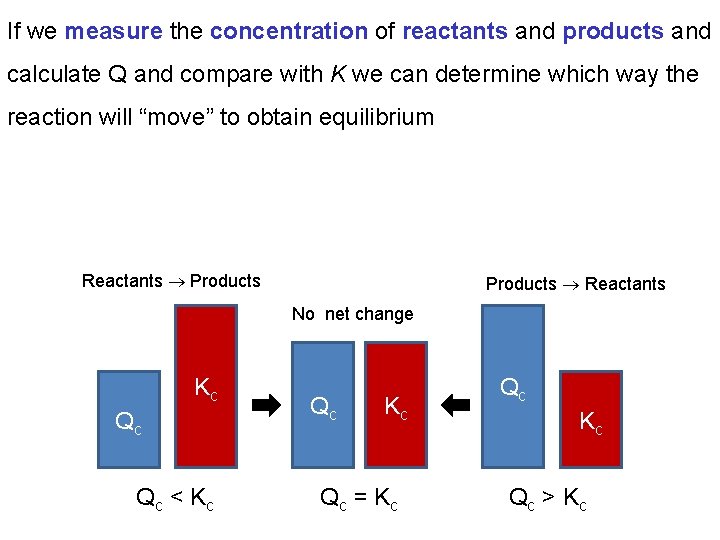
If we measure the concentration of reactants and products and calculate Q and compare with K we can determine which way the reaction will “move” to obtain equilibrium Reactants Products Reactants No net change Kc Qc Qc < K c Qc Kc Qc = K c Qc Kc Qc > K c

Example: The value of Kc for the following reaction: 2 NO 2 N 2 O 4 is 4. 7 at 373 K. Is a mixture of two gases in which [NO 2] = 0. 025 mol/L and [N 2 O 4] = 0. 0014 mol/L at equilibrium? If not in which direction will the reaction proceed? The reaction will proceed in the direction of the products (to the right).

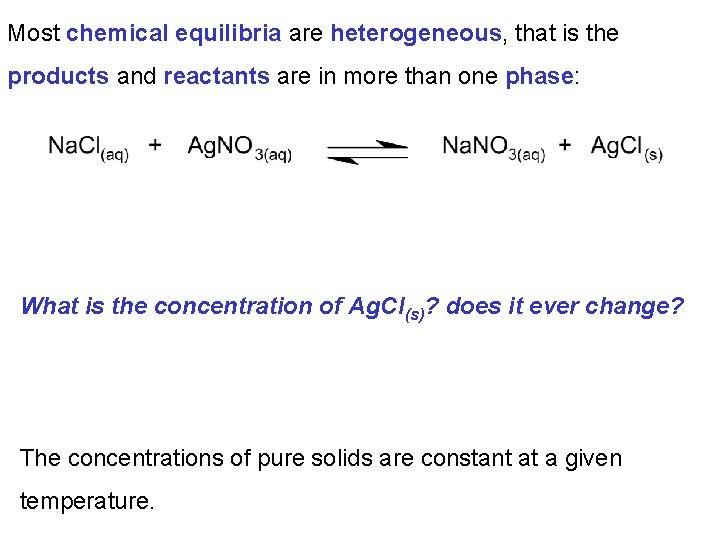
Most chemical equilibria are heterogeneous, that is the products and reactants are in more than one phase: What is the concentration of Ag. Cl(s)? does it ever change? The concentrations of pure solids are constant at a given temperature.

Consider the equilibria: Sucrose(aq) + H 2 O(l) Fructose(aq) + Glucose(aq) What is the concentration of H 2 O(l)? does it ever change? The concentrations of pure liquids are constant at a given temperature.

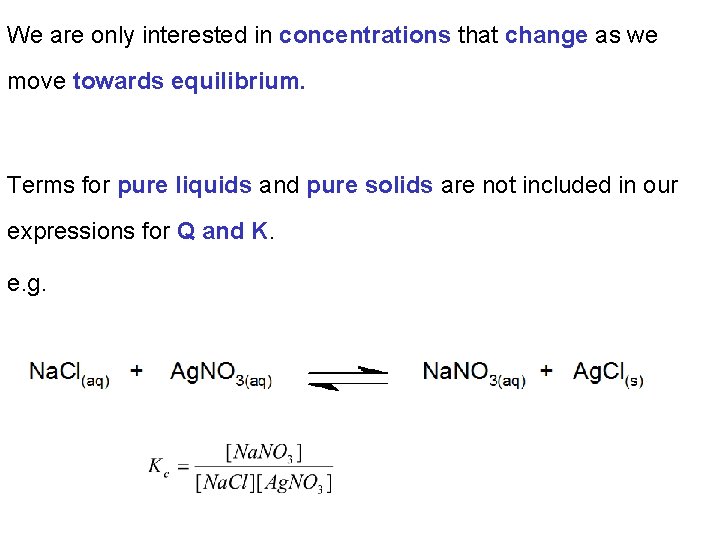
We are only interested in concentrations that change as we move towards equilibrium. Terms for pure liquids and pure solids are not included in our expressions for Q and K. e. g.

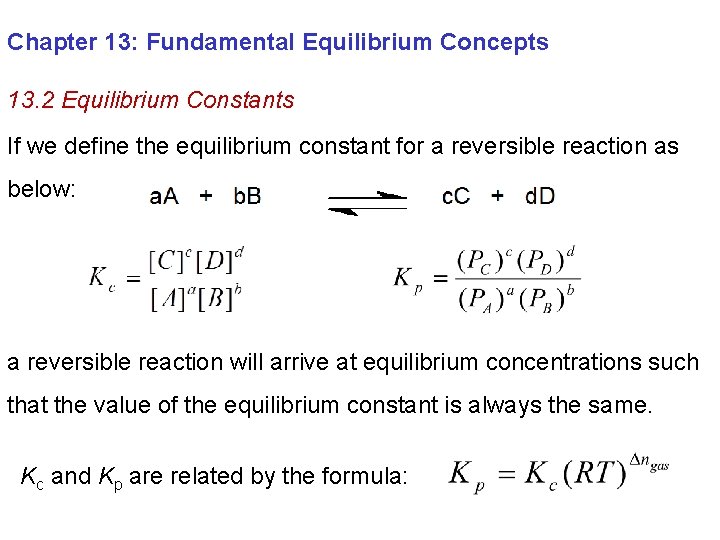
Chapter 13: Fundamental Equilibrium Concepts 13. 2 Equilibrium Constants If we define the equilibrium constant for a reversible reaction as below: a reversible reaction will arrive at equilibrium concentrations such that the value of the equilibrium constant is always the same. Kc and Kp are related by the formula:

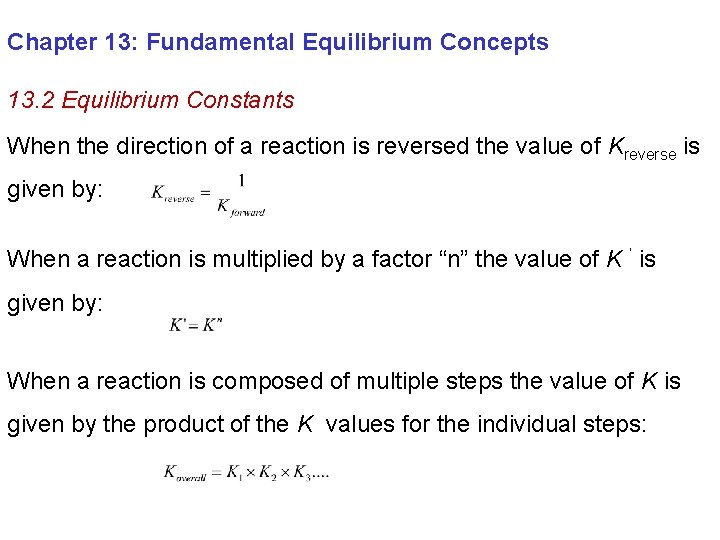
Chapter 13: Fundamental Equilibrium Concepts 13. 2 Equilibrium Constants When the direction of a reaction is reversed the value of Kreverse is given by: When a reaction is multiplied by a factor “n” the value of K ’ is given by: When a reaction is composed of multiple steps the value of K is given by the product of the K values for the individual steps:

Chapter 13: Fundamental Equilibrium Concepts 13. 2 Equilibrium Constants A system not at equilibrium (Q ≠ K) will spontaneously move in the direction to bring it to equilibrium. If Q < K then reactants will be converted into products until equilibrium is achieved. If Q > K then products will be converted into reactants until equilibrium is achieved.

Chapter 13: Fundamental Equilibrium Concepts 13. 2 Equilibrium Constants As the concentrations of pure solids and pure liquids are constants they are not included in the expressions for Q and K.

Chapter 13: Fundamental Equilibrium Concepts 13. 3 Shifting Equilibria: Le Châtelier’s Principle

Le Châtelier’s principle states: “When a change is made to a chemical system at equilibrium the system will spontaneously return to the equilibrium position” Henry Louis Le Châtelier 1850 - 1936

“When a change is made to a chemical system at equilibrium the system will spontaneously return to the equilibrium position” What do we mean by a “change”? Any physical or chemical change that results in Q ≠ K What do we mean by a “chemical system at equilibrium”? We mean Q = K for the system

“When a change is made to a chemical system at equilibrium the system will spontaneously return to the equilibrium position” What do we mean by “spontaneously return to the equilibrium position”? We mean that without intervention the system will adjust so Q=K

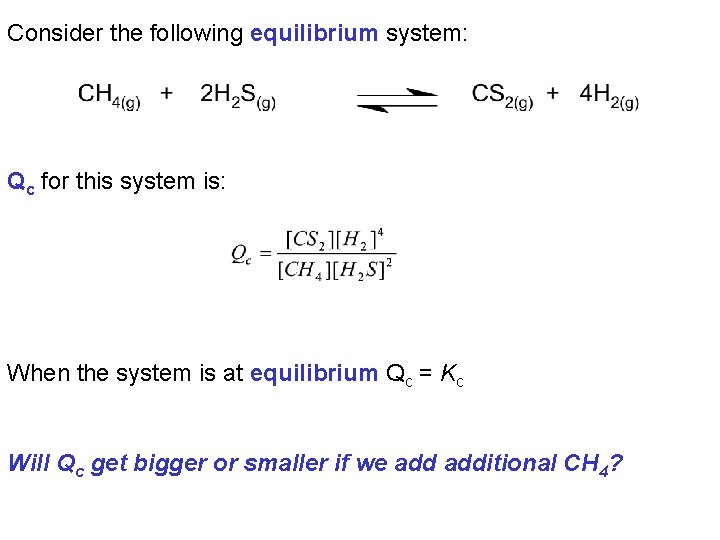
Consider the following equilibrium system: Qc for this system is: When the system is at equilibrium Qc = Kc Will Qc get bigger or smaller if we additional CH 4?
![Initially Qc Kc Increasing the CH 4 results in the denominator of the Initially, Qc = Kc Increasing the [CH 4] results in the denominator of the](https://slidetodoc.com/presentation_image_h/bad7ef2f8342814dd820b6f92521ba1a/image-46.jpg)
Initially, Qc = Kc Increasing the [CH 4] results in the denominator of the expression for Qc getting bigger and Qc becoming smaller. i. e. after addition of CH 4, Qc < Kc

Initially, Qc = Kc After addition of CH 4, Qc < Kc Le Châteliers principle tells us the system will adjust so that Qc = Kc again. So how can we make Qc bigger?

Initially, Qc = Kc. After addition of CH 4, Qc < Kc So how can we make Qc bigger? By making the numerator in Qc smaller (consuming reactants) and making the denominator bigger (producing products).

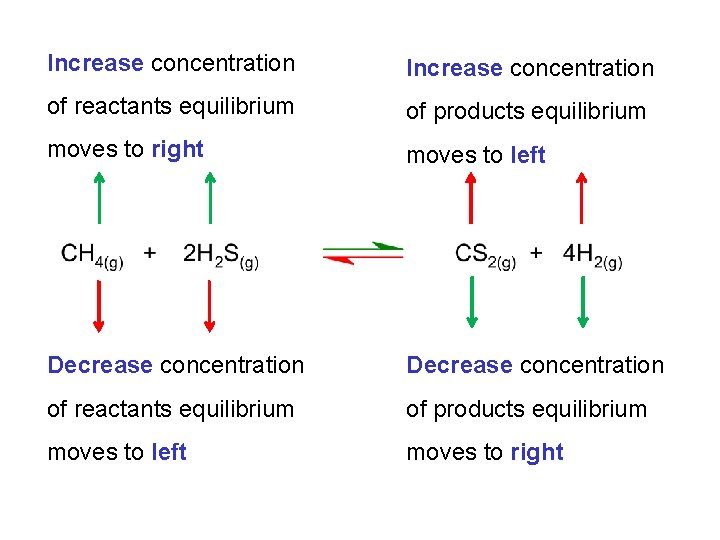
The net result of adding CH 4 to this system was the formation of more products. “the equilibrium moved to the right” Adding reactants always results in the equilibrium moving to the right. Similarly, removing products will also result in the equilibrium moving to the right.

Increase concentration of reactants equilibrium of products equilibrium moves to right moves to left Decrease concentration of reactants equilibrium of products equilibrium moves to left moves to right

The famous “donkey cart” picture takes on new meaning when we think in terms of equilibria.

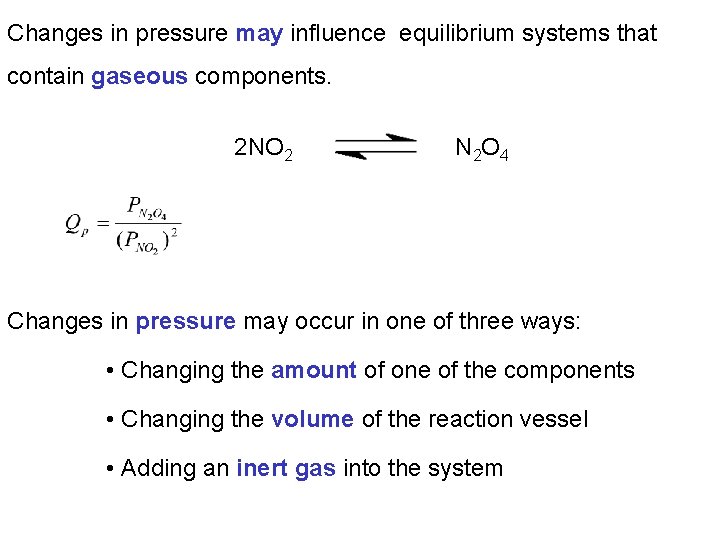
Changes in pressure may influence equilibrium systems that contain gaseous components. 2 NO 2 N 2 O 4 Changes in pressure may occur in one of three ways: • Changing the amount of one of the components • Changing the volume of the reaction vessel • Adding an inert gas into the system

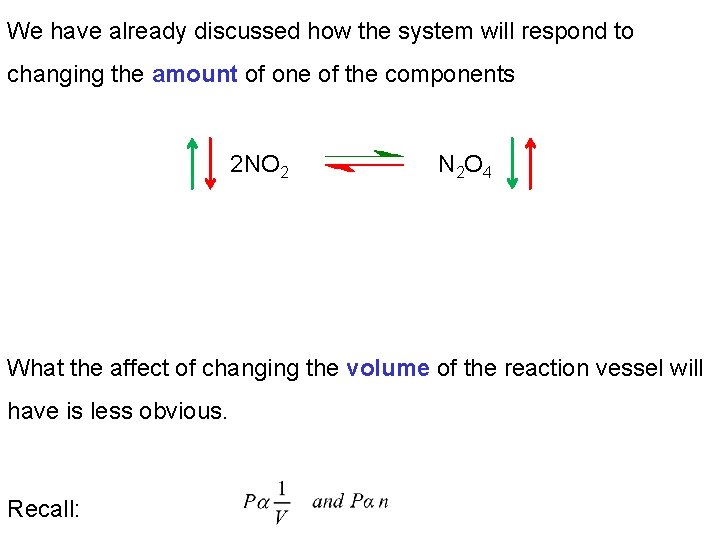
We have already discussed how the system will respond to changing the amount of one of the components 2 NO 2 N 2 O 4 What the affect of changing the volume of the reaction vessel will have is less obvious. Recall:

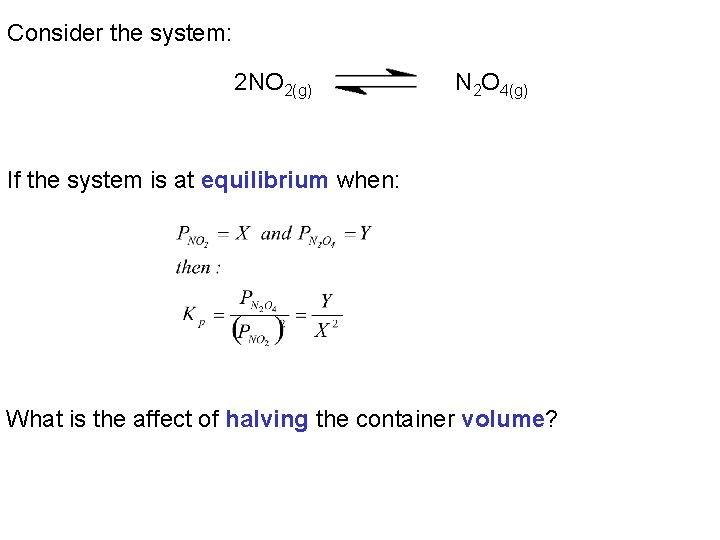
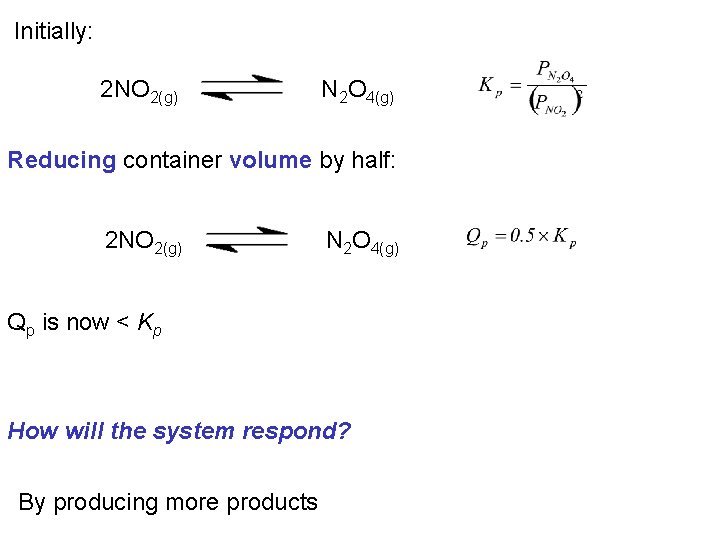
Consider the system: 2 NO 2(g) N 2 O 4(g) If the system is at equilibrium when: What is the affect of halving the container volume?

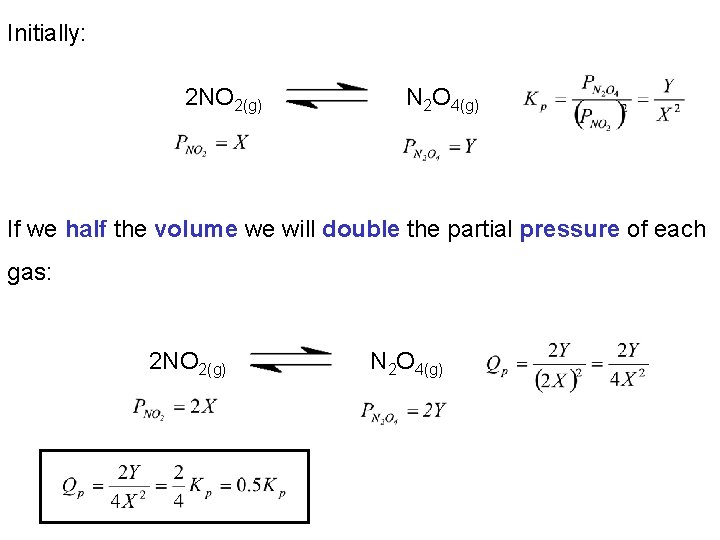
Initially: 2 NO 2(g) N 2 O 4(g) If we half the volume we will double the partial pressure of each gas: 2 NO 2(g) N 2 O 4(g)

Initially: 2 NO 2(g) N 2 O 4(g) Reducing container volume by half: 2 NO 2(g) N 2 O 4(g) Qp is now < Kp How will the system respond? By producing more products

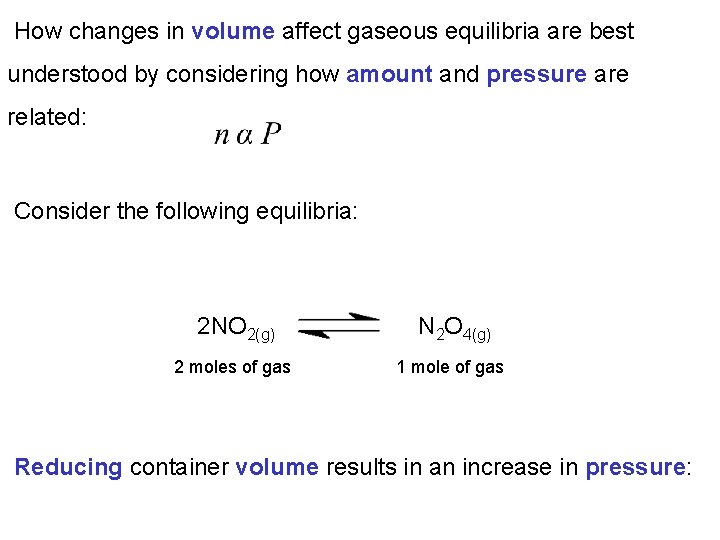
How changes in volume affect gaseous equilibria are best understood by considering how amount and pressure are related: Consider the following equilibria: 2 NO 2(g) N 2 O 4(g) 2 moles of gas 1 mole of gas Reducing container volume results in an increase in pressure:

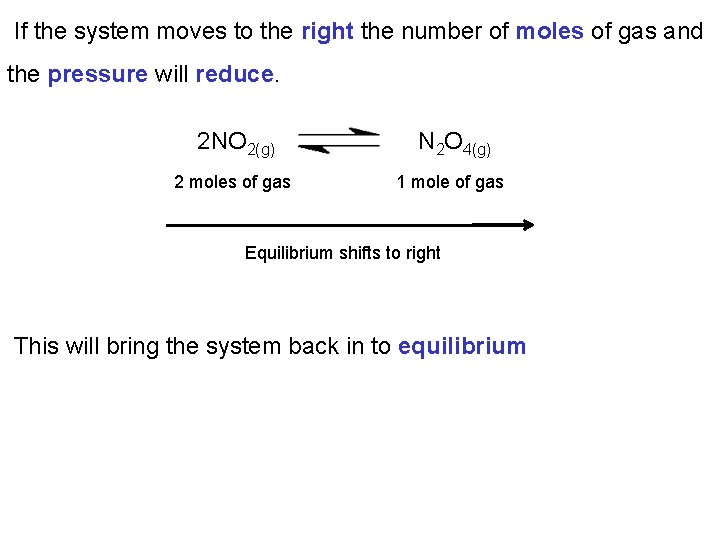
If the system moves to the right the number of moles of gas and the pressure will reduce. 2 NO 2(g) N 2 O 4(g) 2 moles of gas 1 mole of gas Equilibrium shifts to right This will bring the system back in to equilibrium

Example: How will the following system respond to a reduction in volume? 3 moles of gas 5 moles of gas A reduction in volume will increase the pressure. The system will spontaneously adjust to decrease the pressure. The equilibrium will move to the left.

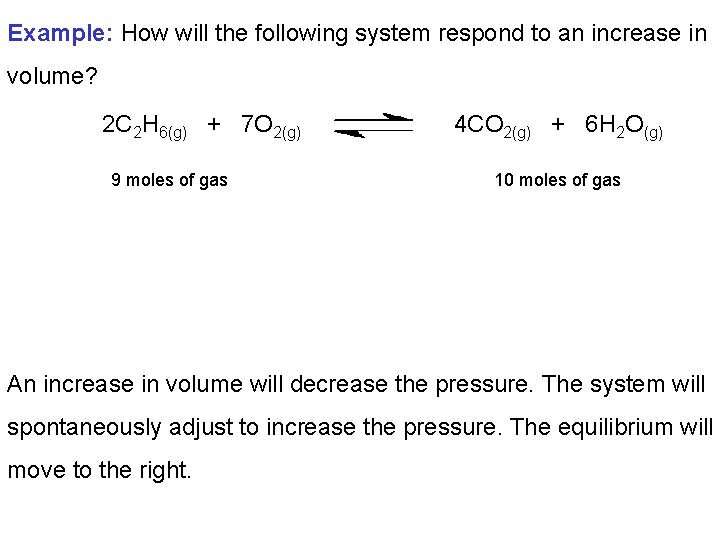
Example: How will the following system respond to an increase in volume? 2 C 2 H 6(g) + 7 O 2(g) 9 moles of gas 4 CO 2(g) + 6 H 2 O(g) 10 moles of gas An increase in volume will decrease the pressure. The system will spontaneously adjust to increase the pressure. The equilibrium will move to the right.

Qp is determined only by the partial pressure of each reactant and product. Adding an inert gas will increase the total pressure but will not change any of the partial pressures. Will adding an inert gas cause the equilibrium position to shift? Adding an inert gas does not affect the equilibria

Temperature changes result in a change in the value of K. For an exothermic process: A + B C + Heat Typically, increasing T decreases K favoring reactants (and vice versa) For an endothermic process: A + B + Heat C Typically, increasing T increases K favoring products (and vice versa)

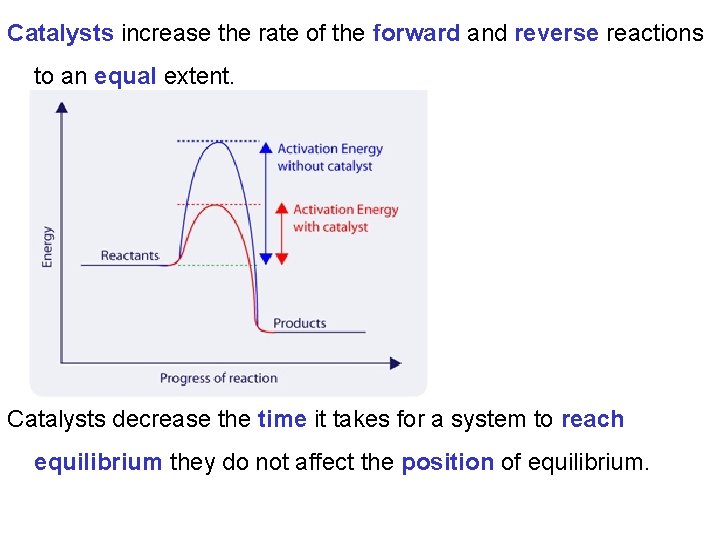
Catalysts increase the rate of the forward and reverse reactions to an equal extent. Catalysts decrease the time it takes for a system to reach equilibrium they do not affect the position of equilibrium.

Chapter 13: Fundamental Equilibrium Concepts 13. 3 Shifting Equilibria: Le Châtelier’s Principle If a system at equilibrium is perturbed the system will adjust to bring it back into equilibrium. This perturbation may include: – Changes in concentration – Changes in partial pressure Changing the temperature of a system changes K, typically. If we consider heat as a reactant/product we can predict the effect of a temperature change. Catalysts do not affect the position of the equilibrium.

Chapter 13: Fundamental Equilibrium Concepts 13. 4 Equilibrium Calculations

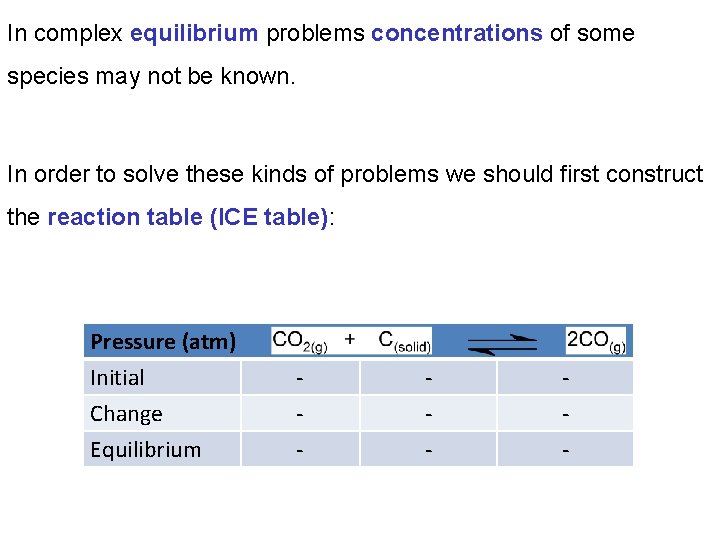
In complex equilibrium problems concentrations of some species may not be known. In order to solve these kinds of problems we should first construct the reaction table (ICE table): Pressure (atm) Initial Change Equilibrium - - -

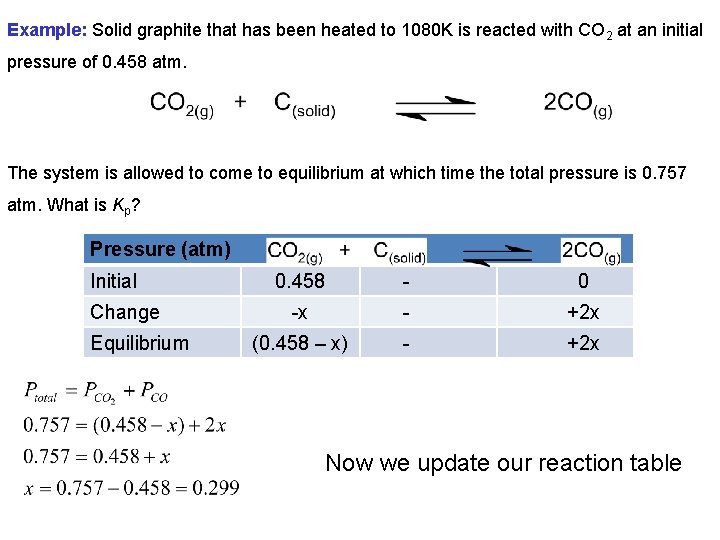
Example: Solid graphite that has been heated to 1080 K is reacted with CO 2 at an initial pressure of 0. 458 atm. The system is allowed to come to equilibrium at which time the total pressure is 0. 757 atm. What is Kp? Pressure (atm) Initial Change Equilibrium 0. 458 - 0 -x - +2 x (0. 458 – x) - +2 x Now we update our reaction table

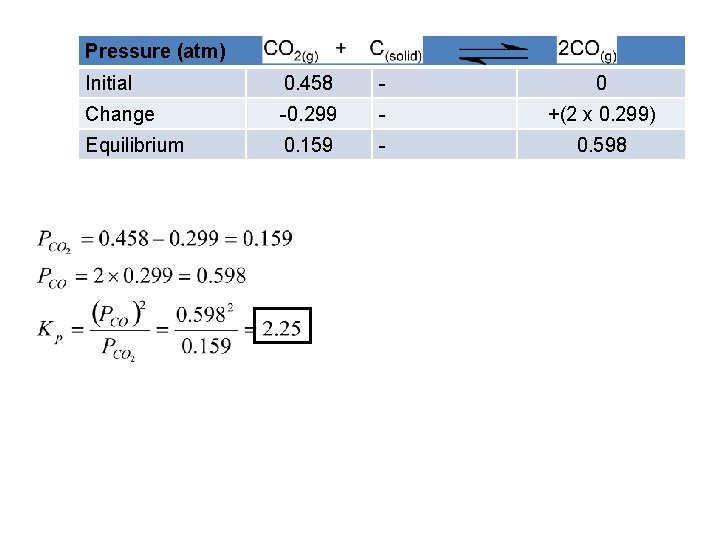
Pressure (atm) Initial 0. 458 - 0 Change -0. 299 - +(2 x 0. 299) Equilibrium 0. 159 - 0. 598

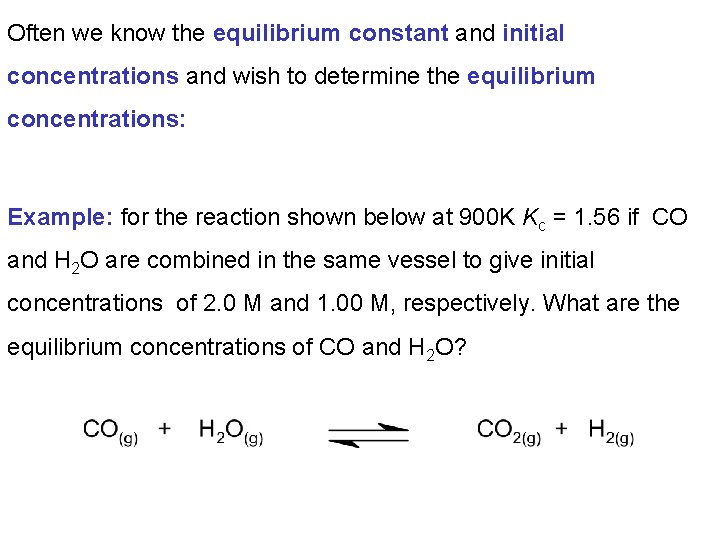
Often we know the equilibrium constant and initial concentrations and wish to determine the equilibrium concentrations: Example: for the reaction shown below at 900 K Kc = 1. 56 if CO and H 2 O are combined in the same vessel to give initial concentrations of 2. 0 M and 1. 00 M, respectively. What are the equilibrium concentrations of CO and H 2 O?

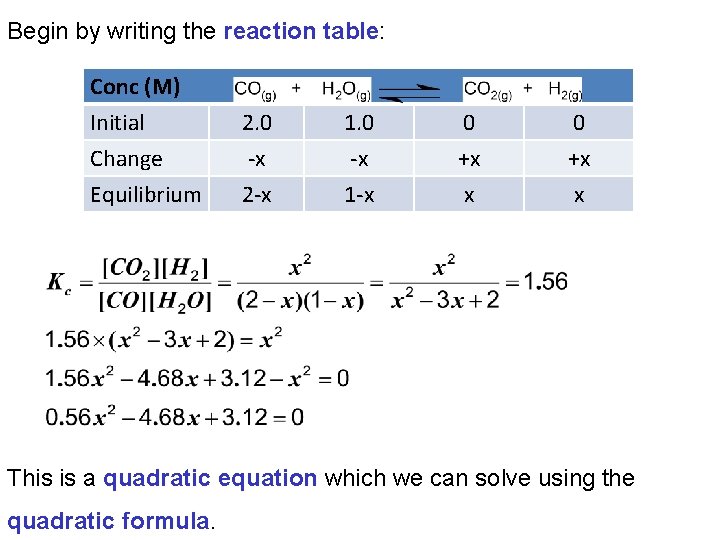
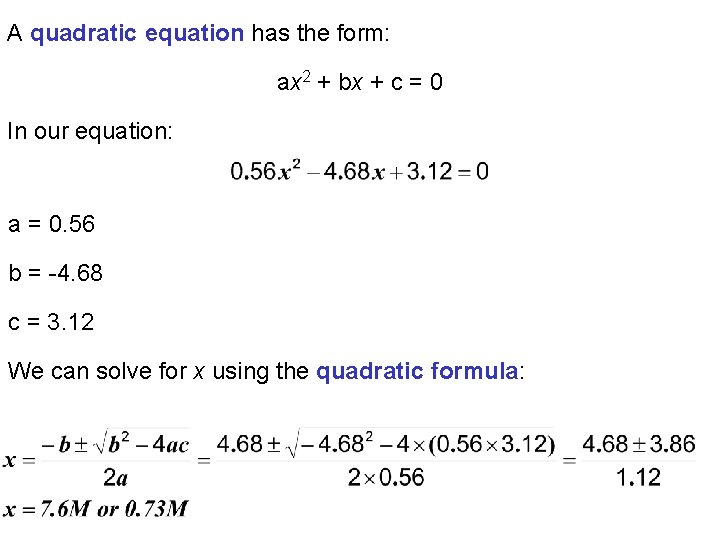
Begin by writing the reaction table: Conc (M) Initial Change Equilibrium 2. 0 -x 2 -x 1. 0 -x 1 -x 0 +x x This is a quadratic equation which we can solve using the quadratic formula.

A quadratic equation has the form: ax 2 + bx + c = 0 In our equation: a = 0. 56 b = -4. 68 c = 3. 12 We can solve for x using the quadratic formula:

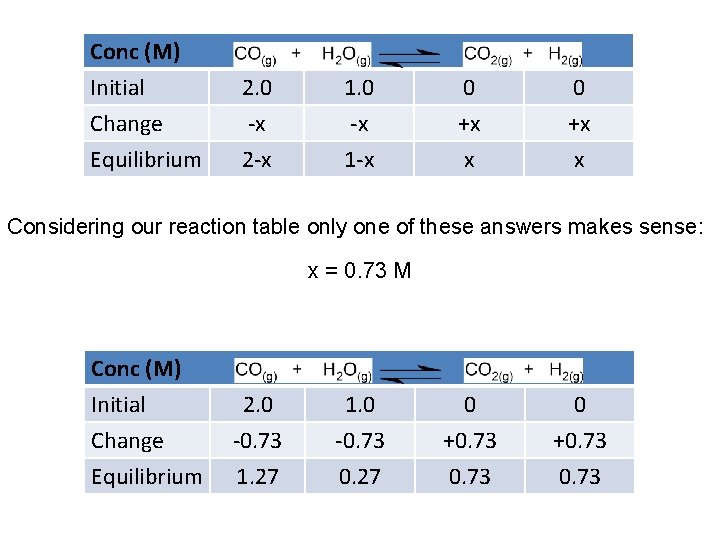
Conc (M) Initial Change Equilibrium 2. 0 -x 2 -x 1. 0 -x 1 -x 0 +x x Considering our reaction table only one of these answers makes sense: x = 0. 73 M Conc (M) Initial Change Equilibrium 2. 0 -0. 73 1. 27 1. 0 -0. 73 0. 27 0 +0. 73

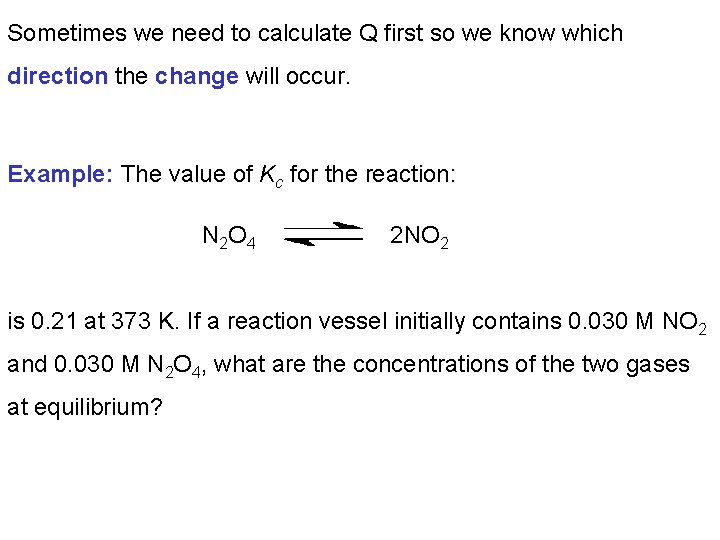
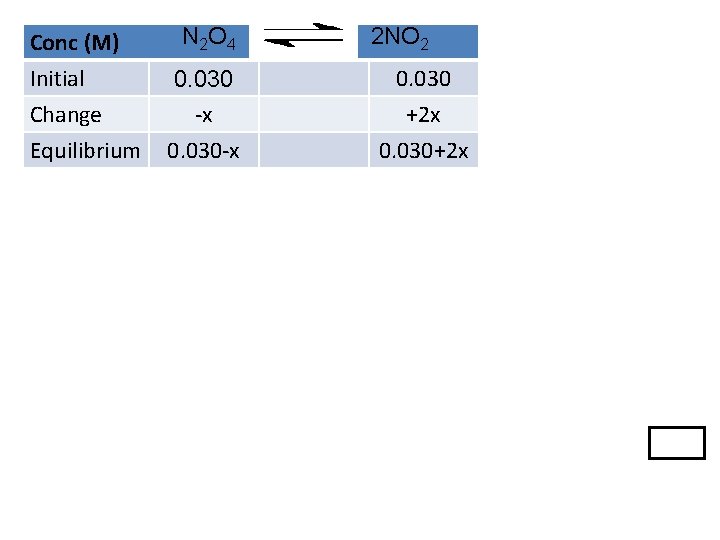
Sometimes we need to calculate Q first so we know which direction the change will occur. Example: The value of Kc for the reaction: N 2 O 4 2 NO 2 is 0. 21 at 373 K. If a reaction vessel initially contains 0. 030 M NO 2 and 0. 030 M N 2 O 4, what are the concentrations of the two gases at equilibrium?

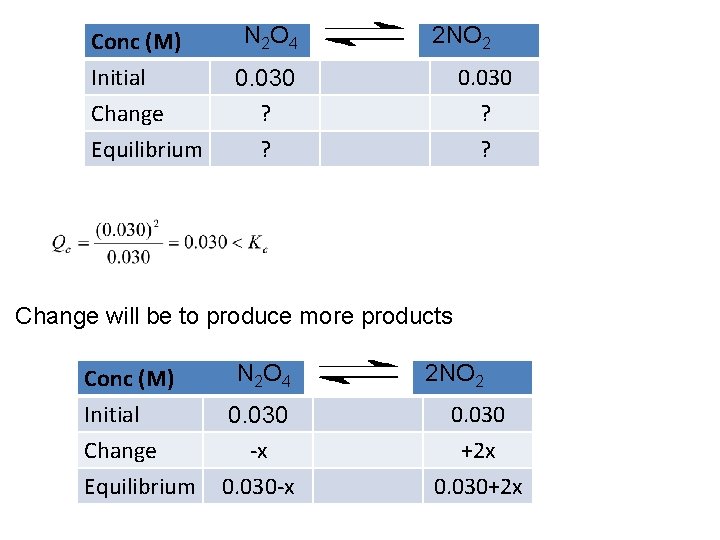
Conc (M) Initial Change Equilibrium N 2 O 4 2 NO 2 0. 030 ? ? Change will be to produce more products Conc (M) Initial Change Equilibrium N 2 O 4 0. 030 -x 0. 030 -x 2 NO 2 0. 030 +2 x 0. 030+2 x

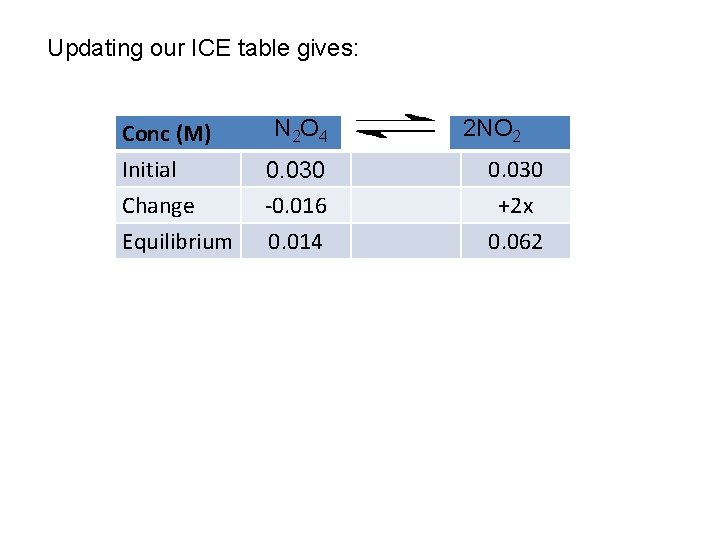
Conc (M) Initial Change Equilibrium N 2 O 4 0. 030 -x 0. 030 -x 2 NO 2 0. 030 +2 x 0. 030+2 x

Updating our ICE table gives: Conc (M) Initial Change Equilibrium N 2 O 4 0. 030 -0. 016 0. 014 2 NO 2 0. 030 +2 x 0. 062

Chapter 13: Fundamental Equilibrium Concepts 13. 4 Equilibrium Calculations Given initial concentrations and an equilibrium constant it is possible to establish a reaction (ICE) table to ease the determination of the equilibrium concentrations of the reactants and products.

Chapter 13: Fundamental Equilibrium Concepts 16. 4 Free Energy

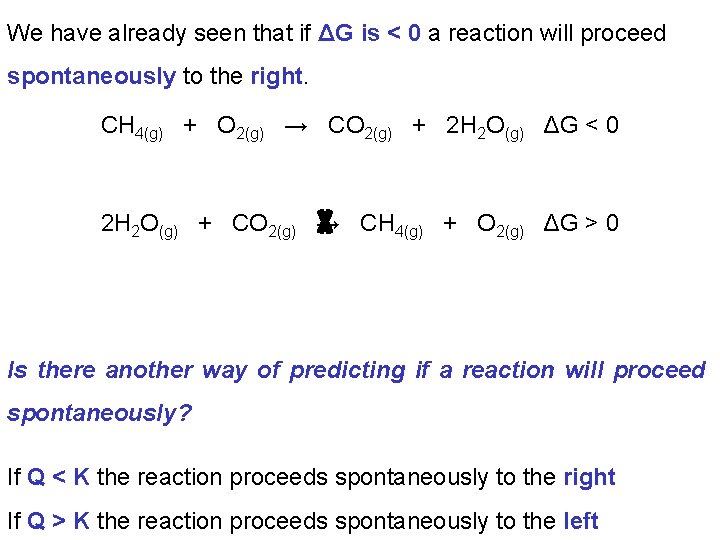
We have already seen that if ΔG is < 0 a reaction will proceed spontaneously to the right. CH 4(g) + O 2(g) → CO 2(g) + 2 H 2 O(g) ΔG < 0 2 H 2 O(g) + CO 2(g) → CH 4(g) + O 2(g) ΔG > 0 Is there another way of predicting if a reaction will proceed spontaneously? If Q < K the reaction proceeds spontaneously to the right If Q > K the reaction proceeds spontaneously to the left

A reaction will proceed spontaneously until ΔG = 0 (no net movement either to products or reactants) Alternatively we can say, A reaction will proceed spontaneously until Q = K i. e. When ΔG = O the system is at equilibrium Q and ΔG are both measures of how far a system is from equilibrium.

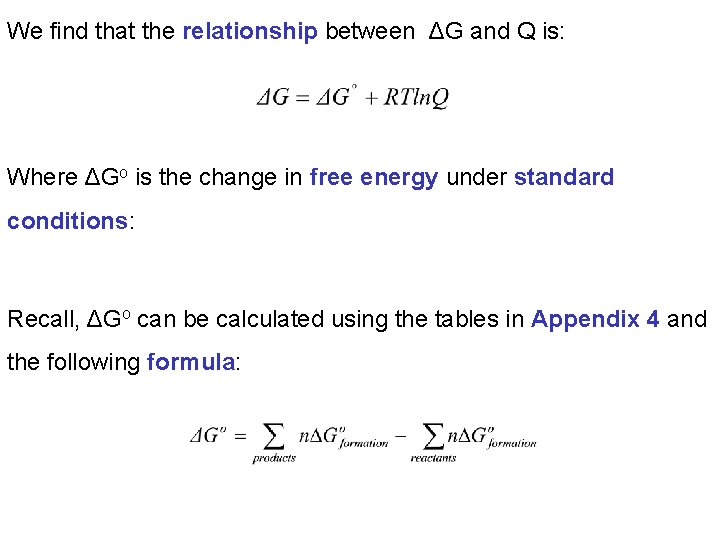
We find that the relationship between ΔG and Q is: Where ΔGo is the change in free energy under standard conditions: Recall, ΔGo can be calculated using the tables in Appendix 4 and the following formula:

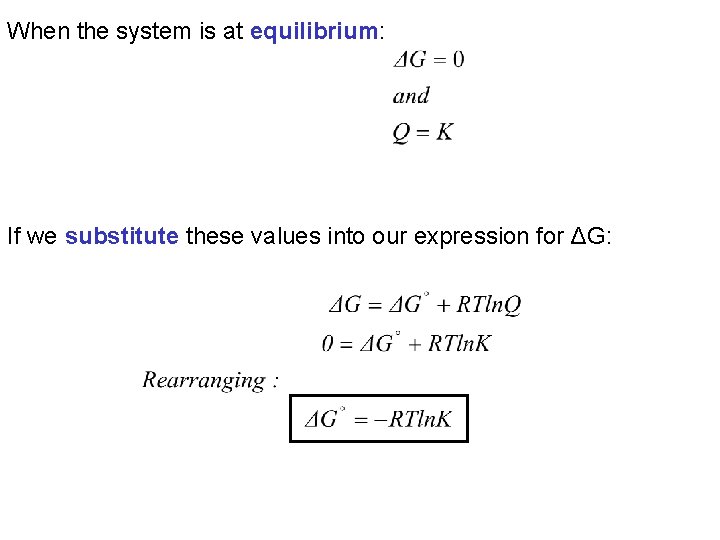
When the system is at equilibrium: If we substitute these values into our expression for ΔG:

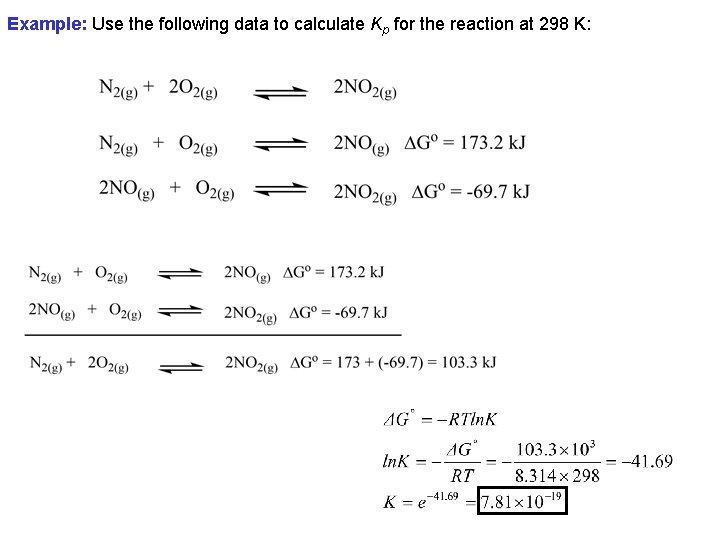
Example: Use the following data to calculate Kp for the reaction at 298 K:

We have noted qualitatively that K is dependent on temperature. e. g. The value of K is clearly dependent on T. Is there a quantitative relationship between T and K?

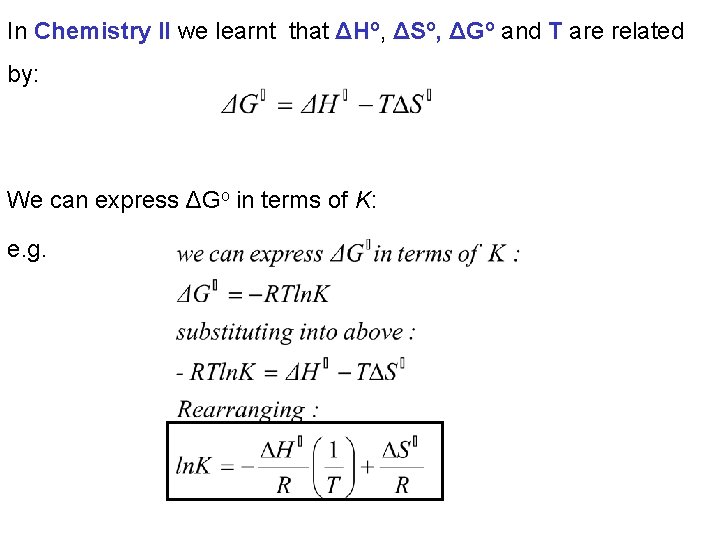
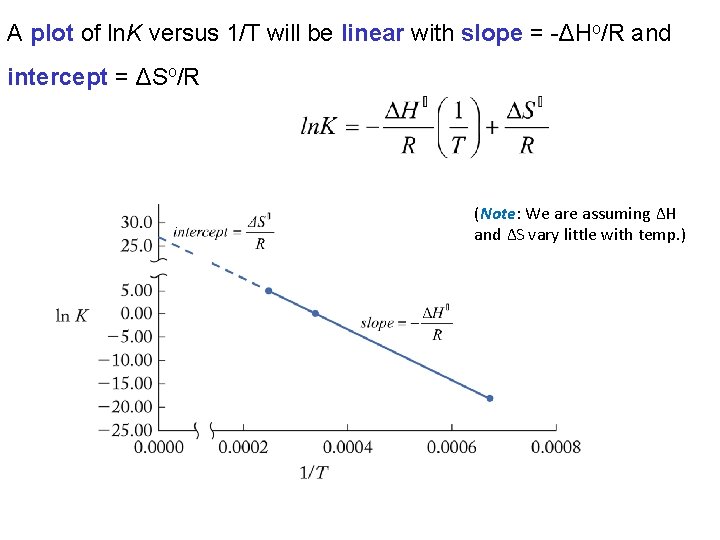
In Chemistry II we learnt that ΔHo, ΔSo, ΔGo and T are related by: We can express ΔGo in terms of K: e. g.

A plot of ln. K versus 1/T will be linear with slope = -ΔHo/R and intercept = ΔSo/R (Note: We are assuming ΔH and ΔS vary little with temp. )

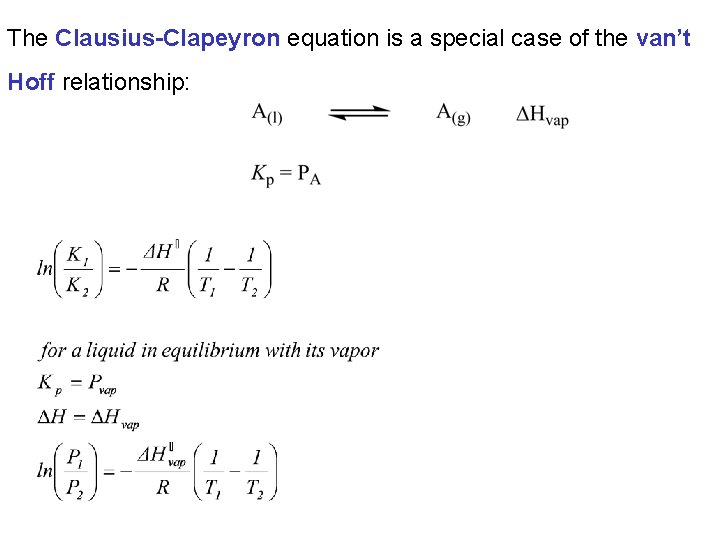
To simplify calculations we can write this expression in a “twopoint form” This formula is known as the “van’t Hoff equation” Note the similarity to the two-point form of the Arrhenius equation and the Clausius-Clapeyron equation.

The Clausius-Clapeyron equation is a special case of the van’t Hoff relationship:

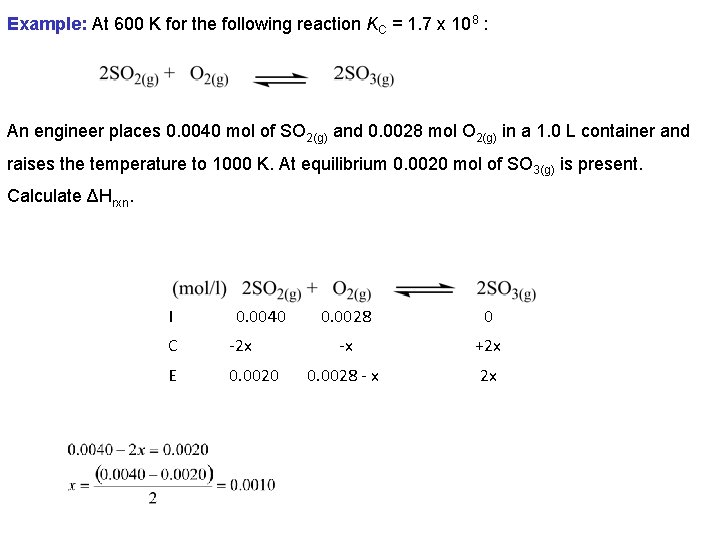
Example: At 600 K for the following reaction KC = 1. 7 x 108 : An engineer places 0. 0040 mol of SO 2(g) and 0. 0028 mol O 2(g) in a 1. 0 L container and raises the temperature to 1000 K. At equilibrium 0. 0020 mol of SO 3(g) is present. Calculate ΔHrxn. I 0. 0040 C -2 x E 0. 0020 0. 0028 0 -x +2 x 0. 0028 - x 2 x

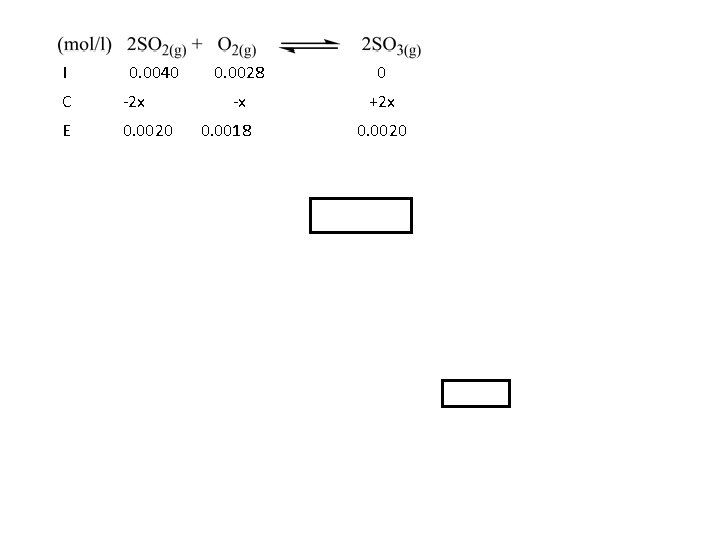
I 0. 0040 C -2 x E 0. 0020 0. 0028 0 -x +2 x 0. 0018 0. 0020

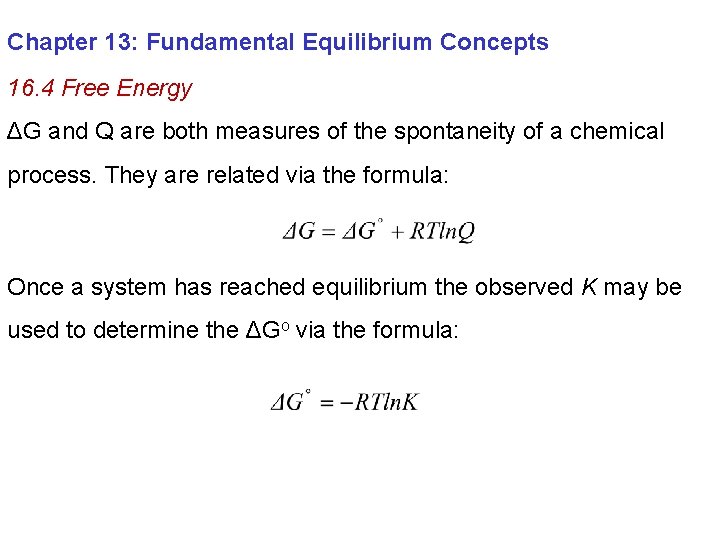
Chapter 13: Fundamental Equilibrium Concepts 16. 4 Free Energy ΔG and Q are both measures of the spontaneity of a chemical process. They are related via the formula: Once a system has reached equilibrium the observed K may be used to determine the ΔGo via the formula:

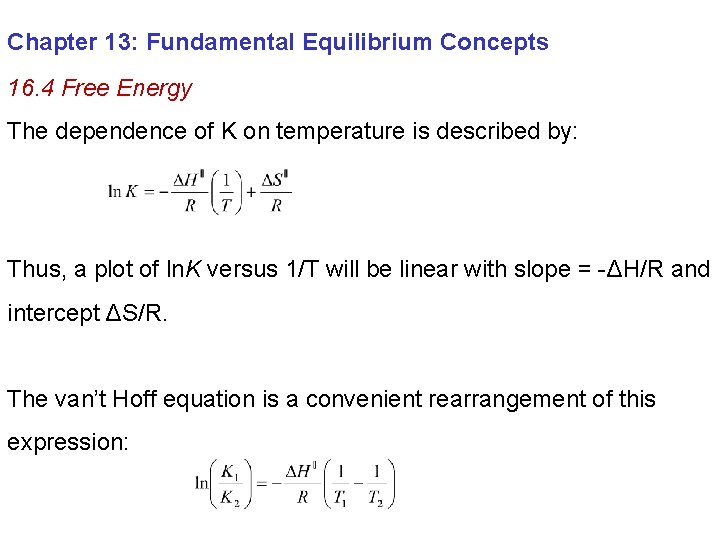
Chapter 13: Fundamental Equilibrium Concepts 16. 4 Free Energy The dependence of K on temperature is described by: Thus, a plot of ln. K versus 1/T will be linear with slope = -ΔH/R and intercept ΔS/R. The van’t Hoff equation is a convenient rearrangement of this expression:
 Pasal 163 is
Pasal 163 is Pasal 163 is
Pasal 163 is Apa itu pluralisme
Apa itu pluralisme Ruang lingkup hpi
Ruang lingkup hpi Pasal 163 is
Pasal 163 is Admittance from impedance
Admittance from impedance Ent.163
Ent.163 Ent.163
Ent.163 Ent.163
Ent.163 Ent163
Ent163 Ent163
Ent163 163 mk
163 mk Mc.163.com download
Mc.163.com download Nabl full form
Nabl full form Ent.163
Ent.163 Ent.163
Ent.163 Ent 163
Ent 163 Youcat 163
Youcat 163 Doctrine and covenants 163
Doctrine and covenants 163 163 ent
163 ent Ent 163
Ent 163 Cs specializations uci
Cs specializations uci State finance law 163
State finance law 163 Gratido
Gratido Design philosophy
Design philosophy Math 163 ccbc
Math 163 ccbc Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry ders notları
General chemistry ders notları General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Fundamentals of nursing chapter 1 notes
Fundamentals of nursing chapter 1 notes Chapter p prerequisites
Chapter p prerequisites Chapter p prerequisites fundamental concepts of algebra
Chapter p prerequisites fundamental concepts of algebra Chapter p prerequisites
Chapter p prerequisites Chapter p prerequisites fundamental concepts of algebra
Chapter p prerequisites fundamental concepts of algebra Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Geometric diagram dental charting
Geometric diagram dental charting Chapter 38 assisting with a general physical examination
Chapter 38 assisting with a general physical examination Chapter 4 posting to a general ledger
Chapter 4 posting to a general ledger General
General Chapter 9 general survey and measurement
Chapter 9 general survey and measurement Chapter 6 general anatomy
Chapter 6 general anatomy Sf4o lewis structure
Sf4o lewis structure Post. ref. accounting
Post. ref. accounting The general and special senses chapter 9
The general and special senses chapter 9 Chapter 6 general anatomy and physiology
Chapter 6 general anatomy and physiology Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Pericyclic
Pericyclic Organic vs inorganic compounds
Organic vs inorganic compounds Chapter 9 review stoichiometry section 1 answer key
Chapter 9 review stoichiometry section 1 answer key Chapter 7 review modern chemistry answers
Chapter 7 review modern chemistry answers Modern chemistry chapter 15
Modern chemistry chapter 15 Modern chemistry chapter 14 review answers
Modern chemistry chapter 14 review answers Modern chemistry chapter 13 review answers
Modern chemistry chapter 13 review answers Modern chemistry chapter 12
Modern chemistry chapter 12 Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key What functional group is ch3
What functional group is ch3 Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Difference between solution and suspension
Difference between solution and suspension Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Modern chemistry chapter 4
Modern chemistry chapter 4 Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Stoichiometry chapter 11 study guide
Stoichiometry chapter 11 study guide Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry Chapter 13 mixtures and solutions answers
Chapter 13 mixtures and solutions answers A balanced chemical equation allows one to determine the
A balanced chemical equation allows one to determine the Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Ap chemistry chapter 11
Ap chemistry chapter 11 Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Introduction to chemistry chapter 1
Introduction to chemistry chapter 1