CHEMICAL FORMULA WRITING Chemical Formulas A statement in

































































- Slides: 91

CHEMICAL FORMULA WRITING

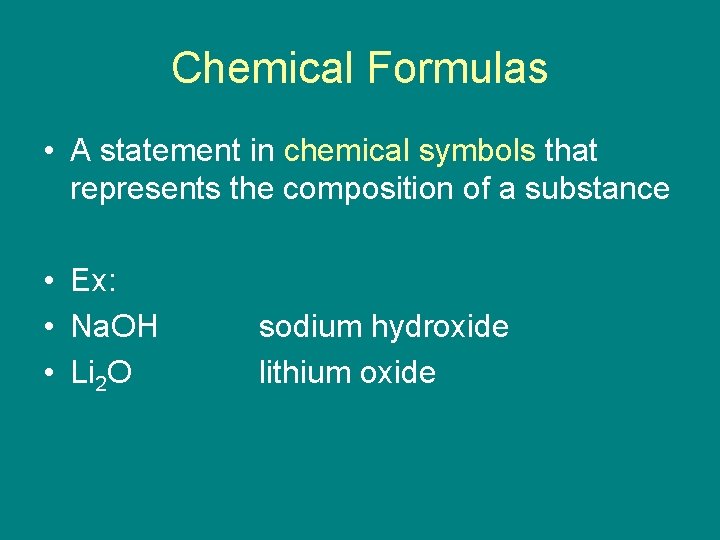
Chemical Formulas • A statement in chemical symbols that represents the composition of a substance • Ex: • Na. OH • Li 2 O sodium hydroxide lithium oxide

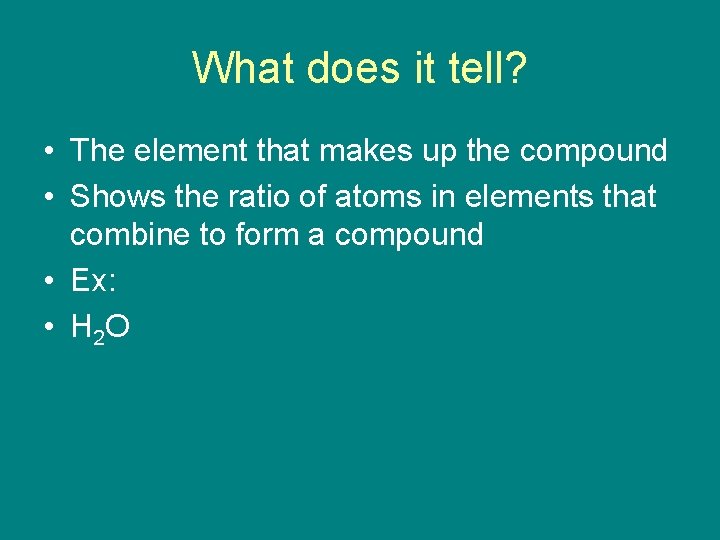
What does it tell? • The element that makes up the compound • Shows the ratio of atoms in elements that combine to form a compound • Ex: • H 2 O

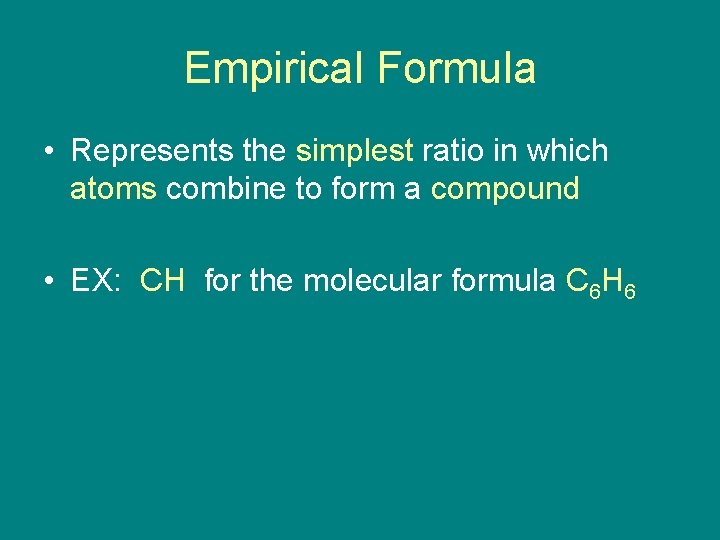
Empirical Formula • Represents the simplest ratio in which atoms combine to form a compound • EX: CH for the molecular formula C 6 H 6

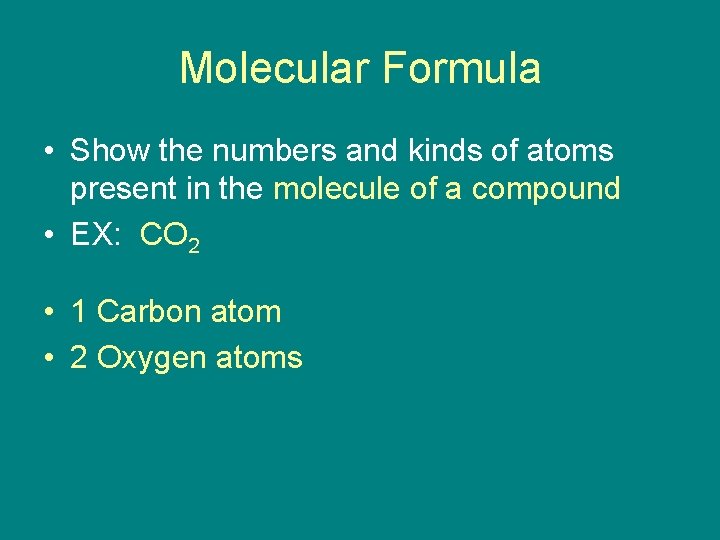
Molecular Formula • Show the numbers and kinds of atoms present in the molecule of a compound • EX: CO 2 • 1 Carbon atom • 2 Oxygen atoms

Empirical, Molecular or Both • C 2 H 2 • C 6 H 6 • H 2 O Molecular Empirical & Molecular

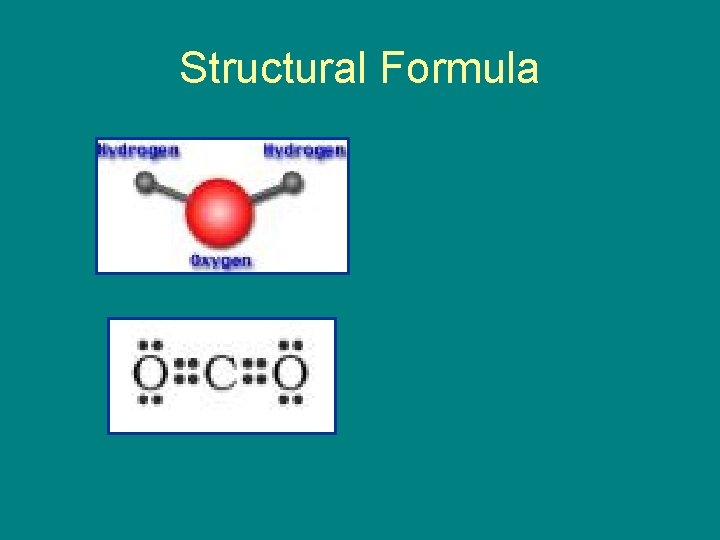
Structural Formula

Formula Units • The lowest whole number ratio of ions in an ionic compound. Ionic formula doesn’t represent molecules • Na. Cl

9. 1 Monatomic Ions • Monatomic Ions – How are the charges of Group A metal and nonmetal ions related to their positions in the periodic table?

9. 1 Monatomic Ions • Monatomic ions consist of a single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons, respectively. • The number of electrons is no longer equal to the number of protons, therefore the atom becomes an ion • Ions are atoms or groups of atoms that have a positive or negative charge

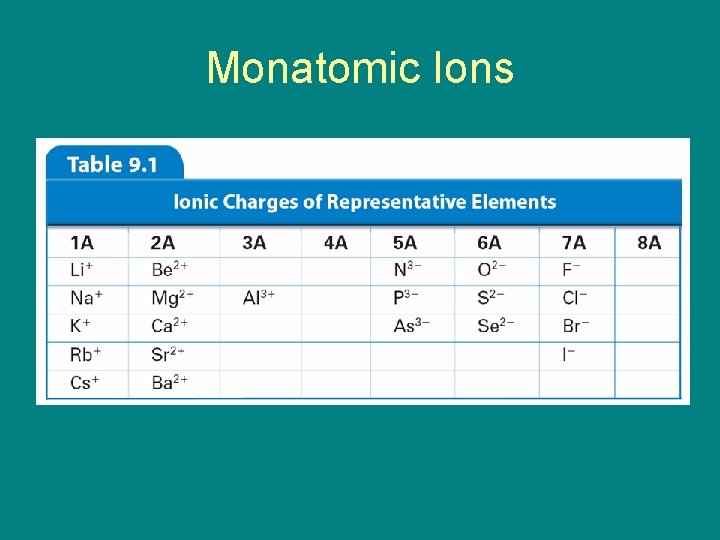
9. 1 Monatomic Ions – Cations – When the metals in Groups 1 A, 2 A, and 3 A lose electrons, they form cations with positive charges equal to their group number. • The names of the cations of the Group 1 A, Group 2 A, and Group 3 A(13) metals are the same as the name of the metal, followed by the word ion or cation.

9. 1 Monatomic Ions • These elements have ionic charges that can be obtained from their group numbers.

9. 1 Monatomic Ions – Anions – Atoms or groups of atoms with a negative charge – Anion names start with the stem of the element name and end in -ide. – Cl becomes Cl– Chloride ion

9. 1 Monatomic Ions • These Group A elements form anions.

9. 1 Monatomic Ions

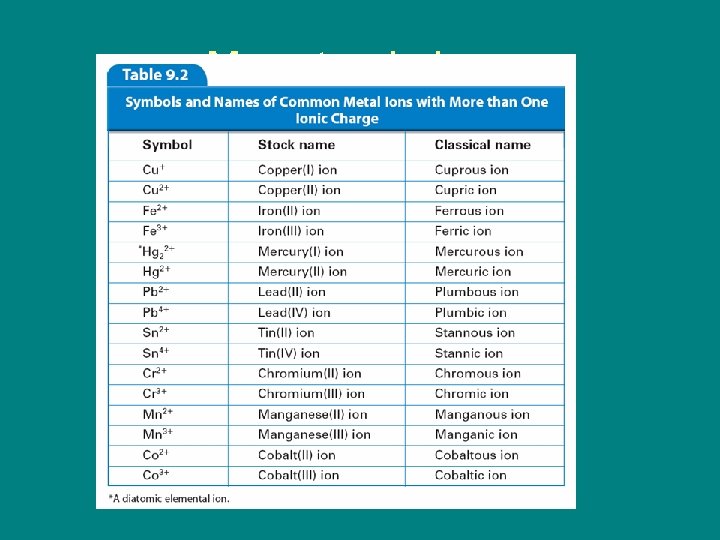
9. 1 Monatomic Ions – The charges of the cations of many transition metal ions must be determined from the number of electrons lost.

9. 1 Monatomic Ions • These colorful solutions contain the transition metal ions Co 3+, Cr 3+, Fe 3+, Ni 2+, and Mn 2+.

9. 1 Monatomic Ions • Many transition metal compounds are colored and can be used as pigments.

9. 1 Monatomic Ions • Two methods are used to name the ions of transition metals. – The Stock system – The classical method

9. 1 Monatomic Ions • In the Stock system, a Roman numeral in parentheses is placed after the name of the element to indicate the numerical value of the charge.

9. 1 Monatomic Ions • In an older less, useful method, the classical name of the element is used to form the root name for the element.

9. 1 Monatomic Ions

1. 1

1. 1

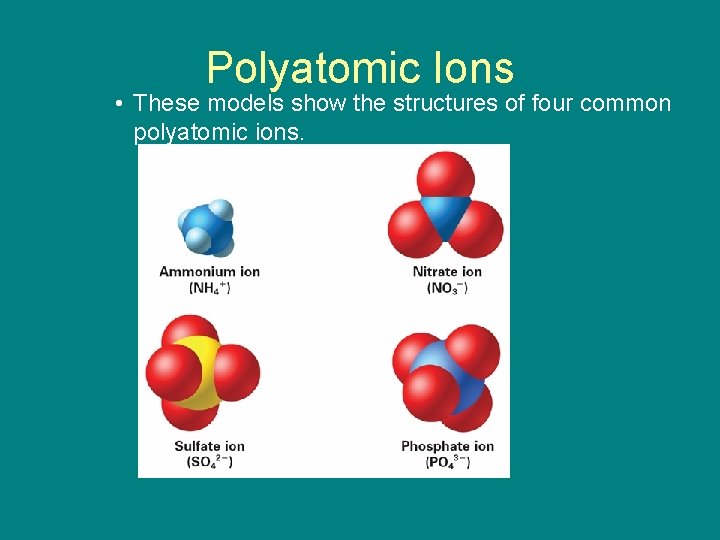
9. 1 Polyatomic Ions • These models show the structures of four common polyatomic ions.

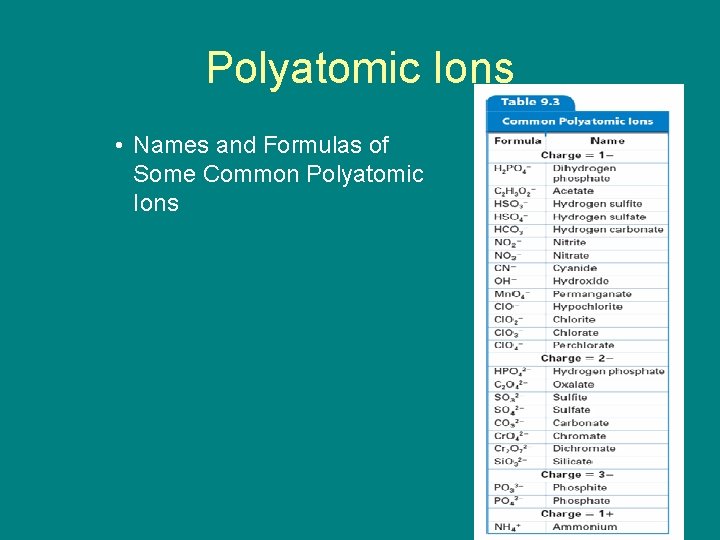
9. 1 Polyatomic Ions • Some ions, called polyatomic ions, are composed of more than one atom. – The names of most polyatomic anions end in -ite or -ate.

9. 1 Polyatomic Ions • Names and Formulas of Some Common Polyatomic Ions

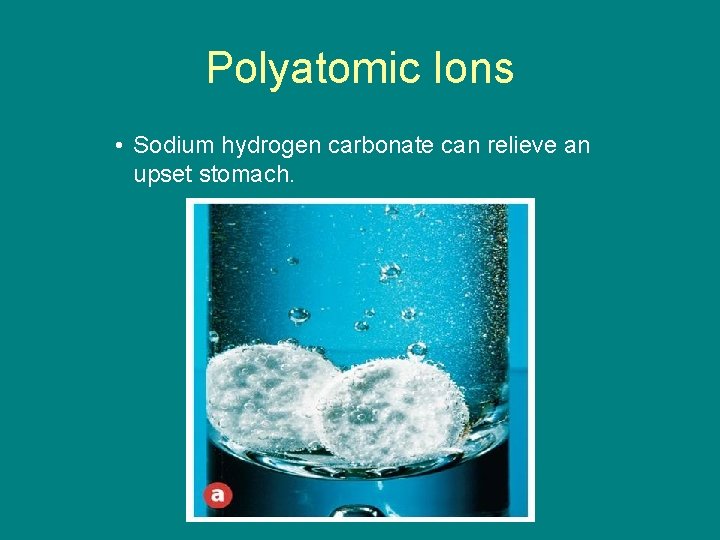
9. 1 Polyatomic Ions • Sodium hydrogen carbonate can relieve an upset stomach.

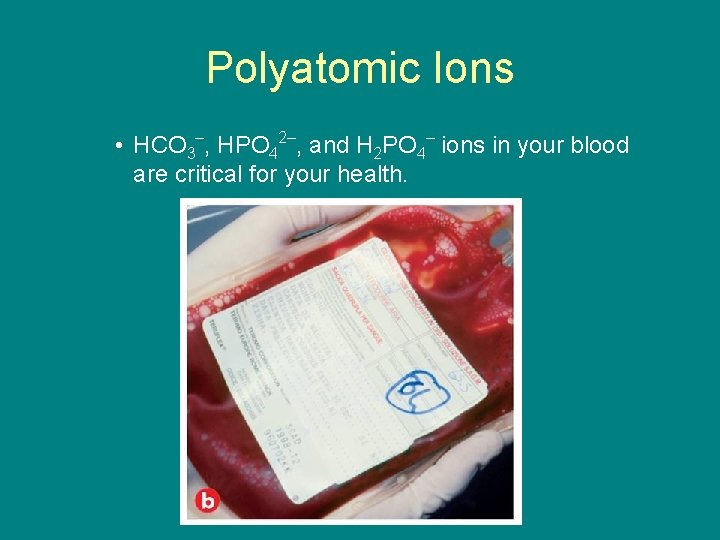
9. 1 Polyatomic Ions • HCO 3–, HPO 42–, and H 2 PO 4– ions in your blood are critical for your health.

9. 1 Polyatomic Ions • Fertilizers contain HPO 42– and H 2 PO 4– ions.

Section Quiz. – 1. When metals from groups 1 A, 2 A, and 3 A form cations, the charge on the ion is equal to a. 8 minus the group number. b. the group number minus 8. c. the period number. d. the group number.

– 2. Which of the following are positively charged polyatomic ions? – (I) ammonium ion – (II) perchlorate ion – (III) ferric ion • I only • III only • I and III

9. 1 Section Quiz – 3. If the name of an ion ends in -ite or ate, the ion is a a. b. c. d. polyatomic cation. polyatomic anion. transition metal cation. monatomic anion.

9. 2 Binary Ionic Compounds • Binary Ionic Compounds – How are the names of binary ionic compounds determined? – How do you write the formulas for binary ionic compounds?

9. 2 Binary Ionic Compounds – Naming Binary Ionic Compounds • A binary compound is composed of two elements and can be either ionic or molecular. – To name any binary ionic compound, place the cation name first, followed by the anion name. – Consist of a metal and non-metal

9. 2 Binary Ionic Compounds • Tin(II) sulfide, or Sn. F 2, is added to toothpastes to prevent cavities.

9. 2 Binary Ionic Compounds • Tin(IV) sulfide, or Sn. S 2, is used in glazes for porcelain fixtures and dishes.

9. 2 Binary Ionic Compounds • Hematite, a common ore of iron, contains iron (III) oxide. The balanced formula is Fe 2 O 3.

9. 2 Binary Ionic Compounds – Writing Formulas for Binary Ionic Compounds – Write the symbol of the cation and then the anion. – Add whatever subscripts are needed to balance the charges + and – charges must cancel out – Less electronegative element goes first

Simulation 9 – Simulation 9 Simulate combining ions and deriving the chemical formulas for several ionic compounds.

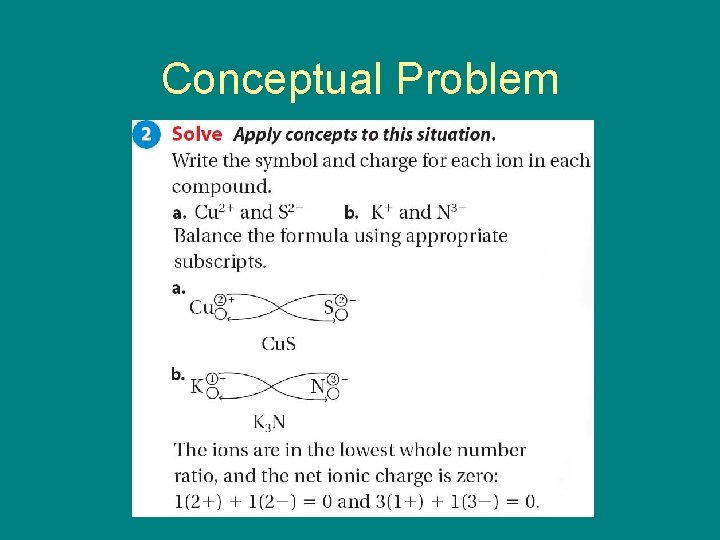
9. 2 Conceptual Problem 9. 2

9. 2 Conceptual Problem


• • A) Na. I B) Sn. Cl 2 C) K 2 S D) Ca. I 2

9. 2 Compounds With Polyatomic Ions • Compounds with Polyatomic Ions – How do you write the formulas and names of compounds containing polyatomic ions?

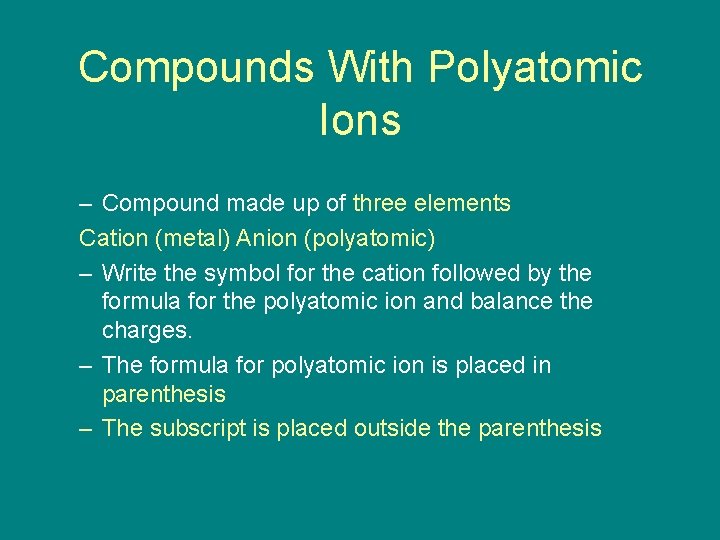
9. 2 Compounds With Polyatomic Ions – Compound made up of three elements Cation (metal) Anion (polyatomic) – Write the symbol for the cation followed by the formula for the polyatomic ion and balance the charges. – The formula for polyatomic ion is placed in parenthesis – The subscript is placed outside the parenthesis

9. 2 Compounds With Polyatomic Ions • Oysters produce calcium carbonate to form their shells and sometimes pearls.

9. 2 Compounds With Polyatomic Ions • Lead(II)sulfate is an important component of an automobile battery.

9. 3

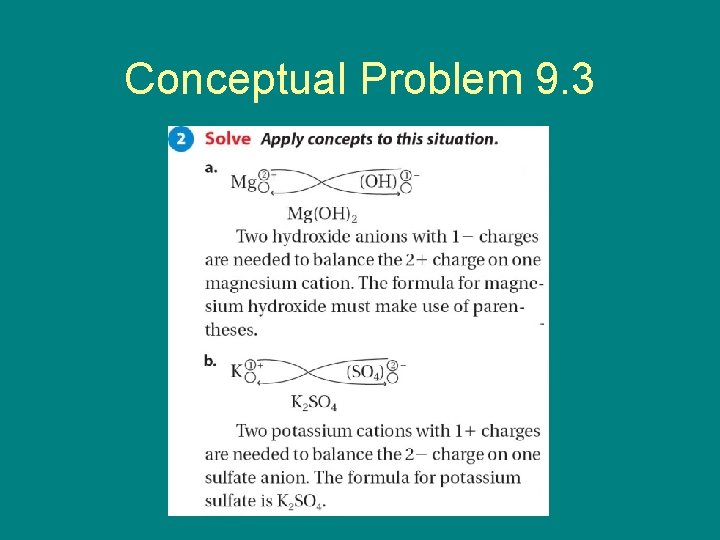
9. 3 Conceptual Problem 9. 3


9. 2 Compounds With Polyatomic Ions – Naming Compounds with Polyatomic Ions – To name a compound containing a polyatomic ion, state the cation first and then the anion, just as you did in naming binary ionic compounds.

9. 2 Compounds With Polyatomic Ions • Sodium hypochlorite (Na. Cl. O) is used as a disinfectant for swimming pools. The metallic cation in this compound is sodium (Na+) so the polyatomic ion must be Cl. O–.

9. 2 Section Quiz – 1. a. b. c. d. The correct name for Cr. Cl 3 is chromium chlorine. chromium(III) chloride. monochromium trichloride. chromium(III) trichloride.

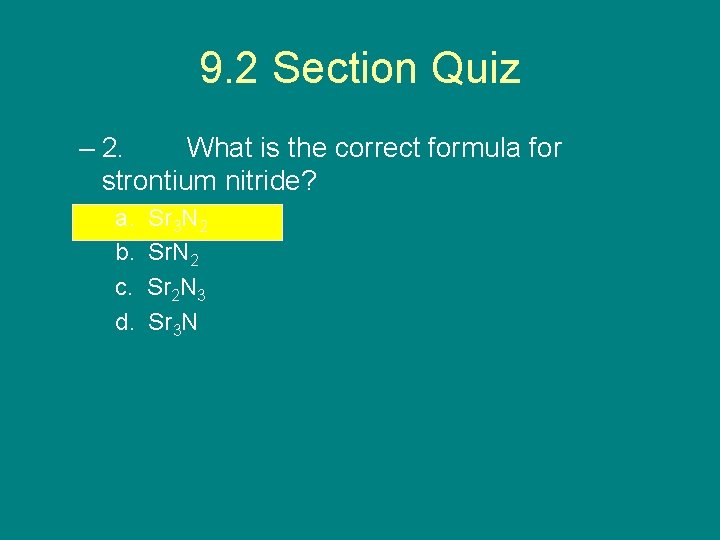
9. 2 Section Quiz – 2. What is the correct formula for strontium nitride? a. b. c. d. Sr 3 N 2 Sr 2 N 3 Sr 3 N

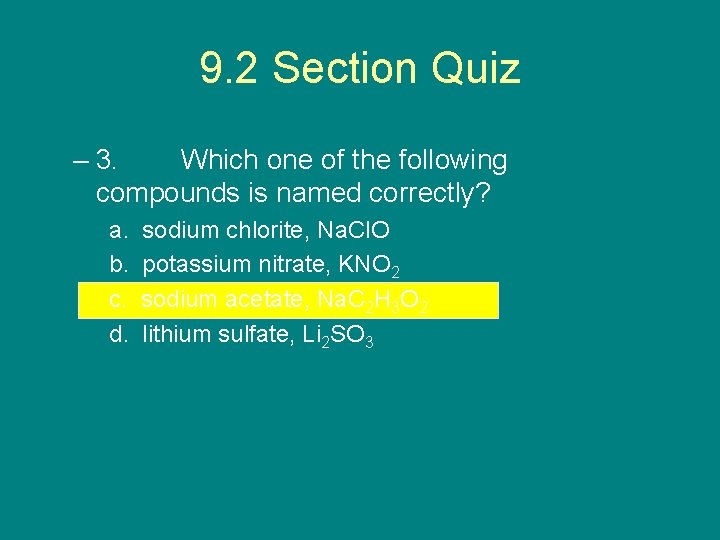
9. 2 Section Quiz – 3. Which one of the following compounds is named correctly? a. b. c. d. sodium chlorite, Na. Cl. O potassium nitrate, KNO 2 sodium acetate, Na. C 2 H 3 O 2 lithium sulfate, Li 2 SO 3

9. 3 Naming Binary Molecular Compounds • Carbon and oxygen combine to form carbon monoxide (CO) and carbon dioxide (CO 2), but these two invisible gases are very different.

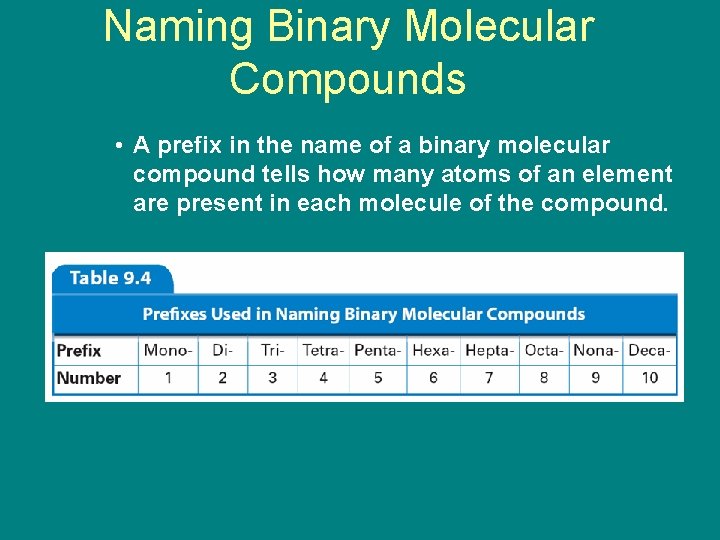
Naming Binary Molecular Compounds 9. 3 • A prefix in the name of a binary molecular compound tells how many atoms of an element are present in each molecule of the compound.

9. 3 Naming Binary Molecular Compounds • Some guidelines for naming binary molecular compounds: • Two non-metallic elements • Compound is of molecules, not ions • Less electronegative elements go first » Name the elements in the order listed in the formula. » Use prefixes to indicate the number of each kind of atom.

9. 3 Naming Binary Molecular Compounds » Omit the prefix mono- when the formula contains only one atom of the first element in the name. » The suffix of the name of the second element is -ide.

9. 3 Writing Formulas for Binary Molecular Compounds • Use the prefixes in the name to tell you the subscript of each element in the formula. Then write the correct symbols for the two elements with the appropriate subscripts.

9. 3 Writing Formulas for Binary Molecular Compounds • Silicon carbide is a hard material like diamond. The name silicon carbide has no prefixes, so the subscripts of silicon and carbon must be one. Thus, the formula for silicon carbide is Si. C.

9. 3 Section Quiz. – 1. Which of the following compounds is named INCORRECTLY? a. b. c. d. CS 2, carbon disulfide BCl 3, boron trichloride IF 7, iodine heptafluoride PCl 5, phosphorus hexachloride

9. 3 Section Quiz. – 2. Which of the following molecular compounds is named INCORRECTLY? a. b. c. d. Sb. Cl 3, antimony trichloride C 2 O 5, dicarbon pentoxide CF 4, carbon tetrafluoride H 3 As, hydrogen arsenide

9. 3 Section Quiz. – 3. The correct formula for tetraphosphorus trisulfide is a. b. c. d. P 3 S 4 S 3 P 4 P 4 S 3 S 4 P 3

9. 4 Naming Acids • Naming Acids – What are three rules for naming acids?

9. 4 Naming Acids • An acid is a compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water. Acids have various uses.

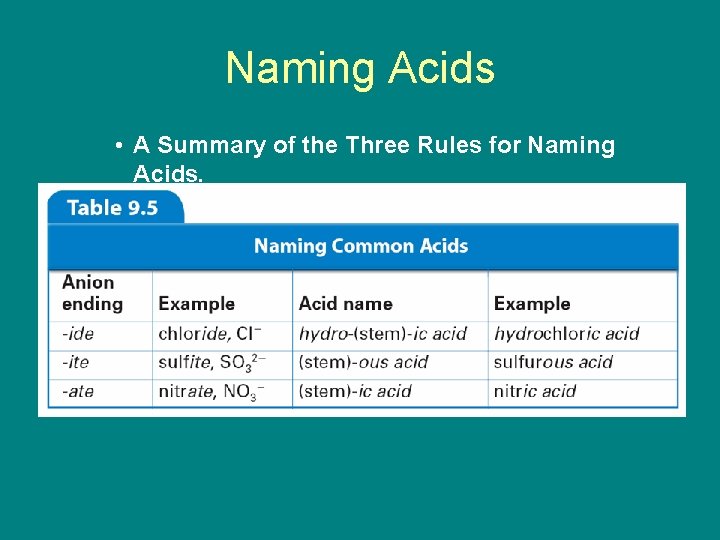
9. 4 Naming Acids • Three rules can help you name an acid with the general formula Hn. X. – When the name of the anion (X) ends in -ide, the acid name begins with the prefix hydro-. The stem of the anion has the suffix -ic and is followed by the word acid.

9. 4 Naming Acids – When the anion name ends in -ite, the acid name is the stem of the anion with the suffix ous, followed by the word acid.

9. 4 Naming Acids – When the anion name ends in -ate, the acid name is the stem of the anion with the suffix ic followed by the word acid.

9. 4 Naming Acids • A Summary of the Three Rules for Naming Acids.

9. 4 Writing Formulas for Acids • Use the rules for writing the names of acids in reverse to write the formulas for acids. – What is the formula for hydrobromic acid? – Following Rule 1, hydrobromic acid (hydro- prefix and -ic suffix) must be a combination of hydrogen ion (H+) and bromide ion (Br–). The formula of hydrobromic acid is HBr.

9. 4 Writing Formulas for Acids

9. 4 Names and Formulas for Bases • Names and Formulas for Bases – How are bases named?

9. 4 Names and Formulas for Bases • Bases are named in the same way as other ionic compounds—the name of the cation is followed by the name of the anion. – For example, aluminum hydroxide consists of the aluminum cation (Al 3+) and the hydroxide anion (OH–). The formula for aluminum hydroxide is Al(OH)3.

9. 4 Names and Formulas for Bases • Sodium hydroxide (Na. OH) is a base that is used to make paper.

9. 4 Names and Formulas for Bases • Cleaners and soap contain sodium hydroxide.

– 1. 9. 4 Section Quiz The name for H 2 S(aq) is a. sulfuric acid. b. hydrosulfuric acid. c. sulfurous acid. d. hydrosulfurous acid.

– 2. 9. 4 Section Quiz The chemical formula for chlorous acid is a. HCl. O 2. b. HCl. O 3. c. HCl. O 4. d. HCl.

– 3. 9. 4 Section Quiz The correct chemical name for NH 4 OH is a. nitrogen tetrahydrogen hydroxide. b. nitrogen pentahydrogen oxide. c. ammonium oxyhydride. d. ammonium hydroxide.

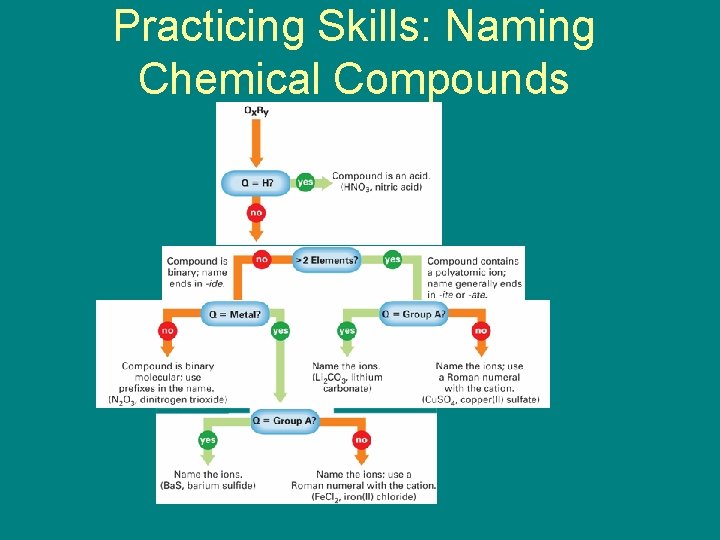
9. 5 Naming Chemical Compounds • Practicing Skills: • Naming Chemical Compounds – How do you use a flowchart to write the name of a chemical compound?

9. 5 Naming Chemical Compounds – Follow the arrows and answer the questions on the flowchart to write the correct name for a compound.

9. 5 Practicing Skills: Naming Chemical Compounds

9. 5 Naming Chemical Compounds • Cu. SO 4 is an example from the flowchart. The compound will end in -ite or -ate. Cu is not part of Group A, so you must name the ions and use a Roman numeral to identify the charge of the transition metal. The name is copper(II) sulfate.

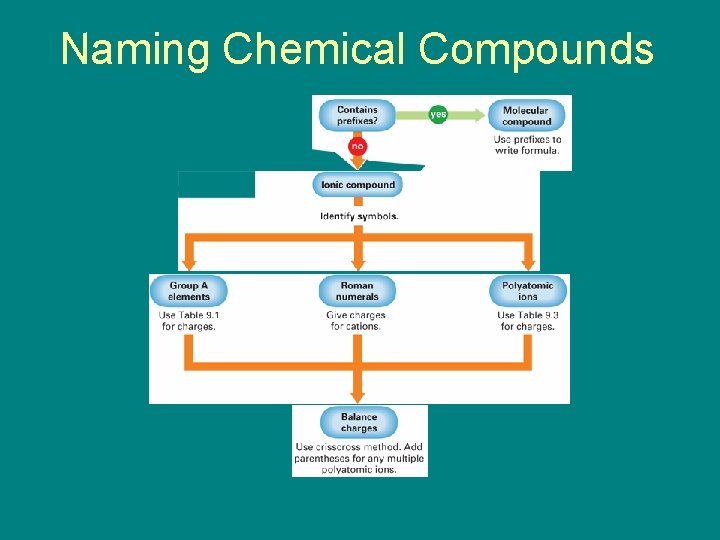
Naming Chemical Compounds 9. 4 • Writing Chemical Formulas – What four guidelines should you follow to write the formula of a chemical compound?

9. 5 : Naming Chemical Compounds • In writing a chemical formula from a chemical name, it is helpful to remember the following guidelines. – An -ide ending generally indicates a binary compound. – An -ite or -ate ending means a polyatomic ion that includes oxygen is in the formula.

9. 5 : Naming Chemical Compounds – Prefixes in a name generally indicate that the compound is molecular. – A Roman numeral after the name of a cation shows the ionic charge of the cation.

9. 5 Naming Chemical Compounds

Name the following Compounds • Ca. Br 2 • Sn. F 2 • Al 2(SO 3)3 • Fe. Cr 2 O 7

Write the formulas for the following Compounds • Copper(II) Nitrate • Magnesium oxide • Sodium Carbonate

Determine the # of atoms in the following formulas and the molar mass of each compound • Ba. SO 4 • 3 Al. PO 4 • Al 2 O 3