Chemical Reactions Topic 7 Chemical Reaction happen all









- Slides: 16

Chemical Reactions Topic 7

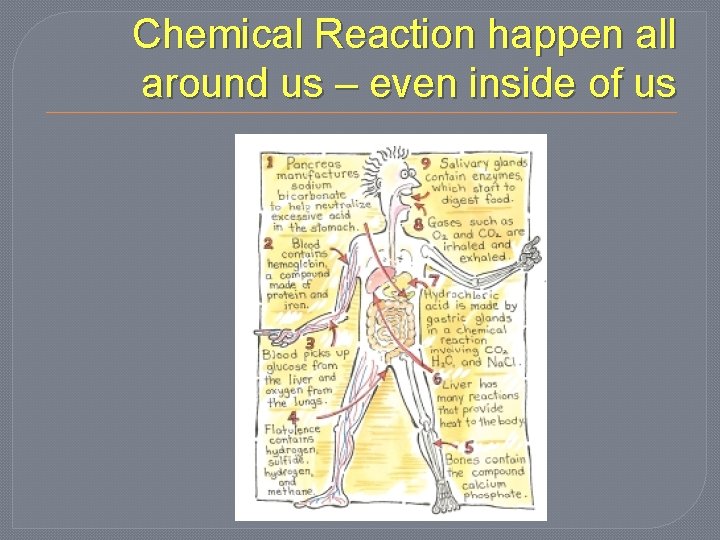
Chemical Reaction happen all around us – even inside of us

Chemical Reactions �Chemical Reactions • Two or more substances undergo a reorganization of atoms to form other substances. �Reactants • The substances that go into a chemical reaction �Products • The substances that are produced from a chemical reaction Reactant + Reactant Products

Give It a Try

Chemical Reactions � When iodine is placed on a potato - a chemical reaction occurs and a blue-black colour is left. • Colour change indicates a chemical reaction � When two solutions are put together – they may form a precipitate (solid) • A precipitate and/or gas forming indicates a chemical reaction

Chemical Reactions �When charcoal is burned, a chemical reaction occurs. • Heat and light production indicates a chemical reaction �When food spoils, its odour may change. • Odour change can indicate a chemical reaction

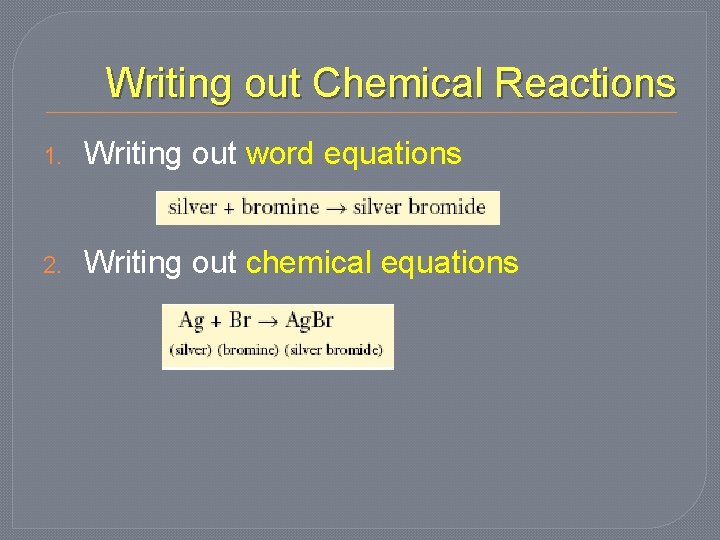
Writing out Chemical Reactions 1. Writing out word equations 2. Writing out chemical equations

Baking Soda and Vinegar �We know Baking soda and vinegar go through a chemical reaction. �Word Equation • Baking soda + Vinegar Sodium acetate + water + carbon dioxide �Chemical Equation

Try it out �Situation: �Word Burning solid magnesium. Equation �Chemical Equation �http: //www. youtube. com/watch? v=dxl. Wts. Fi n. TM&feature=related

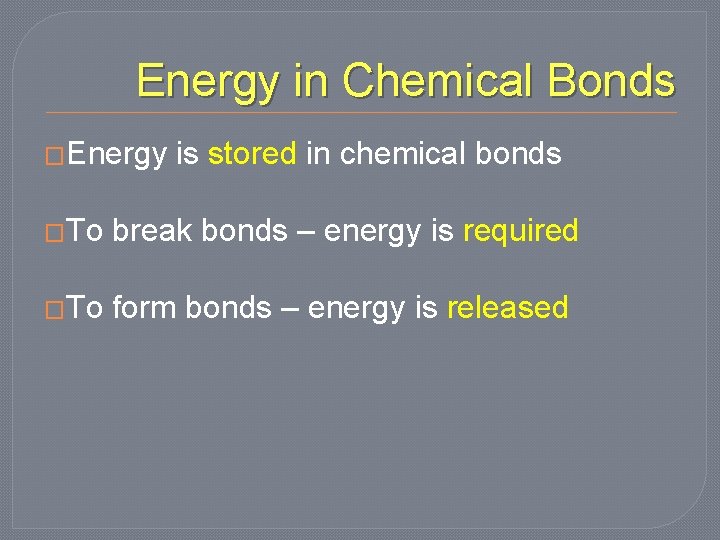
Energy in Chemical Bonds �Energy is stored in chemical bonds �To break bonds – energy is required �To form bonds – energy is released

Exothermic and Endothermic �Even though many reactions take and release energy, we look at whether it takes more or loses more. �Exothermic – Heat exits the chemical reaction �Endothermic reaction – Heat enters the chemical

Example � Burning gas in the car � Word equation • Propane + oxygen carbon dioxide + water � Chemical equation • C 3 H 8(g) + O 2(g) CO 2(g) + H 2 O(g) + energy � Even though you need to put a little energy in to start the chemical reaction, the heat produced is enough to keep the reaction going and more.

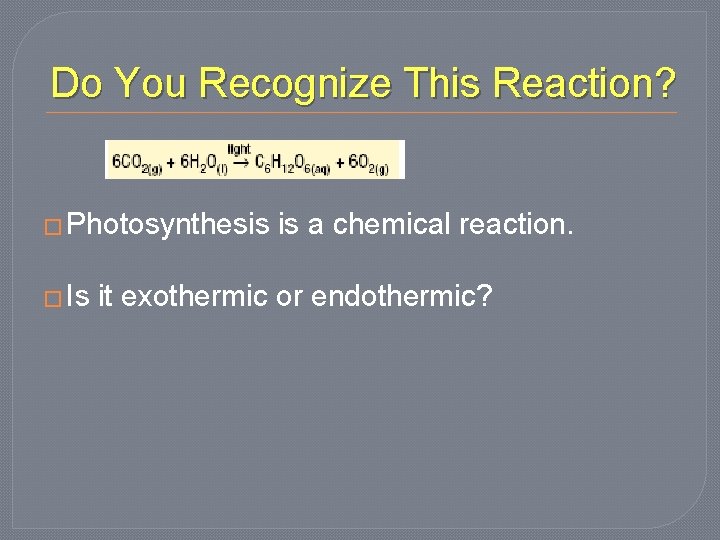
Do You Recognize This Reaction? � Photosynthesis � Is is a chemical reaction. it exothermic or endothermic?

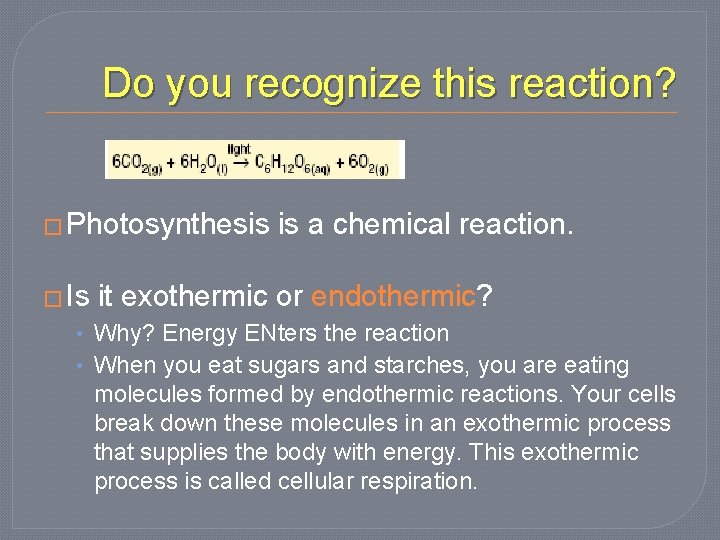
Do you recognize this reaction? � Photosynthesis � Is is a chemical reaction. it exothermic or endothermic? • Why? Energy ENters the reaction • When you eat sugars and starches, you are eating molecules formed by endothermic reactions. Your cells break down these molecules in an exothermic process that supplies the body with energy. This exothermic process is called cellular respiration.

Hot and Cold Packs �Both hot and cold packs go through chemical reactions �Which goes through an endothermic reaction? �Which goes through an exothermic reaction?

Hot and Cold Packs �Both hot and cold packs go through chemical reactions �Which goes through an endothermic reaction? Cold Packs �Which goes through an exothermic reaction? Hot packs
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions What are some clue words for sequencing?
What are some clue words for sequencing? Are all chemical reactions reversible
Are all chemical reactions reversible Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Neutron emission
Neutron emission Redox examples
Redox examples Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Name a point that is collinear with the given points
Name a point that is collinear with the given points Clueing topic sentence
Clueing topic sentence Broad and specific topic examples
Broad and specific topic examples Neutralization equation
Neutralization equation Stoichiometry mole island diagram
Stoichiometry mole island diagram I intro
I intro Types of chemical reactions redox
Types of chemical reactions redox