Naming Binary Molecular Compounds 2 elements covalent bond
Naming Binary Molecular Compounds 2 elements covalent bond

3 Rules to Name 1. Name the first element using the entire name 2. Second element in the formula- use the root word and end in –ide ex: Oxygen Oxide Sulfur Hydride Hydrogen

3. Add a prefix to both words to indicate the number of atoms 1. mono 6. hexa 2. di 7. hepta 3. tri 8. octa 4. tetra 9. nona 5. penta 10. deca-
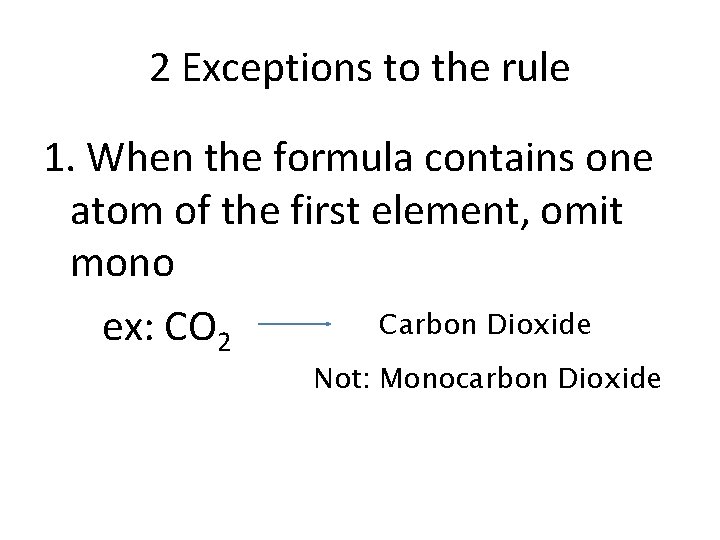
2 Exceptions to the rule 1. When the formula contains one atom of the first element, omit mono Carbon Dioxide ex: CO 2 Not: Monocarbon Dioxide

2 Exceptions to the rule 2. Drop the final letter in the prefix if the element begins with a vowel - For prefixes 1 & 4 -9 ex: CO Carbon monoxide Not: Carbon monooxide
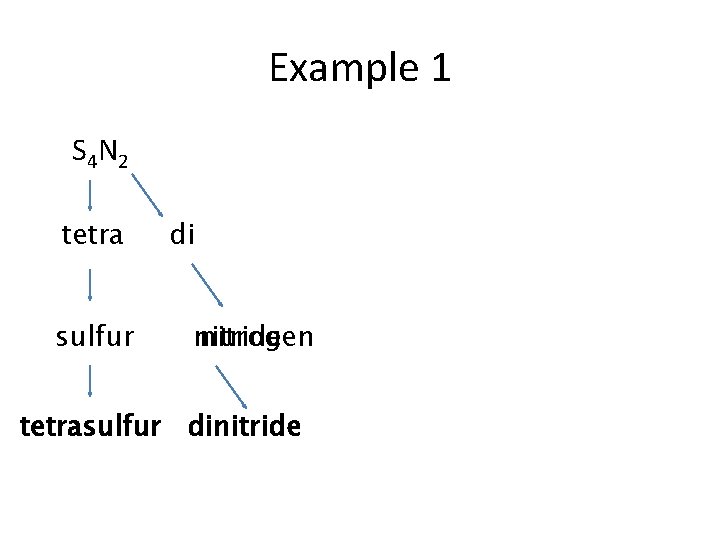
Example 1 S 4 N 2 tetra sulfur di nitride nitrogen tetrasulfur dinitride
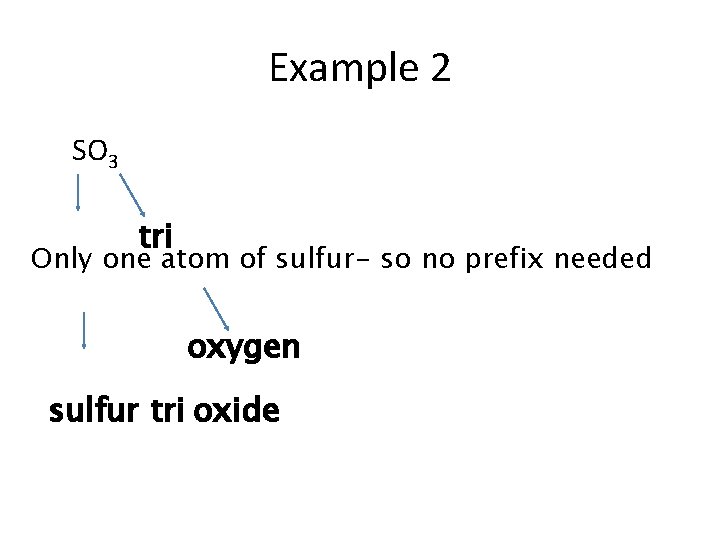
Example 2 SO 3 tri Only one atom of sulfur - so no prefix needed oxygen sulfur tri oxide
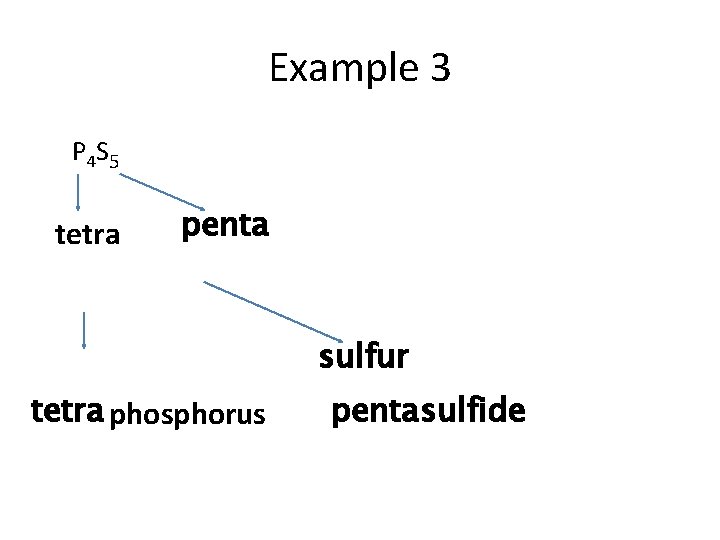
Example 3 P 4 S 5 tetra penta sulfur tetra phosphorus penta sulfide
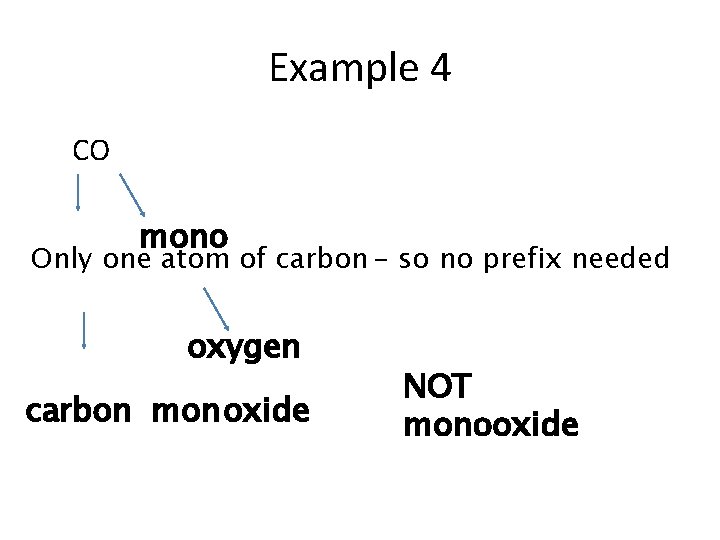
Example 4 CO mono Only one atom of carbon - so no prefix needed oxygen carbon mon oxide NOT monooxide
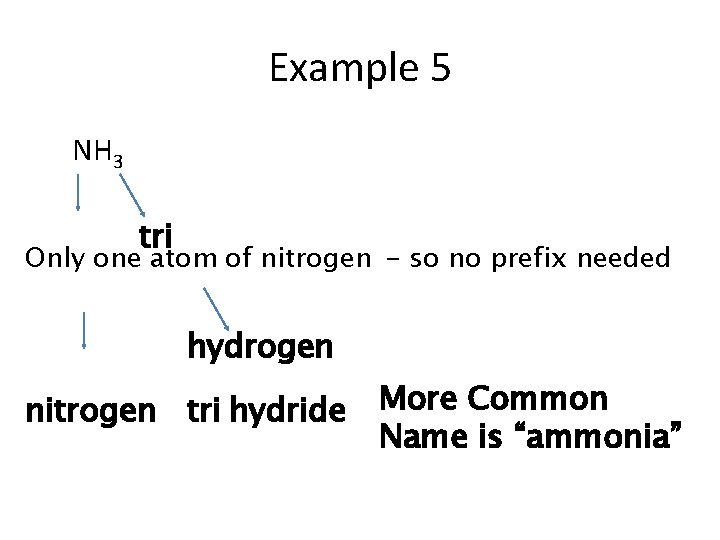
Example 5 NH 3 tri Only one atom of nitrogen - so no prefix needed hydrogen nitrogen tri hydride More Common Name is “ammonia”
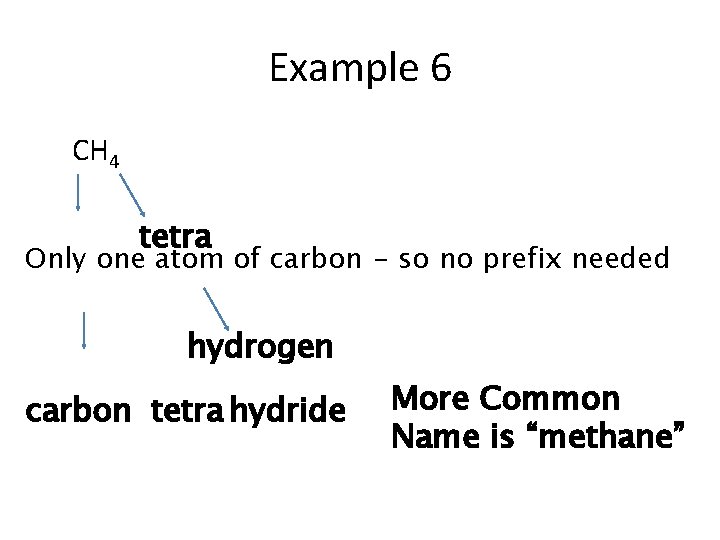
Example 6 CH 4 tetra Only one atom of carbon - so no prefix needed hydrogen carbon tetra hydride More Common Name is “methane”
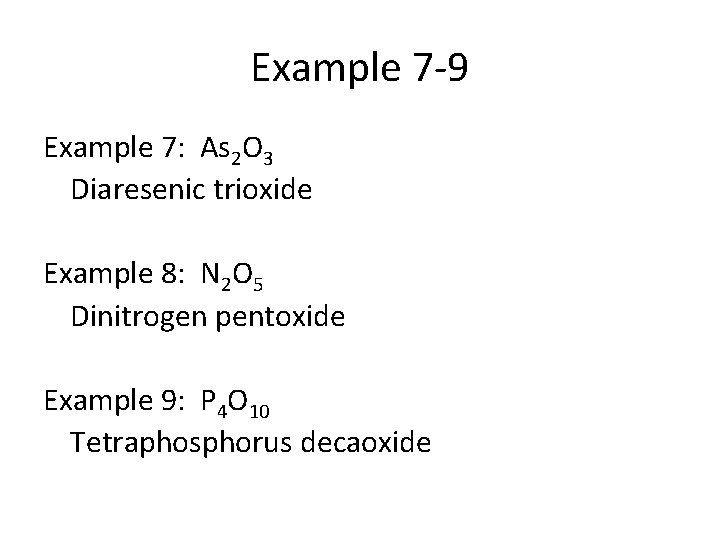
Example 7 -9 Example 7: As 2 O 3 Diaresenic trioxide Example 8: N 2 O 5 Dinitrogen pentoxide Example 9: P 4 O 10 Tetraphosphorus decaoxide
- Slides: 12