Chemical Nomenclature Formula Writing Naming Compounds Chemical Formula





















- Slides: 23

Chemical Nomenclature: Formula Writing & Naming Compounds

Chemical Formula • The representation of a substance using symbols (letters) and subscripts (numbers) H 2 O The subscripts tell you how many atoms a compound is composed of. Water is made of 2 atoms of Hydrogen and 1 atom of oxygen

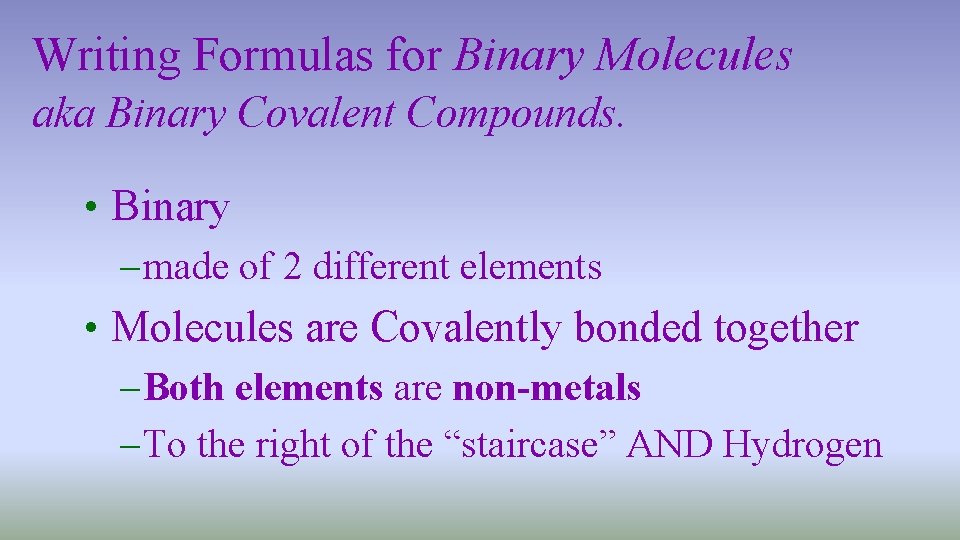
Writing Formulas for Binary Molecules aka Binary Covalent Compounds. • Binary – made of 2 different elements • Molecules are Covalently bonded together – Both elements are non-metals – To the right of the “staircase” AND Hydrogen

Ex. Phosphorus pentoxide PO 5 • Write the symbols for the elements in order – ide refers to an element except peroxide & hydroxide • Subscripts are written from prefixes and follow the symbol – Mono = one – Tetra = four Di = two Penta = 5 Tri = three Hexa = 6

Practice Mono never STARTS a name! • Carbon dioxide • CO 2 • Dihydrogen monoxide • H 2 O • Sulfur hexafluoride • SF 6 • Phosphorous pentachloride • PCl 5

Formula Writing for Ionic Compounds • All ionic formulas are made of 2 parts A • A is positive A can be: Metal or Polyatomic ion / B B is negative B can be: Non-Metal or Polyatomic ion

Ionic Names with Roman Numerals Copper II Chloride Copper II Chlorate 1. Write the symbols ( -ate is a polyatomic ion) 2. The positive oxidation state for the metal is given to you as a Roman Numeral You know the charge on the anion. It’s based on the number of valence e-

3. Make your charges cancel out 4. Write formula & reduce if you can • “ 1” doesn’t need to be written Cu. Cl 2 Cu(Cl. O 3)2

Practice • Lead II oxide • Pb. O • Lead IV oxide • Pb. O 2

Ionic Names without Roman Numerals Potassium Nitride Potassium Nitrate 1. Write down the symbols • If polyatomic ion look it up and write it in parenthesis. Don’t change it! 2. Assign oxidation states / charges Part A will be positive: you should know it! Part B will be negative and you should know them or it’s a PAI (look it up)

3. “Crisscross” 4. Write formula - REDUCE! • “ 1” doesn’t need to be written K 3 N KNO 3

Practice • Calcium Phosphate Ca 3(PO 4)2 • Magnesium Hydroxide Mg(OH)2

Formula Writing for 2 polyatomic ions Ammonium Phosphate 1. Write separate formulas in parenthesis – NEVER change what’s inside!!!!! 2. Assign charges 3. Make the charges cancel out with subscripts & write your final answer……. . (NH 4)3 PO 4

Practice • Ammonium Nitrate NH 4 NO 3 • Ammonium Hydroxide NH 4 OH

Naming……. .

Naming Binary Molecules. . . both elements are non-metals 1. Count the number of atoms as a prefix mono = one di = two tri = three tetra = four. . . 2. Write the prefix before the names of the elements • Mono never STARTS a name

3. Write the name of the second element – end the name with • • hydrogen oxygen nitrogen sulfur fluorine chlorine bromine -ide hydride oxide nitride sulfide fluoride chloride bromide Practice • CO 2 carbon dioxide • N 2 O dinitrogen monoxide • SCl 5 sulfur pentachloride • H 2 O dihydrogen monoxide • CCl 4 carbon tetrachloride

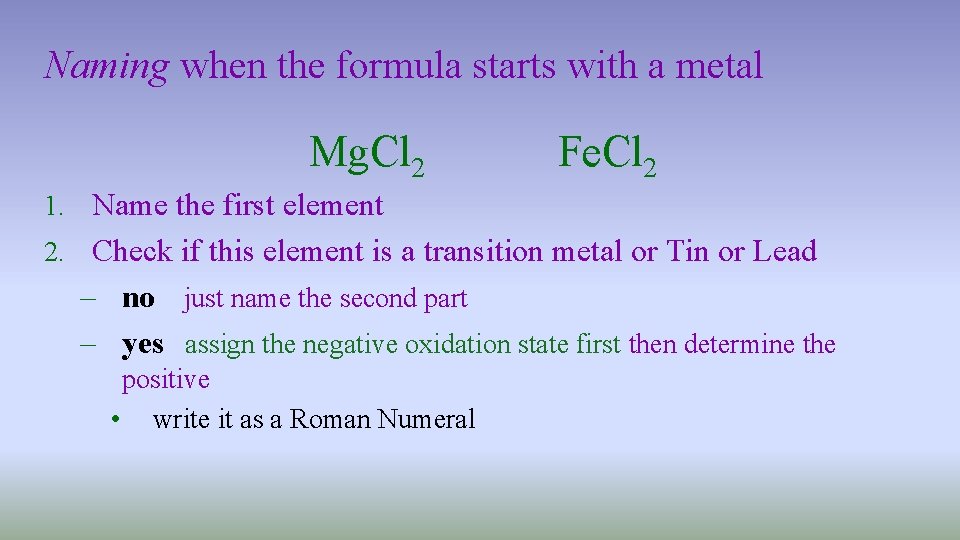
Naming when the formula starts with a metal Mg. Cl 2 Fe. Cl 2 1. Name the first element 2. Check if this element is a transition metal or Tin or Lead – no just name the second part – yes assign the negative oxidation state first then determine the positive • write it as a Roman Numeral

Practice • Cu. SO 4 Copper II sulfate • Ba(OH)2 Barium hydroxide • Al 2 S 3 aluminum sulfide • Cu. Cl copper I chloride • Cu. CO 3 copper II carbonate Remember: You only need a roman numeral if you have a transition metal, or Sn or Pb…. but not Zn or Ag

Naming polyatomic ion - element compounds NH 4 Cl 1. No prefixes 2. No roman numerals just name it! • Practice NH 4 F ammonium fluoride (NH 4)2 S ammonium sulfide

Naming compounds with 2 polyatomic ions • Just name them! Practice • (NH 4)2 SO 4 ammonium sulfate • (NH 4)2 Cr. O 4 ammonium chromate • (NH 4)3 PO 4 ammonium phosphate

The End

 Chemical formulas and names
Chemical formulas and names How to name ionic compounds
How to name ionic compounds Systematic approach to naming chemical compounds
Systematic approach to naming chemical compounds Naming chemical compounds flowchart
Naming chemical compounds flowchart Naming and writing formulas for molecular compounds
Naming and writing formulas for molecular compounds Prefixes for hydrates
Prefixes for hydrates Ionic compounds list
Ionic compounds list Naming compounds and writing formulas
Naming compounds and writing formulas Section 3 writing formulas and naming compounds
Section 3 writing formulas and naming compounds Writing and naming chemical formulas
Writing and naming chemical formulas Functional groups class 10
Functional groups class 10 Coordination compound nomenclature
Coordination compound nomenclature Heterocyclic compounds nomenclature
Heterocyclic compounds nomenclature Nomenclature of heterocyclic compounds
Nomenclature of heterocyclic compounds Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Love chemical formula
Love chemical formula How to name a metallic bond
How to name a metallic bond Cl-1 ion name
Cl-1 ion name Naming ionic compounds
Naming ionic compounds How do you name covalent bonds
How do you name covalent bonds Emperical formual
Emperical formual When to use prefixes for naming compounds
When to use prefixes for naming compounds Naming binary ionic compounds
Naming binary ionic compounds