Ionic Compound Nomenclature v Nomenclature naming and formula




- Slides: 12

Ionic Compound Nomenclature v. Nomenclature – naming and formula writing of chemical compounds

Ionic Nomenclature § Ionic bonding occurs between… § a metal & a non-metal § To name an ionic compound: § name the metal § name the non-metal, adding an –ide ending § name the cation, name the anion

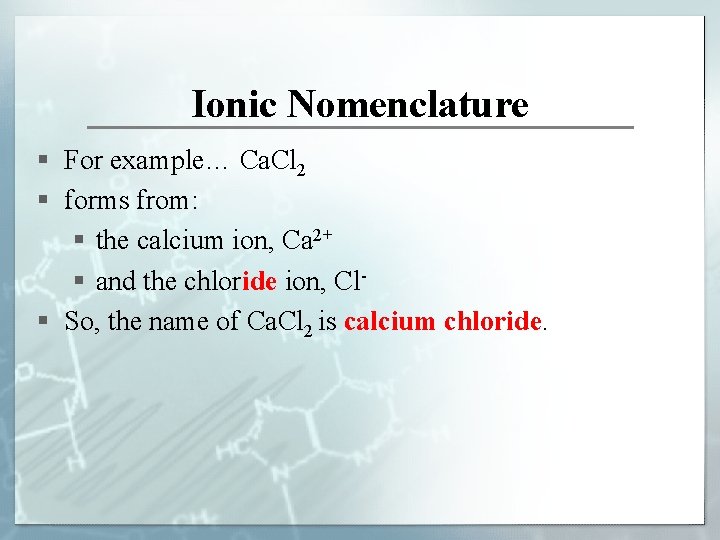
Ionic Nomenclature § For example… Ca. Cl 2 § forms from: § the calcium ion, Ca 2+ § and the chloride ion, Cl§ So, the name of Ca. Cl 2 is calcium chloride.

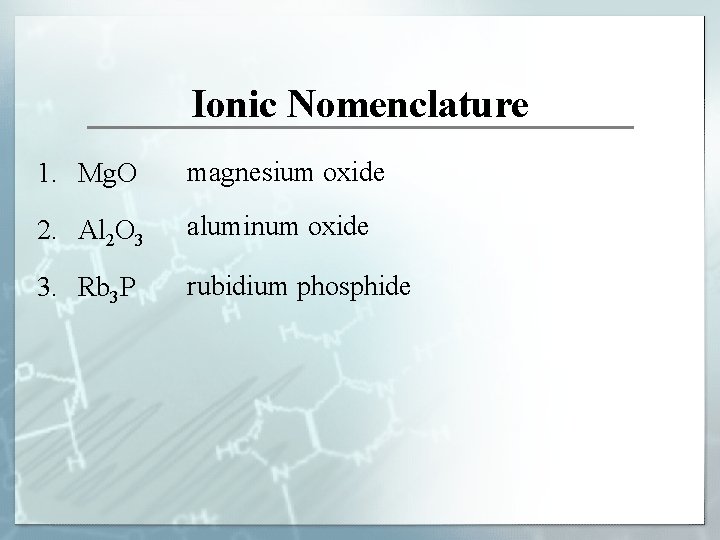
Ionic Nomenclature 1. Mg. O magnesium oxide 2. Al 2 O 3 aluminum oxide 3. Rb 3 P rubidium phosphide

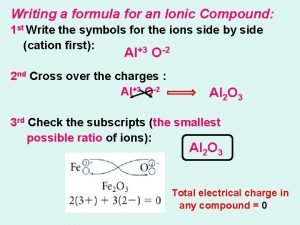
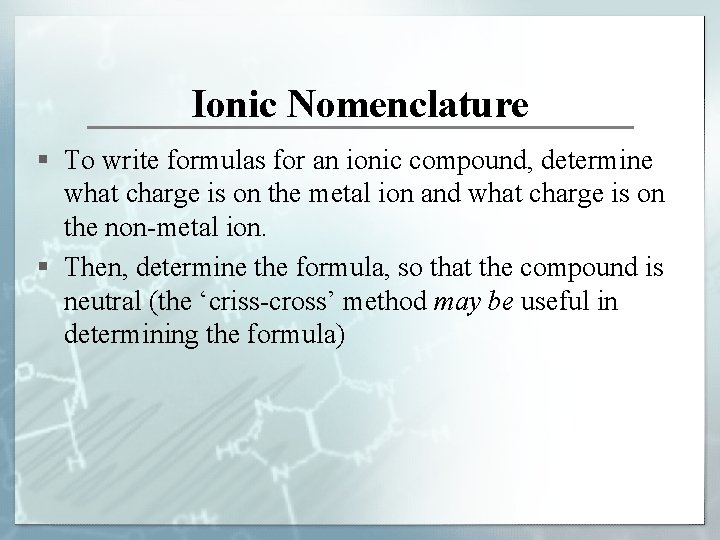
Ionic Nomenclature § To write formulas for an ionic compound, determine what charge is on the metal ion and what charge is on the non-metal ion. § Then, determine the formula, so that the compound is neutral (the ‘criss-cross’ method may be useful in determining the formula)

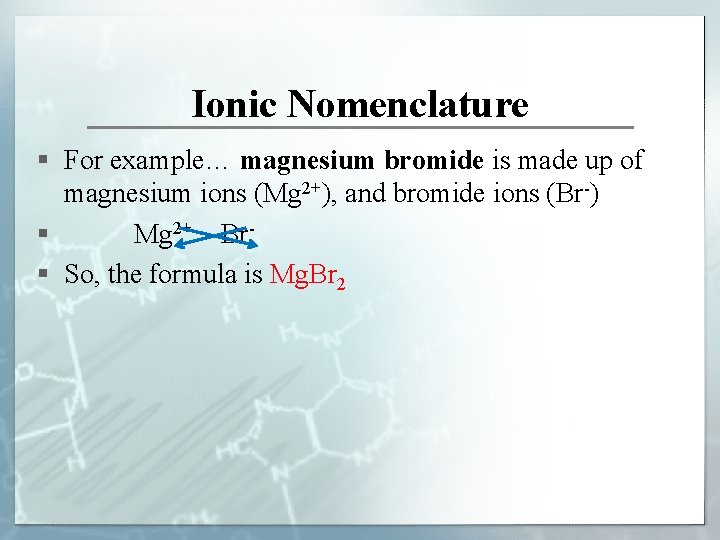
Ionic Nomenclature § For example… magnesium bromide is made up of magnesium ions (Mg 2+), and bromide ions (Br-) § Mg 2+ Br§ So, the formula is Mg. Br 2

Ionic Nomenclature 1. sodium chloride 2. barium fluoride 3. potassium sulfide 4. magnesium phosphide 5. calcium oxide (try some more…)

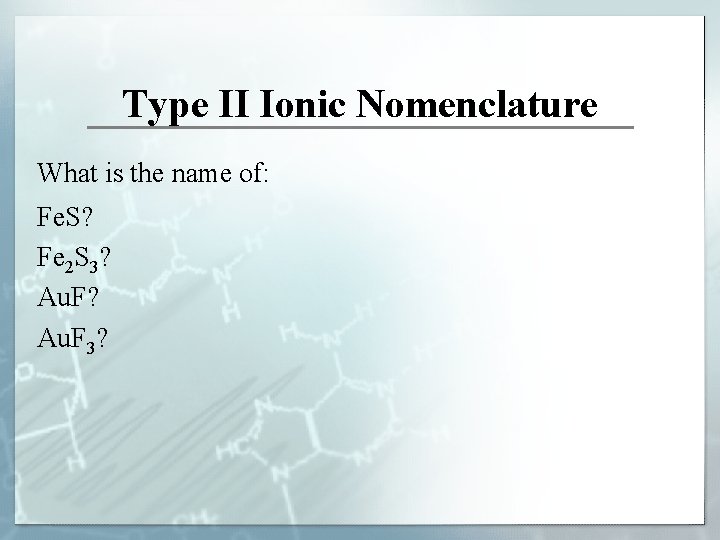
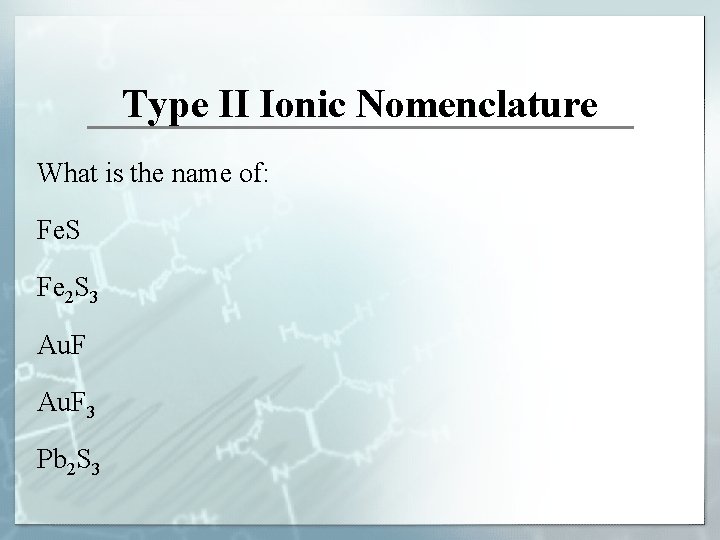
Type II Ionic Nomenclature What is the name of: Fe. S? Fe 2 S 3? Au. F 3?

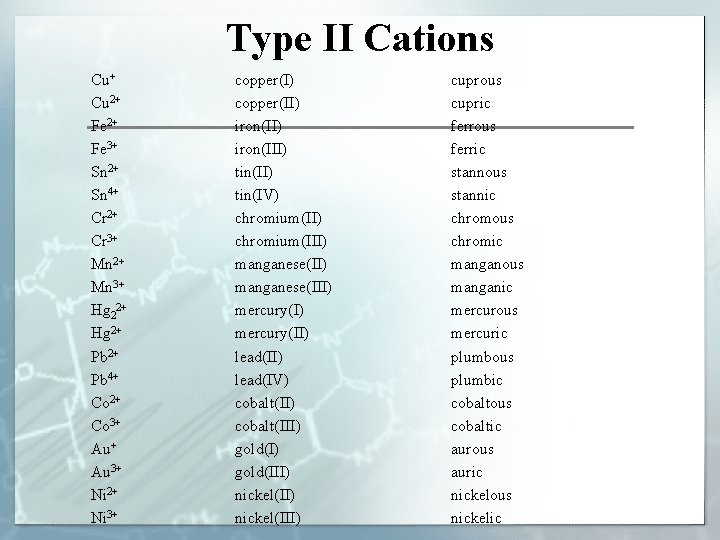
Type II Cations Cu+ Cu 2+ Fe 3+ Sn 2+ Sn 4+ Cr 2+ Cr 3+ Mn 2+ Mn 3+ Hg 22+ Hg 2+ Pb 4+ Co 2+ Co 3+ Au 3+ Ni 2+ Ni 3+ copper(I) copper(II) iron(III) tin(IV) chromium(III) manganese(III) mercury(II) lead(IV) cobalt(III) gold(III) nickel(III) cuprous cupric ferrous ferric stannous stannic chromous chromic manganous manganic mercurous mercuric plumbous plumbic cobaltous cobaltic aurous auric nickelous nickelic

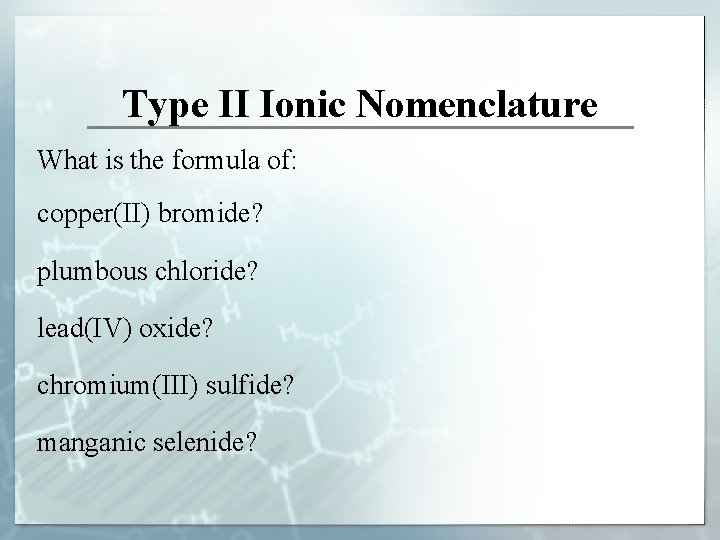
Type II Ionic Nomenclature What is the formula of: copper(II) bromide? plumbous chloride? lead(IV) oxide? chromium(III) sulfide? manganic selenide?

Type II Ionic Nomenclature What is the name of: Fe. S Fe 2 S 3 Au. F 3 Pb 2 S 3

 Aluminum and oxygen ionic compound
Aluminum and oxygen ionic compound Mixed ionic and covalent naming
Mixed ionic and covalent naming Ionic compounds list
Ionic compounds list Naming ionic and covalent bonds
Naming ionic and covalent bonds Predicting and naming ionic compounds
Predicting and naming ionic compounds Lithium chloride formula of ionic compound
Lithium chloride formula of ionic compound Writing and naming chemical formulas
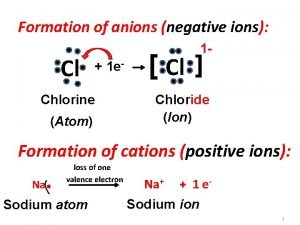
Writing and naming chemical formulas The formation of negative ion is:
The formation of negative ion is: Chemical.formula
Chemical.formula How to write an ionic compound formula
How to write an ionic compound formula The chemical formula for an ionic compound represents the
The chemical formula for an ionic compound represents the Ni ionic charge
Ni ionic charge Naming ionic compounds
Naming ionic compounds