Naming Ionic or Covalent Compounds Naming Ionic and






- Slides: 10

Naming Ionic or Covalent Compounds Naming Ionic and Covalent compounds follow different rules We will begin by learning the rules of naming ionic compounds first.

Naming Ionic Compounds 1. Start with the name of the first atom in the molecule. 2. Take the next atom in the molecule and replace its ending with an “ide” suffix. 3. Putting those two names together gives you the compound’s name. That is why Na. Cl is called Sodium chloride. It gets more complicated but for now stick with the rules.

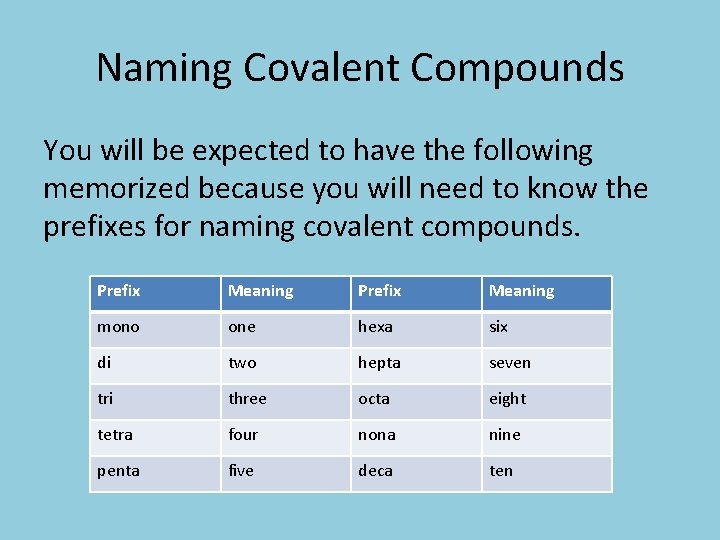
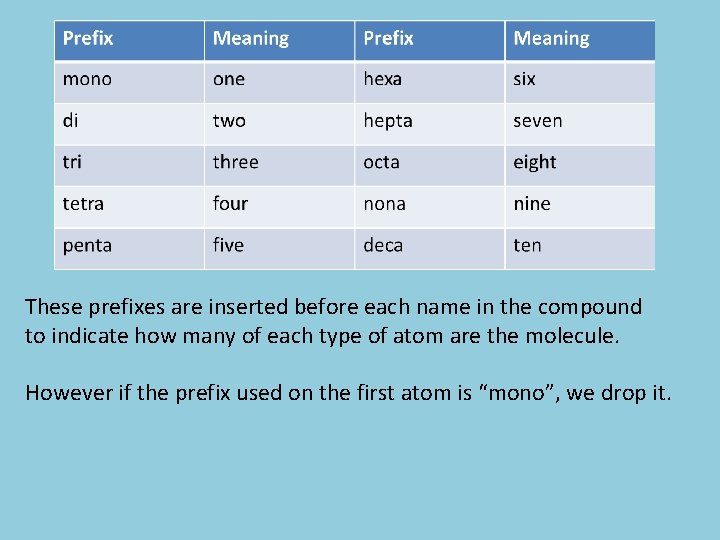
Naming Covalent Compounds You will be expected to have the following memorized because you will need to know the prefixes for naming covalent compounds. Prefix Meaning mono one hexa six di two hepta seven tri three octa eight tetra four nona nine penta five deca ten

These prefixes are inserted before each name in the compound to indicate how many of each type of atom are the molecule. However if the prefix used on the first atom is “mono”, we drop it.

Example of naming a covalent compound CO 2 has the name carbon dioxide. Since there is only one carbon in the molecule we should use the name mono before carbon. But we drop mono and just say carbon. However we have to put a “di” prefix in front of oxygen because we have 2 oxygen atoms present. Just like naming the ionic compounds, we change the last atom’s name to an “ide” ending.

Dihydrogen Monoxide • BAN DIHYDROGEN MONOXIDE - THE INVISIBLE KILLER! • Dihydrogen monoxide is colorless, odorless, tasteless, and kills uncounted thousands of people every year. • What are the dangers of Dihydrogen Monoxide? • Most of these deaths are caused by accidental inhalation of DHMO, but the dangers of dihydrogen monoxide do not end there. Prolonged exposure to its solid form causes severe tissue damage. Symptoms of DHMO ingestion can include excessive sweating and urination, and possibly a bloated feeling, nausea, vomiting and body electrolyte imbalance. For those who have become dependent, DHMO withdrawal means certain death. • What is your solution to this dangerous problem?

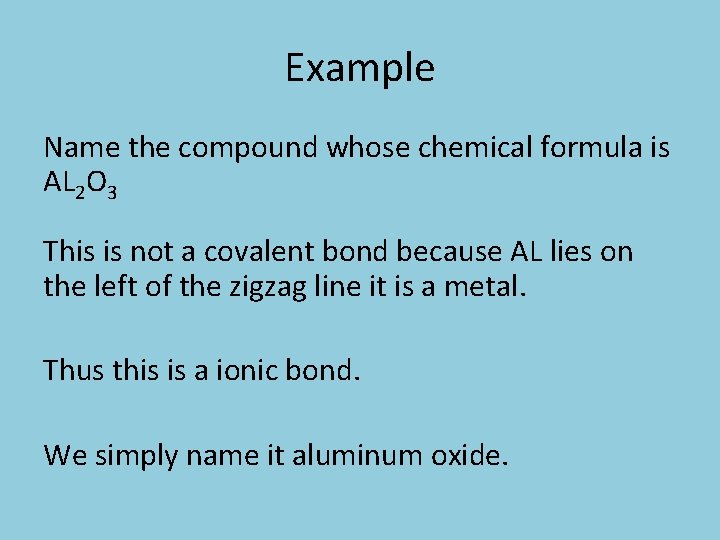
Example Name the compound whose chemical formula is AL 2 O 3 This is not a covalent bond because AL lies on the left of the zigzag line it is a metal. Thus this is a ionic bond. We simply name it aluminum oxide.

Example What is the name of the molecule PH 3 Since P and H are both non metals this is a covalent bond. The prefix for phosphorus (P) is “mono” but since it is the first atom we drop the mono. The prefix for hydrogen is tri and don’t forget to add the ide to the end of hydrogen. So we name this phosphorus trihydride.

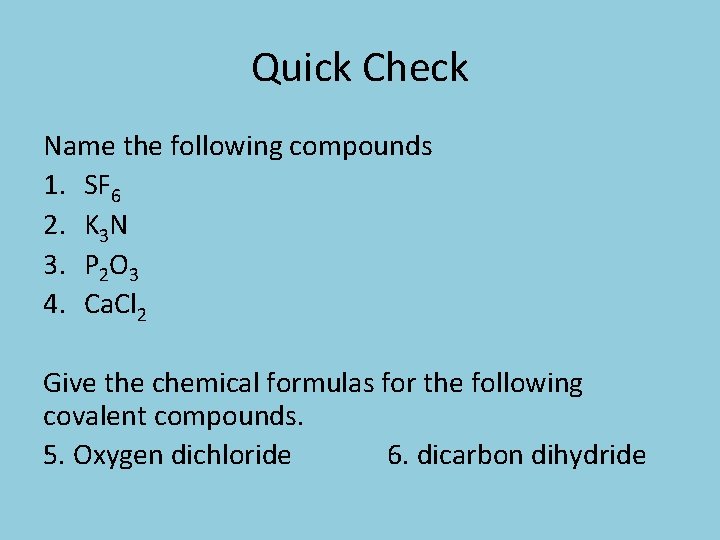
Quick Check Name the following compounds 1. SF 6 2. K 3 N 3. P 2 O 3 4. Ca. Cl 2 Give the chemical formulas for the following covalent compounds. 5. Oxygen dichloride 6. dicarbon dihydride

Quick Check answers Name the following compounds 1. SF 6 sulfur hexafluoride 2. K 3 N potassium nitride 3. P 2 O 3 diphosphorus trioxide 4. Ca. Cl 2 calcium chloride Give the chemical formulas for the following covalent compounds. 5. Oxygen dichloride 6. dicarbon dihydride OCl 2 C 2 H 2