4 2 Ionic and Covalent Compound Naming Ionic

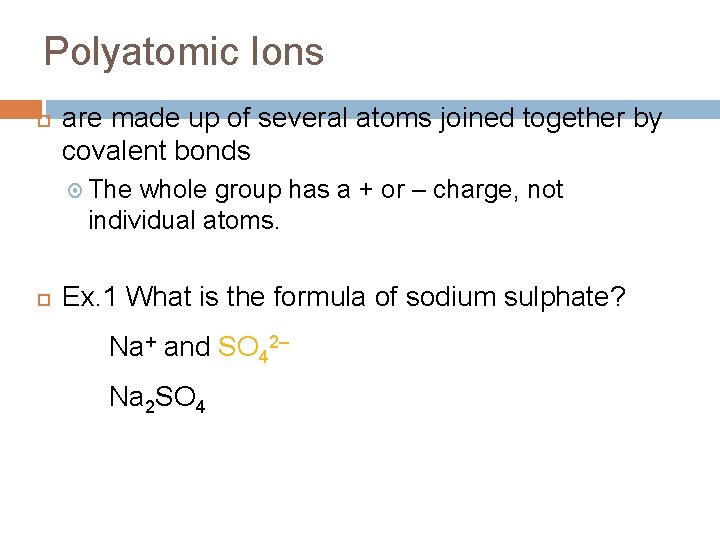



- Slides: 11

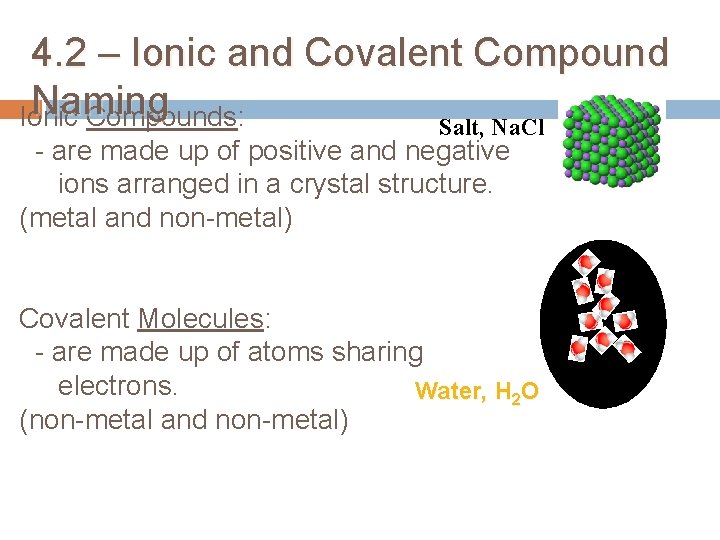
4. 2 – Ionic and Covalent Compound Naming Ionic Compounds: Salt, Na. Cl - are made up of positive and negative ions arranged in a crystal structure. (metal and non-metal) Covalent Molecules: - are made up of atoms sharing electrons. Water, H 2 O (non-metal and non-metal)

Naming Ionic Compounds: name of an ionic compound = positive ion negative ion-ide Ex. 1: magnesium and oxygen positive ion negative ion-ide Magnesium ox + ide Magnesium oxide Ex. 2: what is the name of Ca 3 N 2? Magnesium oxide is used as a drying agent. Ca = calcium; N = nitrogen Drop the end of the negative ion and add –ide Calcium nitride Ex. 3: What is the name of Ba. Cl 2? Barium chloride

Writing formulas for Ionic Compounds Remember: positive charges must = negative charges Ex. 1: What is the formula for magnesium phosphide? § Magnesium is Mg 2+ Phosphorous is P 3– § Lowest common multiple of 2 and 3 is 6 § 3 Mg 2+ ions & 2 P 3– ions (6 +ve’s & 6 –ve’s) § Magnesium phosphide = Mg 3 P 2 Ex. 2: What is the formula for calcium oxide? Calcium is Ca 2+ Oxygen is O 2– 1 Ca 2+ ion & 1 O 2– ion Calcium oxide = Ca. O

Drawing Formula Diagrams More Examples: Lithium nitride Barium sulphide

Ionic Compound with a Multivalent Metal Multivalent: some transition metals have more than one charge. Roman numerals are used after the metal name to indicate which ion was used Ex. 1 What is the formula manganese(III) sulphide? This manganese is Mn 3+ Sulphur is S 2– Lowest common multiple of 3 and 2 is 6 2 Mn 3+ ions and 3 S 2– ions Mn 2 S 3

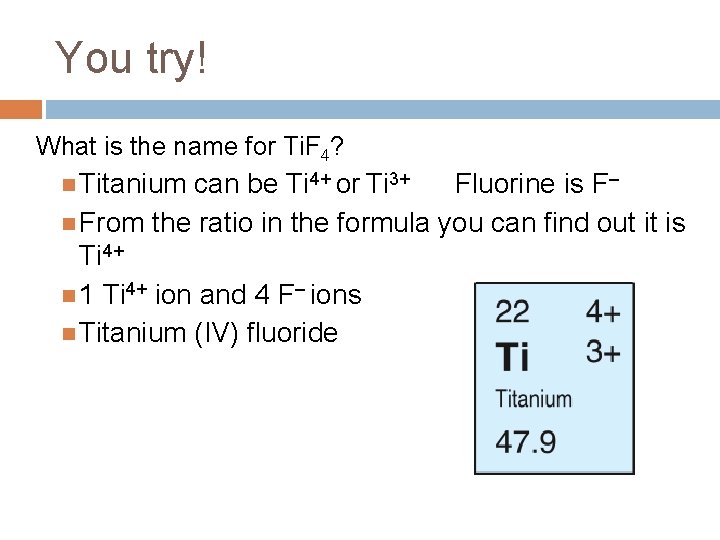
You try! What is the name for Ti. F 4? Titanium can be Ti 4+ or Ti 3+ Fluorine is F– From the ratio in the formula you can find out it is Ti 4+ 1 Ti 4+ ion and 4 F– ions Titanium (IV) fluoride
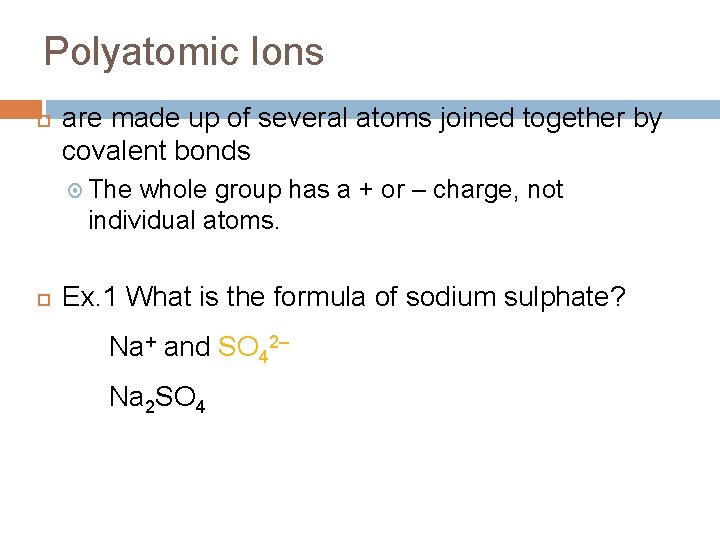
Polyatomic Ions are made up of several atoms joined together by covalent bonds The whole group has a + or – charge, not individual atoms. Ex. 1 What is the formula of sodium sulphate? Na+ and SO 42– Na 2 SO 4

Ex. 2: What is the name of the compound KCl. O? K+ = potassium Cl. O– = hypochlorite Potassium hypochlorite Ex. 3: What is the formula for Calcium nitrate? Ca 2+ and NO 3 - Ca(NO 3)2 * Note the brackets around NO 3 show there are two of the nitrate ions present

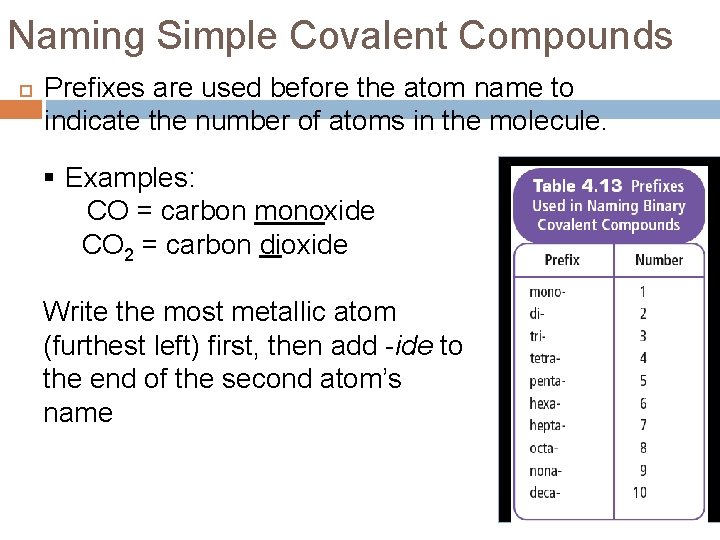
Naming Simple Covalent Compounds Prefixes are used before the atom name to indicate the number of atoms in the molecule. § Examples: CO = carbon monoxide CO 2 = carbon dioxide Write the most metallic atom (furthest left) first, then add -ide to the end of the second atom’s name

Examples: What is the name of the molecule Si 3 P 6? Trisilicon hexaphosphide What is the chemical formula for the molecule trinitrogen tetrachloride? N 3 Cl 4

How do you know which type it is? To determine whether a compound is ionic or covalent: - Examine the formula • • Ionic compounds start with a metal or the ammonium ion Covalent compounds start with a non-metal Take the Section 4. 2 Quiz