Naming and Formula Writing Covalent Compounds n Naming








- Slides: 17

Naming and Formula Writing

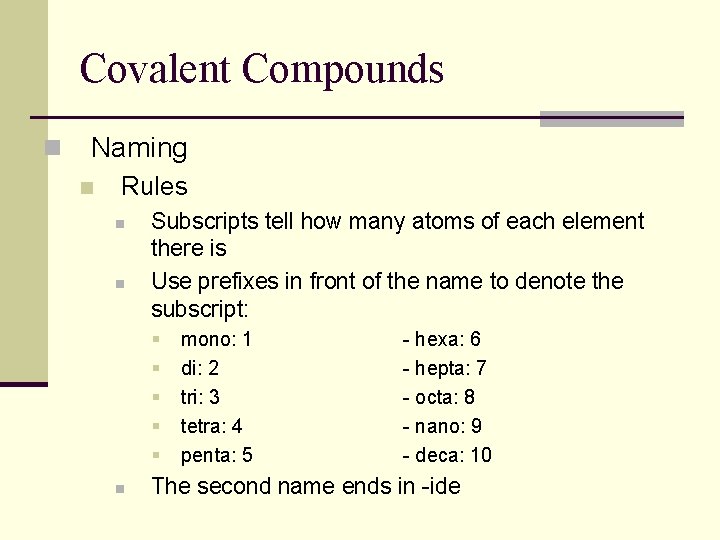
Covalent Compounds n Naming n Rules n n Subscripts tell how many atoms of each element there is Use prefixes in front of the name to denote the subscript: § § § n mono: 1 di: 2 tri: 3 tetra: 4 penta: 5 - hexa: 6 - hepta: 7 - octa: 8 - nano: 9 - deca: 10 The second name ends in -ide

Covalent Compounds n Naming n Examples n NO 2 – Nitrogen dioxide § Mono is only used in the second part of the name. n N 2 O 3 – Dinitrogen trioxide

Covalent Compounds n Formula Writing n n Write the symbols of the elements Write the subscripts for each element from the prefix

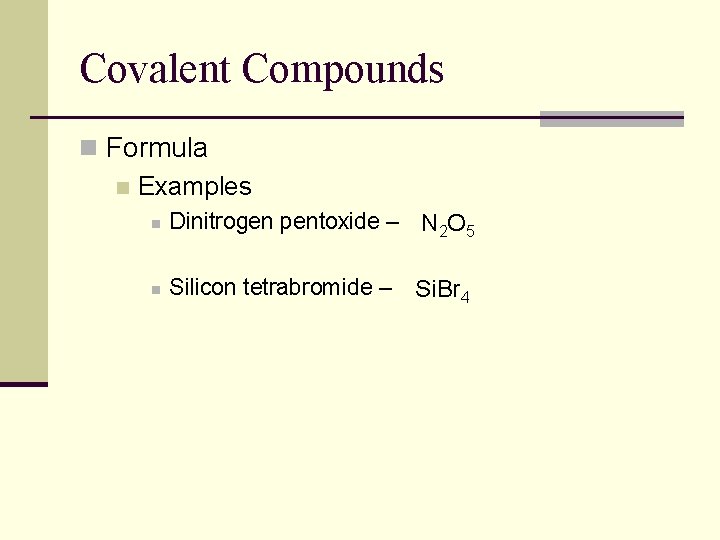
Covalent Compounds n Formula n Examples n Dinitrogen pentoxide – N 2 O 5 n Silicon tetrabromide – Si. Br 4

Ionic Compounds n Naming n Rules n Cation is named first (metal) – keep original name § n E. g. : Mg+2 is Magnesium Anion is named second (non-metal)- ending of the name is replaced with –ide § E. g. : Cl-1 is Chloride

Ionic Compounds n Naming n Examples n Na 2 S – Sodium sulfide n Al. Cl 3 – Aluminum Chloride

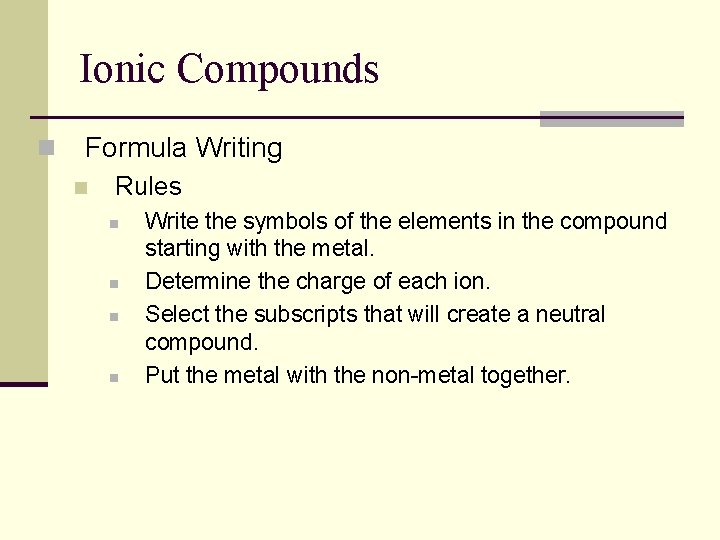
Ionic Compounds n Formula Writing n Rules n n Write the symbols of the elements in the compound starting with the metal. Determine the charge of each ion. Select the subscripts that will create a neutral compound. Put the metal with the non-metal together.

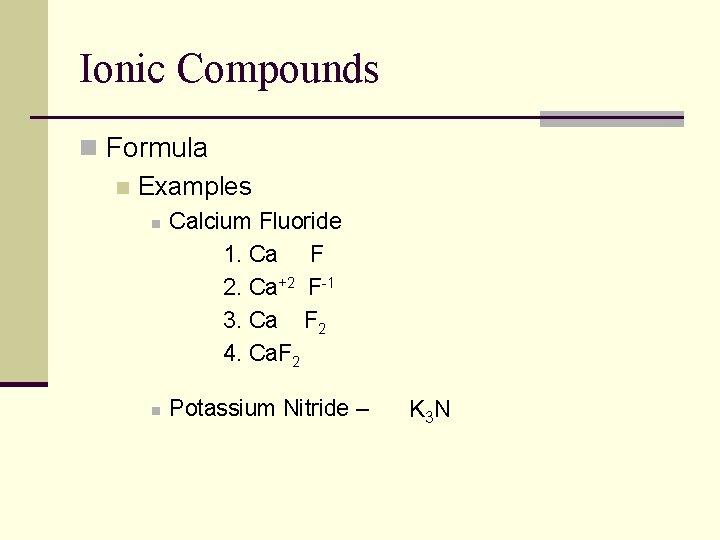
Ionic Compounds n Formula n Examples n n Calcium Fluoride 1. Ca F 2. Ca+2 F-1 3. Ca F 2 4. Ca. F 2 Potassium Nitride – K 3 N

Transition Metals n Naming n Rules n Cation is named first (metal) – keep original name § n Determine the charge of the transition metal and the charge becomes a roman numeral after the name. § n E. g. : Fe+3 is Iron looking at the subscript of the non-metal and make that the charge of the transition metal Anion is named second (non-metal)- ending of the name is replaced with –ide § E. g. : Cl-1 is Chloride

Transition Metals n Naming n Examples n Mn. F 2 – Mangenese (II) flouride n Ni. S – Nickel (II) sulfide

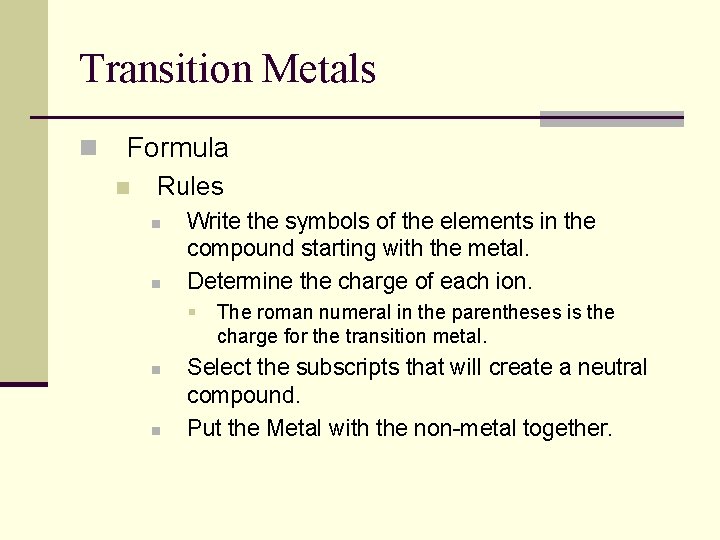
Transition Metals n Formula n Rules n n Write the symbols of the elements in the compound starting with the metal. Determine the charge of each ion. § n n The roman numeral in the parentheses is the charge for the transition metal. Select the subscripts that will create a neutral compound. Put the Metal with the non-metal together.

Transition Metals n Formula n Examples n Chromium (III) Sulfide – n Iron (II) nitride – Fe 3 N 2 Cr 2 S 3

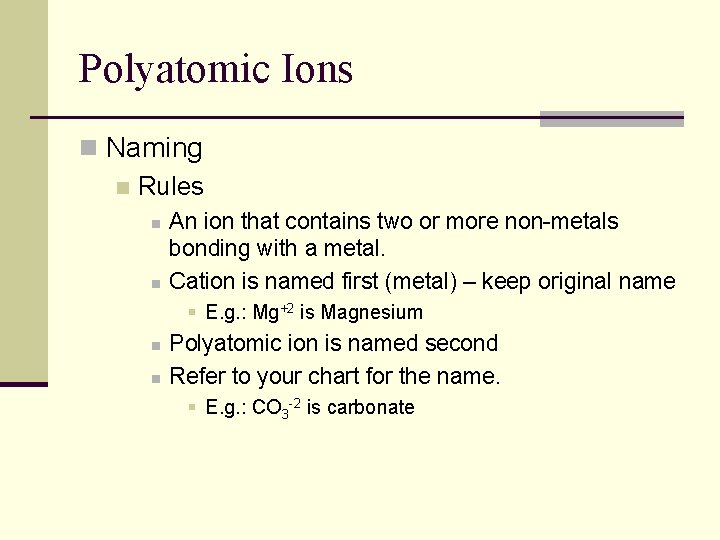
Polyatomic Ions n Naming n Rules n n An ion that contains two or more non-metals bonding with a metal. Cation is named first (metal) – keep original name § E. g. : Mg+2 is Magnesium n n Polyatomic ion is named second Refer to your chart for the name. § E. g. : CO 3 -2 is carbonate

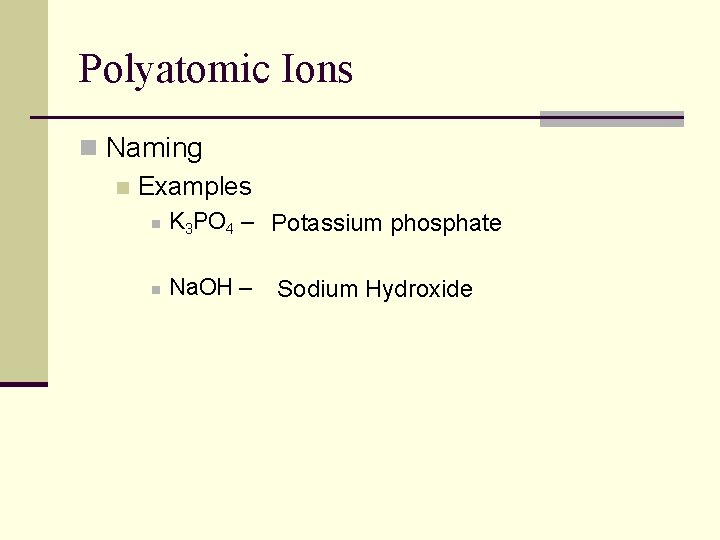
Polyatomic Ions n Naming n Examples n K 3 PO 4 – Potassium phosphate n Na. OH – Sodium Hydroxide

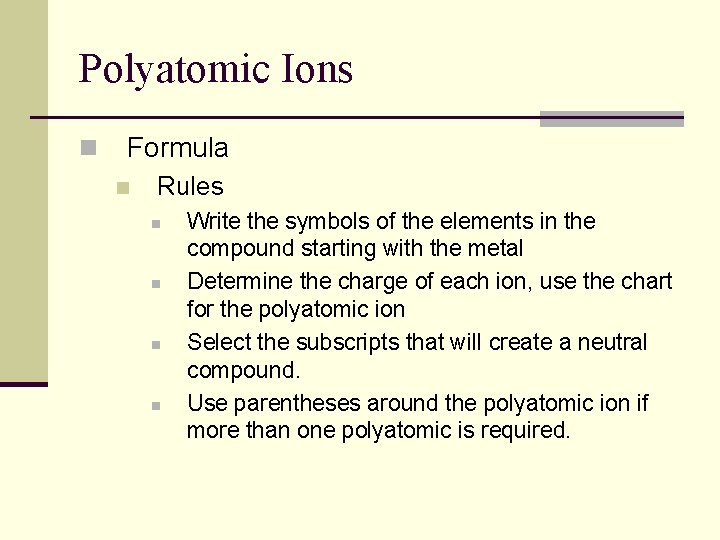
Polyatomic Ions n Formula n Rules n n Write the symbols of the elements in the compound starting with the metal Determine the charge of each ion, use the chart for the polyatomic ion Select the subscripts that will create a neutral compound. Use parentheses around the polyatomic ion if more than one polyatomic is required.

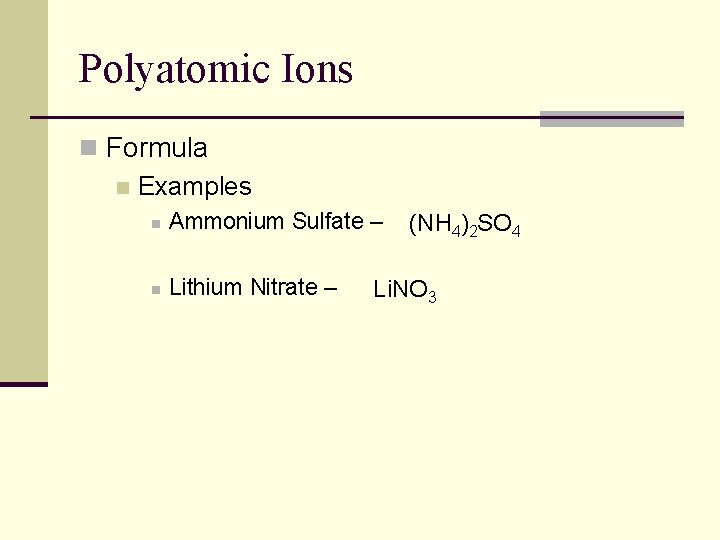
Polyatomic Ions n Formula n Examples n Ammonium Sulfate – n Lithium Nitrate – (NH 4)2 SO 4 Li. NO 3