Limiting Reactants Pancake Recipe makes 10 pancakes 1
















- Slides: 24

Limiting Reactants

Pancake Recipe (makes 10 pancakes) • 1 cup flour • 1 tablespoon sugar • 1 teaspoon baking powder • 1 cup buttermilk • 1 egg • 3 tablespoons melted butter

How many pancakes could you make if you had the following quantities of ingredients? • 2 cups flour • 2 tablespoons sugar • 2 teaspoons baking powder • 2 cups buttermilk • 2 eggs • 6 tablespoons melted butter Answer: 20 pancakes Original Recipe 1 cup flour 1 tablespoon sugar 1 teaspoon baking powder 1 cup buttermilk 1 egg 3 tablespoons melted butter

How many pancakes could you make if you had the following quantities of ingredients? • 2 cups flour • 2 tablespoons sugar • 2 teaspoons baking powder • 2 cups buttermilk • 1 egg • 6 tablespoons melted butter Answer: 10 pancakes Original Recipe 1 cup flour 1 tablespoon sugar 1 teaspoon baking powder 1 cup buttermilk 1 egg 3 tablespoons melted butter

How many pancakes could you make if you had the following quantities of ingredients? Original Recipe • 3 cups flour 1 cup flour 1 tablespoon sugar • 2 tablespoons sugar 1 teaspoon baking powder • 3 teaspoons baking powder 1 cup buttermilk 1 egg 3 tablespoons melted butter • 4 cups buttermilk • 3 eggs • 10 tablespoons melted butter Answer: 20 pancakes

Use of Ingredients • • Ingredients 3 cups flour 2 tablespoons sugar 3 teaspoons baking powder • 4 cups buttermilk 3 eggs • 10 tablespoons melted butter Inventory of Which ingredient would get used ingredients up completely (limiting ingredient)? Which ingredients would you have extra left over (excess ingredients)? How much extra would you have left over for each of the excess ingredients? Original Recipe 1 cup flour 1 tablespoon sugar 1 teaspoon baking powder 1 cup buttermilk 1 egg 3 tablespoons melted butter

Answer check • Which ingredient would get used up completely (limiting ingredient)? sugar • Which ingredients would you have extra left over (excess ingredients)? flour, baking powder, buttermilk, eggs, butter • How much extra would you have left over for each of the excess ingredients? flour (1 cup), baking powder (1 teaspoon), buttermilk (2 cups), eggs (1), butter (4 tablespoons)

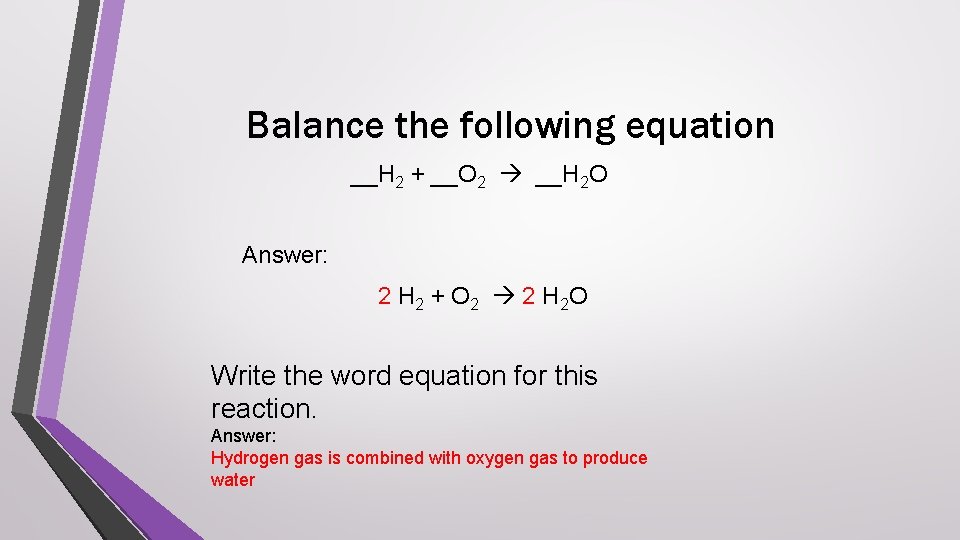
Balance the following equation __H 2 + __O 2 __H 2 O Answer: 2 H 2 + O 2 2 H 2 O Write the word equation for this reaction. Answer: Hydrogen gas is combined with oxygen gas to produce water

Use of Ingredients Inventory of Ingredients • 4 moles H 2 • 4 moles O 2 ingredients • Which ingredient would get used up completely (limiting ingredient)? • Which ingredients would you have extra left over (excess ingredients)? • How much extra would you have left over for each of the excess ingredients?

Answer check • Which ingredient would get used up completely • • (limiting ingredient)? hydrogen Which ingredients would you have extra left over (excess ingredients)? oxygen How much extra would you have left over for each of the excess ingredients? 2 moles oxygen

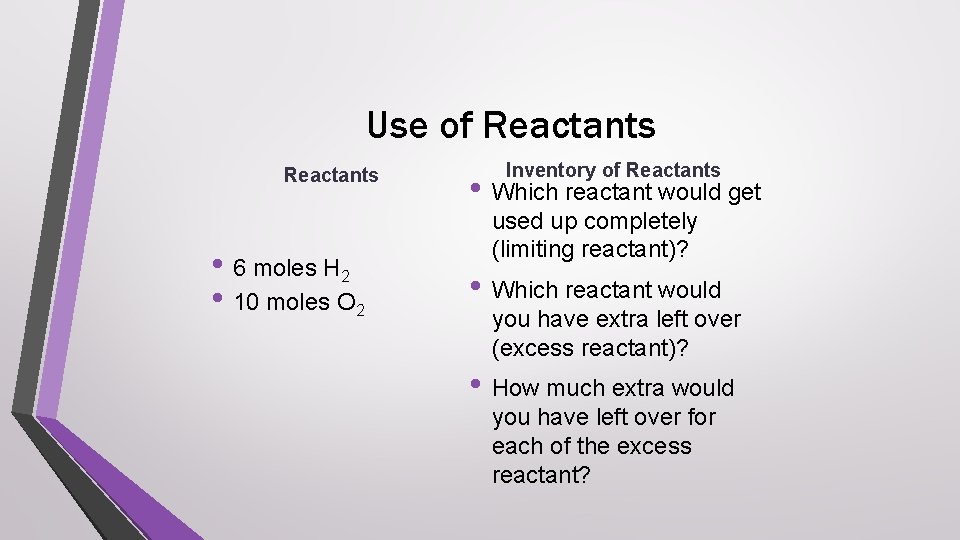
Use of Reactants • 6 moles H 2 • 10 moles O 2 Inventory of Reactants • Which reactant would get used up completely (limiting reactant)? • Which reactant would you have extra left over (excess reactant)? • How much extra would you have left over for each of the excess reactant?

Answer check • Which reactant would get used up completely • • (limiting reactant)? hydrogen Which reactant would you have extra left over (excess reactant)? oxygen How much extra would you have left over of the excess reactant? 7 moles oxygen

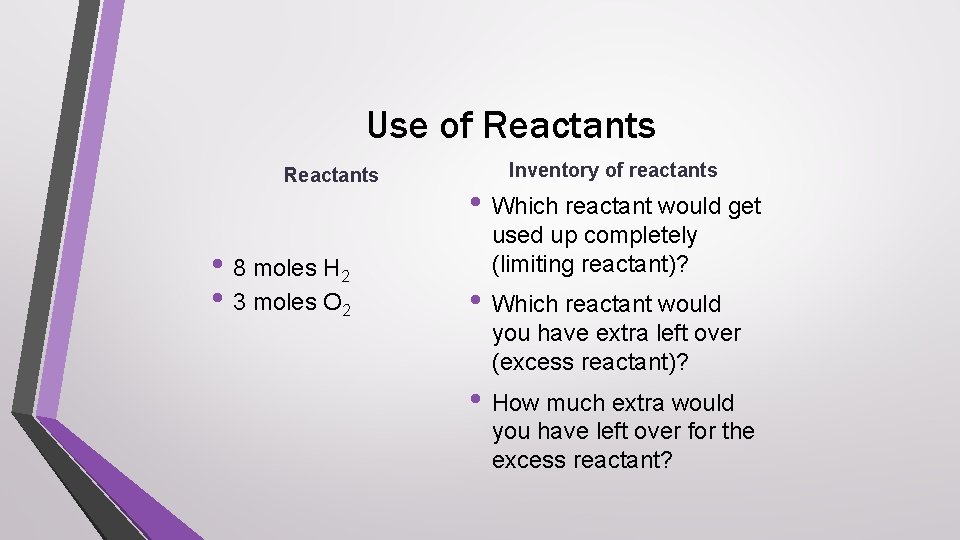
Use of Reactants • 8 moles H 2 • 3 moles O 2 Inventory of reactants • Which reactant would get used up completely (limiting reactant)? • Which reactant would you have extra left over (excess reactant)? • How much extra would you have left over for the excess reactant?

Answer check • Which reactant would get used up completely • • (limiting reactant)? oxygen Which reactant would you have extra left over (excess reactant)? hydrogen How much extra would you have left over for the excess reactant? 2 moles hydrogen

Practice O Balance the equation 2 Li. OH + H 2 SO 4 2 H 2 O + Li 2 SO 4 O Given 10 grams of each reactant. How many grams of each product could be produced? O Which reactant is limiting? Which is in excess? O O Find out how many moles are in 10 grams of each reactant. O How many grams of H 2 O is produced? How many grams of Li 2 SO 4? How many moles of H 2 O does each make? Now we can determine which reactant is limiting. Using 10 g of H 2 SO 4 (the limiting reactant) how many moles of H 2 O is produced? How many moles of Li 2 SO 4?

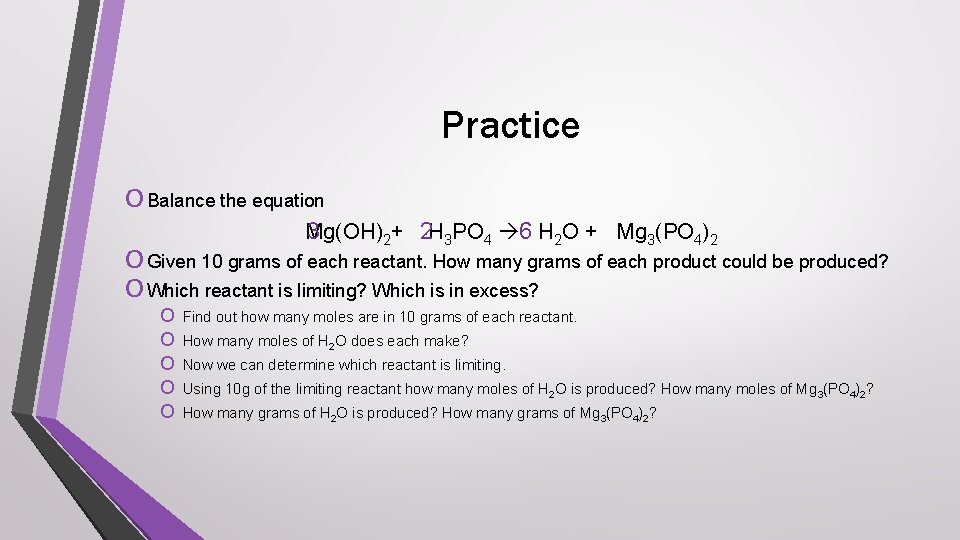
Practice O Balance the equation Mg(OH) 3 2+ 2 H 3 PO 4 6 H 2 O + Mg 3(PO 4)2 O Given 10 grams of each reactant. How many grams of each product could be produced? O Which reactant is limiting? Which is in excess? O O O Find out how many moles are in 10 grams of each reactant. How many moles of H 2 O does each make? Now we can determine which reactant is limiting. Using 10 g of the limiting reactant how many moles of H 2 O is produced? How many moles of Mg 3(PO 4)2? How many grams of H 2 O is produced? How many grams of Mg 3(PO 4)2?

Practice O Balance the equation CH 4+ 2 O 2 2 H 2 O + CO 2 O Given 10 grams of each reactant. How many grams of each product could be produced? O Which reactant is limiting? Which is in excess? O O O Find out how many moles are in 10 grams of each reactant. How many moles of H 2 O does each make? Now we can determine which reactant is limiting. Using 10 g of the limiting reactant how many moles of H 2 O is produced? How many moles of CO 2? How many grams of H 2 O is produced? How many grams of CO 2?

Practice O Balance the equation C 8 H 18+ O 2 H 2 O + CO 2 O Given 10 grams of each reactant. How many grams of each product could be produced? O Which reactant is limiting? Which is in excess? O O O Find out how many moles are in 10 grams of each reactant. How many moles of H 2 O does each make? Now we can determine which reactant is limiting. Using 10 g of the limiting reactant how many moles of H 2 O is produced? How many moles of CO 2? How many grams of H 2 O is produced? How many grams of CO 2?

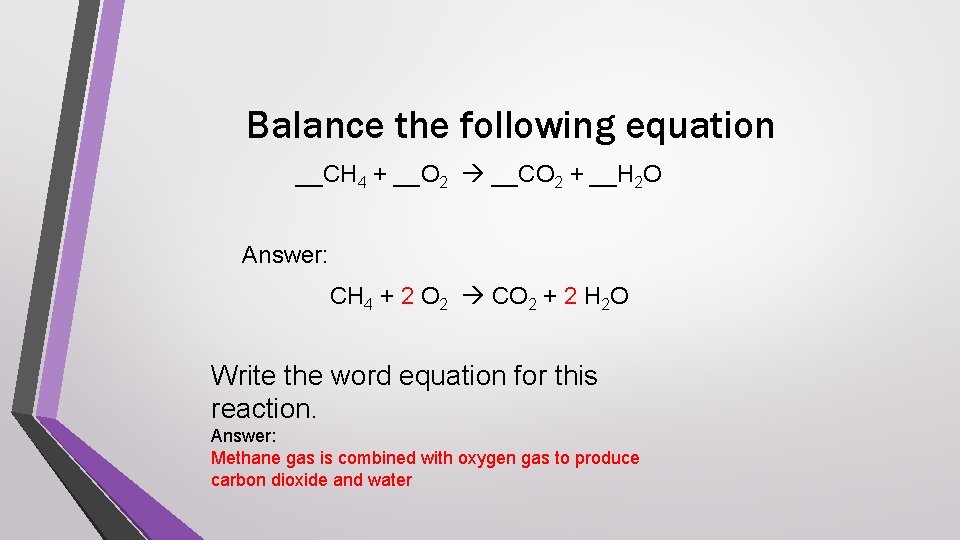
Balance the following equation __CH 4 + __O 2 __CO 2 + __H 2 O Answer: CH 4 + 2 O 2 CO 2 + 2 H 2 O Write the word equation for this reaction. Answer: Methane gas is combined with oxygen gas to produce carbon dioxide and water

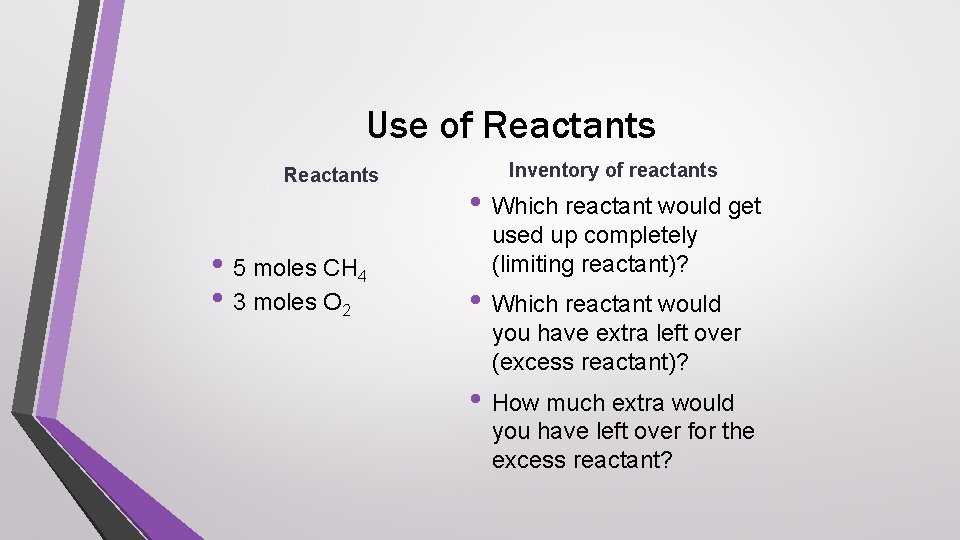
Use of Reactants • 5 moles CH 4 • 3 moles O 2 Inventory of reactants • Which reactant would get used up completely (limiting reactant)? • Which reactant would you have extra left over (excess reactant)? • How much extra would you have left over for the excess reactant?

Answer check • Which reactant would get used up completely • • (limiting reactant)? oxygen Which reactant would you have extra left over (excess reactant)? methane How much extra would you have left over for the excess reactant? 3. 5 moles methane

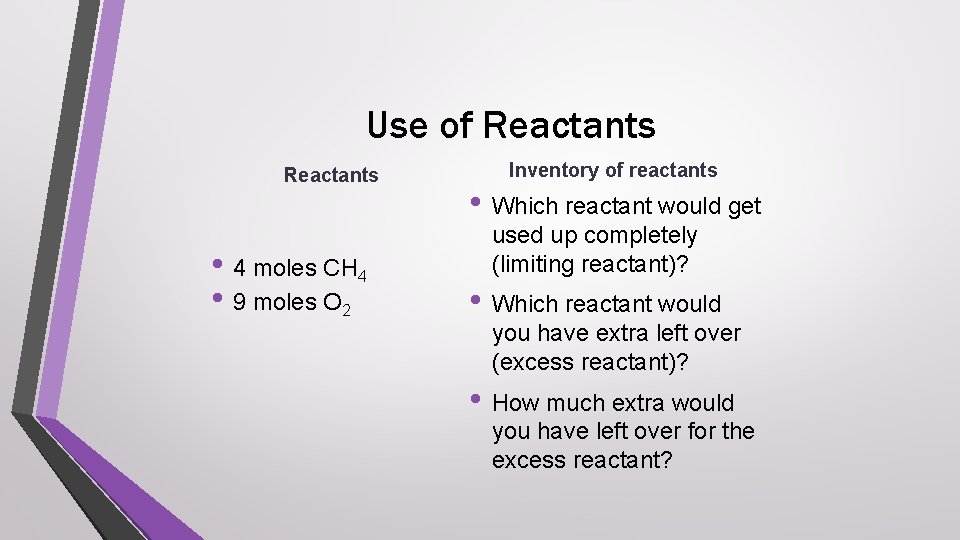
Use of Reactants • 4 moles CH 4 • 9 moles O 2 Inventory of reactants • Which reactant would get used up completely (limiting reactant)? • Which reactant would you have extra left over (excess reactant)? • How much extra would you have left over for the excess reactant?

Answer check • Which reactant would get used up completely • • (limiting reactant)? methane Which reactant would you have extra left over (excess reactant)? oxygen How much extra would you have left over for the excess reactant? 1 mole oxygen

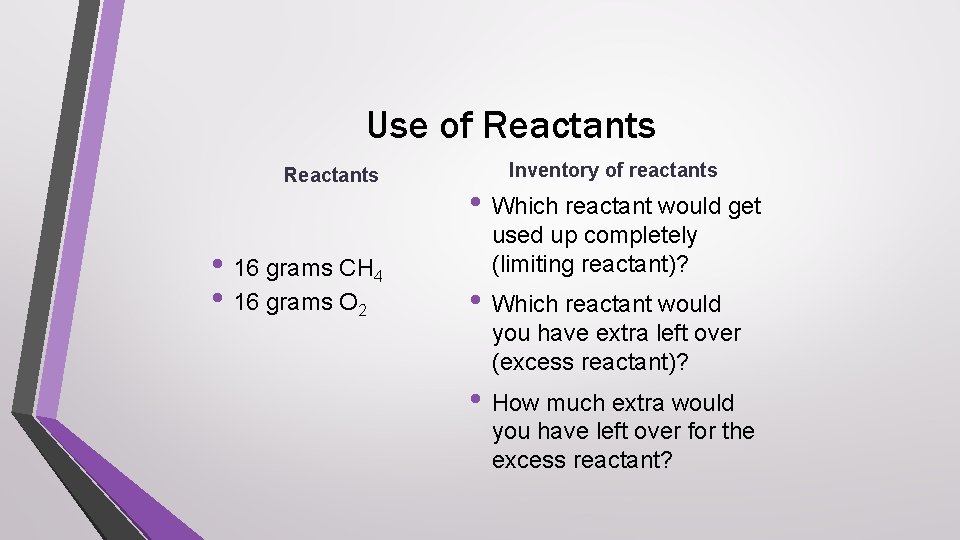
Use of Reactants • 16 grams CH 4 • 16 grams O 2 Inventory of reactants • Which reactant would get used up completely (limiting reactant)? • Which reactant would you have extra left over (excess reactant)? • How much extra would you have left over for the excess reactant?