LIMITING AND EXCESS REACTANTS YOU HAVE 23 PIECES










- Slides: 21

LIMITING AND EXCESS REACTANTS

YOU HAVE 23 PIECES OF BREAD, 18 PIECES OF HAM, AND 29 PIECES OF CHEESEH. OW MANY HAM AND CHEESE SANDWICHES COULD YOU MAKE? (ASSUME 1 HAM AND 2 CHEESE IN EACH SANDWICH…MMMM…) What is the limiting factor in the sandwich making? ? How much ham is excess? How much cheese is excess?

YOU ARE THE YOUNGEST PERSON (17 YEARS OLD) TO WIN THE LOCAL LOTTERY …$120, 000!!!! You find your way to the used car dealer who has 8 beautiful cars for sale to be driven off the lot after sale!!! … each only $25, 000 (all taxes in!). How many cars can you buy? What is in Excess? What is the limiting factor? Your age is the limiting reactant…

IF 2 MOLES OF NITROGEN REACT WITH 5 MOLES OF HYDROGEN, HOW MANY MOLES OF AMMONIA (N 2 H 3) WILL BE PRODUCED? (DETERMINE THE LIMITING REACTANT AND HOW MUCH OF THE EXCESS REACTANT WILL BE LEFT OVER. ) 2 N 2 + 3 H 2 2 N 2 H 3 2. 00 mol 5. 00 mol ? ? mol You only need 2 mols N 2 to react with 3 mols of H 2 in order to get NH 3… A 2: 3 ratio needed. But you’ve got 5 mols of H 2 available… A 2: 5 ratio… Which is the excess reactant? ? H 2!!

1. CHEMICAL EQUATIONS DO NOT TELL THE WHOLE STORY ABOUT A REACTION: 1. 2. 3. The exact conditions for the reaction (e. g. temperature, stirring, pressure, etc. ) The behavior of the atoms during the reaction The nature of the reaction (i. e. fast or slow)

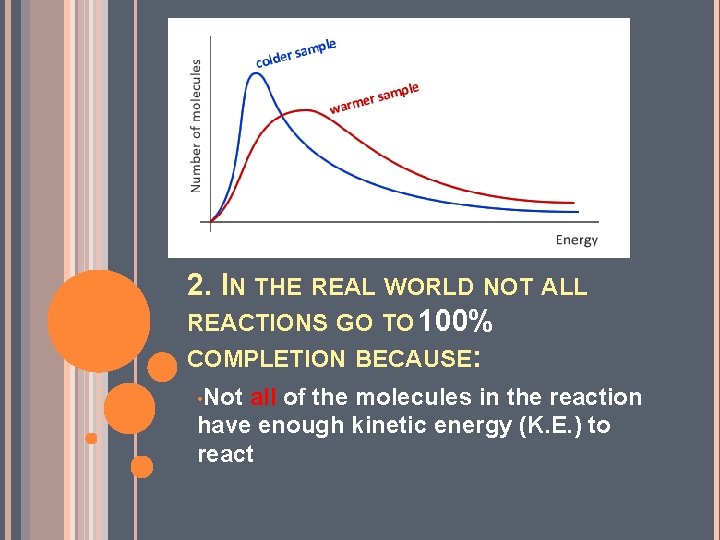
2. IN THE REAL WORLD NOT ALL REACTIONS GO TO 100% COMPLETION BECAUSE: • Not all of the molecules in the reaction have enough kinetic energy (K. E. ) to react

3. TO GET A MORE COMPLETE REACTION USING THE MOST “IDEAL” CONDITIONS POSSIBLE AN EXCESS OF ONE OF THE REACTANTS IS ADDED This is called the “Excess Reactant”. There is more of it than you actually need! 1.

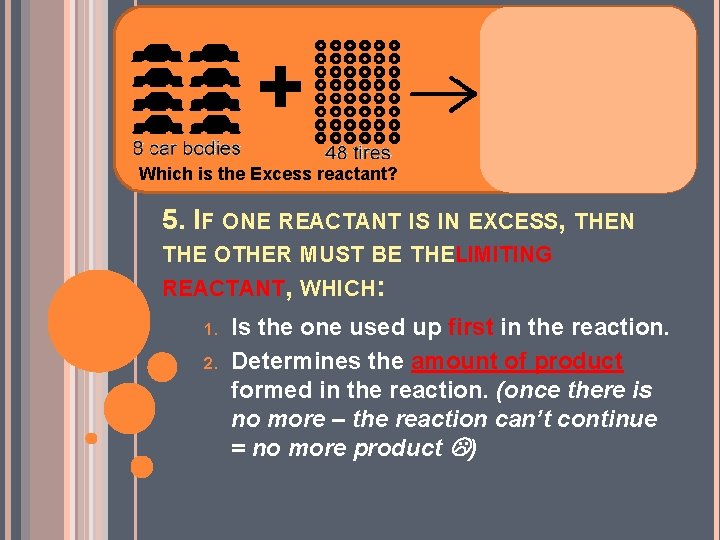
4. THE CRITERIA FOR CHOOSING THE EXCESS REACTANT ARE: 1. 2. The cheaper or the most abundant one The one most easily separated after the reaction is finished.

Which is the Excess reactant? 5. IF ONE REACTANT IS IN EXCESS, THEN THE OTHER MUST BE THEL IMITING REACTANT, WHICH: 1. 2. Is the one used up first in the reaction. Determines the amount of product formed in the reaction. (once there is no more – the reaction can’t continue = no more product )

YOU WORK AT HOME DEPOT IN THE PAINT DEPARTMENT. TO MIX UP PINK PAINT YOU NEED 3 PARTSW HITE AND 1 PARTR ED PAINT MIXED… Suppose you have 6 L of white paint and 1. 5 L of red paint. 1) Which color of paint is limiting the amount of pink paint produced? 3 LWhite : 1 LRed Red!! 6 LWhite : ? LRed 2) How many Litres of pink paint can you make with what you have? ? 3 LWhite : 1 LRed ? LWhite : 1. 5 LRed So 4. 5 L of White paint to use = 6 L of Pink Paint!

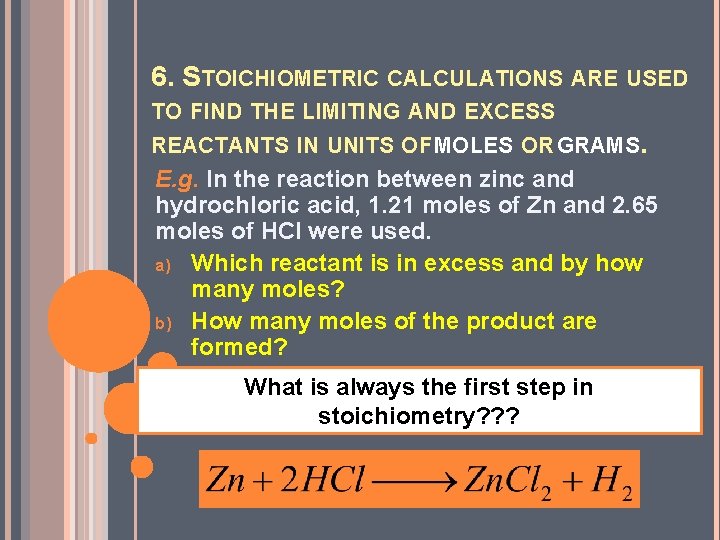
6. STOICHIOMETRIC CALCULATIONS ARE USED TO FIND THE LIMITING AND EXCESS REACTANTS IN UNITS OF MOLES OR GRAMS. E. g. In the reaction between zinc and hydrochloric acid, 1. 21 moles of Zn and 2. 65 moles of HCl were used. a) Which reactant is in excess and by how many moles? b) How many moles of the product are formed? What is always the first step in stoichiometry? ? ?

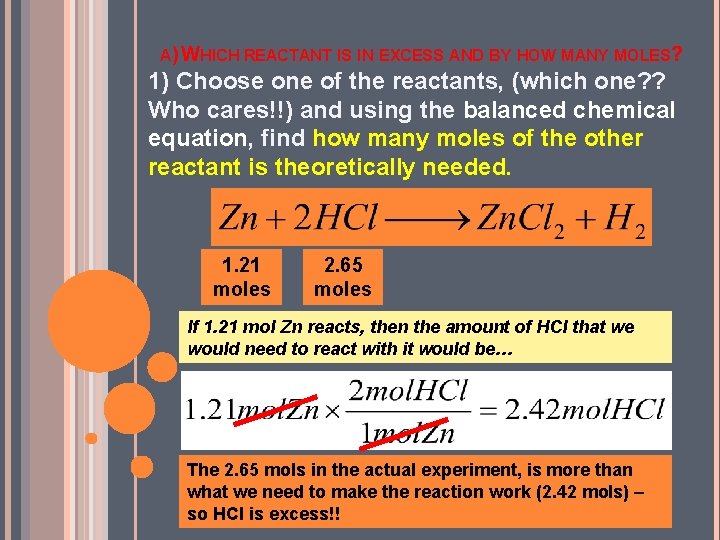
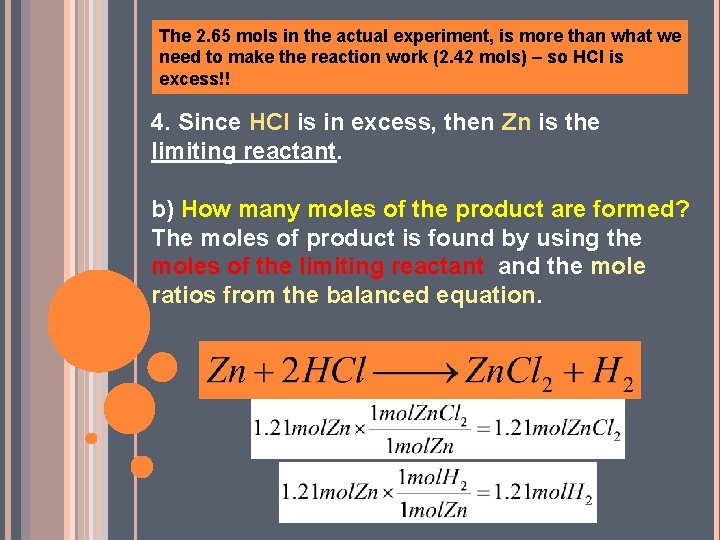
A) WHICH REACTANT IS IN EXCESS AND BY HOW MANY MOLES? 1) Choose one of the reactants, (which one? ? Who cares!!) and using the balanced chemical equation, find how many moles of the other reactant is theoretically needed. 1. 21 moles 2. 65 moles If 1. 21 mol Zn reacts, then the amount of HCl that we would need to react with it would be… The 2. 65 mols in the actual experiment, is more than what we need to make the reaction work (2. 42 mols) – so HCl is excess!!

The 2. 65 mols in the actual experiment, is more than what we need to make the reaction work (2. 42 mols) – so HCl is excess!! 4. Since HCl is in excess, then Zn is the limiting reactant. b) How many moles of the product are formed? The moles of product is found by using the moles of the limiting reactant and the mole ratios from the balanced equation.

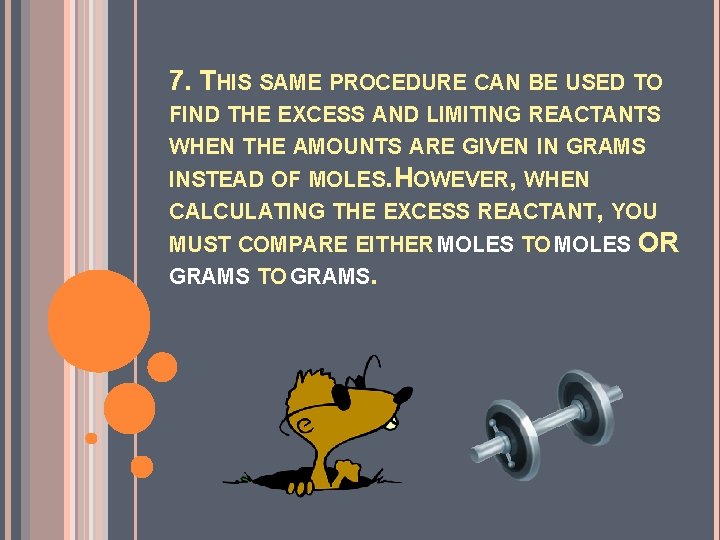
7. THIS SAME PROCEDURE CAN BE USED TO FIND THE EXCESS AND LIMITING REACTANTS WHEN THE AMOUNTS ARE GIVEN IN GRAMS INSTEAD OF MOLES. HOWEVER, WHEN CALCULATING THE EXCESS REACTANT, YOU MUST COMPARE EITHER MOLES TO MOLES OR GRAMS TO GRAMS.

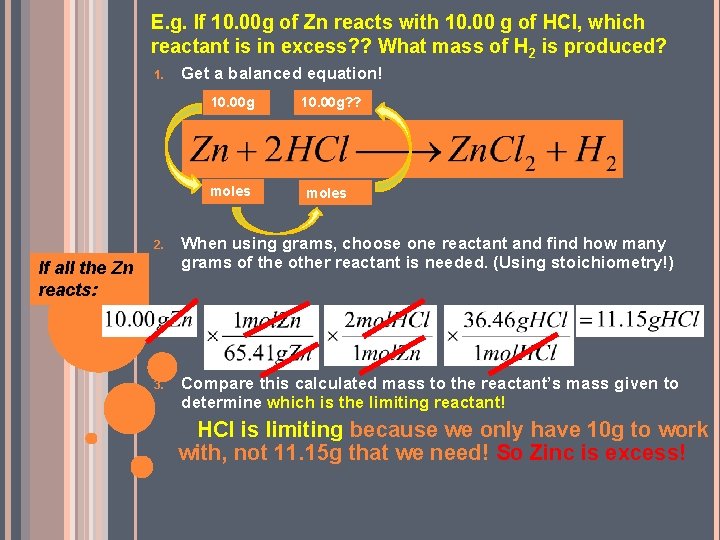
E. g. If 10. 00 g of Zn reacts with 10. 00 g of HCl, which reactant is in excess? ? What mass of H 2 is produced? 1. Get a balanced equation! 10. 00 g moles 10. 00 g? ? moles 2. When using grams, choose one reactant and find how many grams of the other reactant is needed. (Using stoichiometry!) 3. Compare this calculated mass to the reactant’s mass given to determine which is the limiting reactant! If all the Zn reacts: HCl is limiting because we only have 10 g to work with, not 11. 15 g that we need! So Zinc is excess!

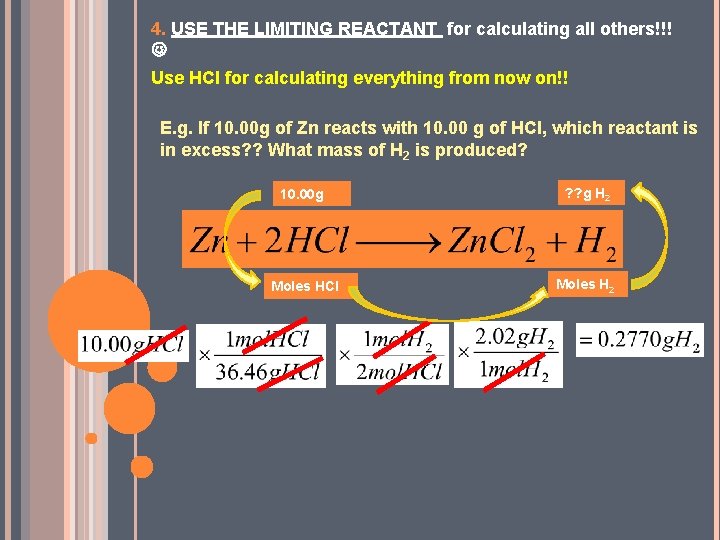
4. USE THE LIMITING REACTANT for calculating all others!!! Use HCl for calculating everything from now on!! E. g. If 10. 00 g of Zn reacts with 10. 00 g of HCl, which reactant is in excess? ? What mass of H 2 is produced? 10. 00 g ? ? g H 2 Moles HCl Moles H 2

LET’S PRACTICE !!! TRY THE QUESTIONS ON THE NOTES!

PERCENT YIELD THE AMOUNT OF PRODUCT “ACTUALLY” PRODUCED IN YOUR EXPERIMENT, AS A % OF WHAT YOU SHOULD HAVE GOT! 1. Since not all conditions in a lab situation are going to be “perfect”, it is unlikely that 100% yield will be obtained from a reaction. Reasons for this could be … 1. Some portion of the reactants may simply not react (not enough K. E. ) 2. Side reactions may take place making a different product than we want 3. Some product may be lost during recovery or transfer

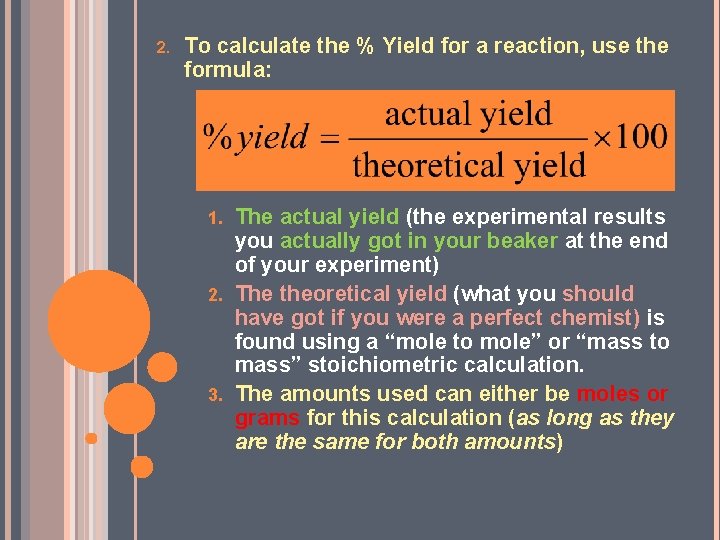
2. To calculate the % Yield for a reaction, use the formula: The actual yield (the experimental results you actually got in your beaker at the end of your experiment) 2. The theoretical yield (what you should have got if you were a perfect chemist) is found using a “mole to mole” or “mass to mass” stoichiometric calculation. 3. The amounts used can either be moles or grams for this calculation (as long as they are the same for both amounts) 1.

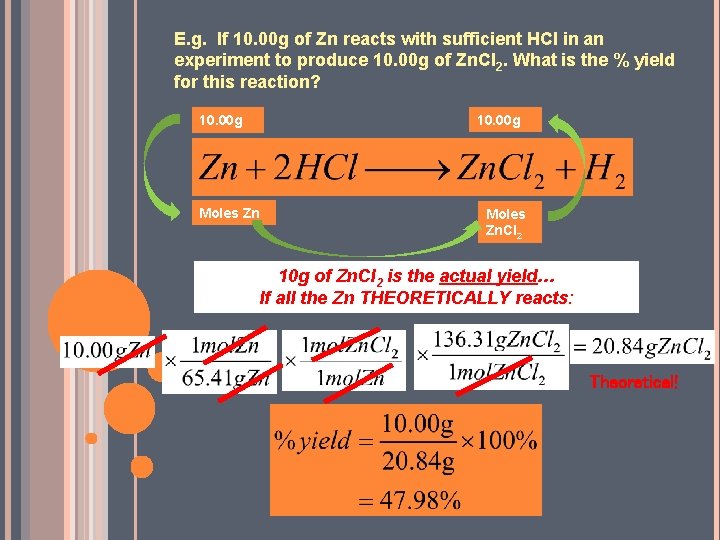
E. g. If 10. 00 g of Zn reacts with sufficient HCl in an experiment to produce 10. 00 g of Zn. Cl 2. What is the % yield for this reaction? 10. 00 g Moles Zn. Cl 2 10 g of Zn. Cl 2 is the actual yield… If all the Zn THEORETICALLY reacts: Theoretical!

YOU TRY SOME!! TRY THE NEXT 2 QUESTIONS ON YOUR NOTES!