Limiting Reactants and Percentage Yield 9 2 Reactants




- Slides: 12

Limiting Reactants and Percentage Yield 9. 2

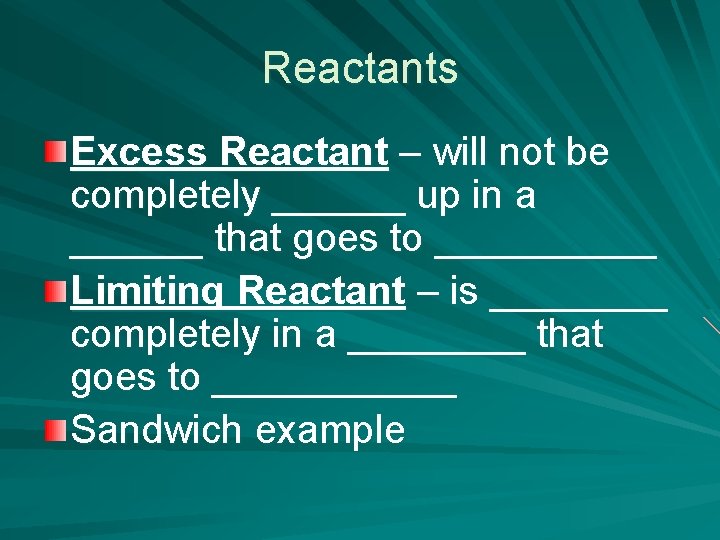
Reactants Excess Reactant – will not be completely ______ up in a ______ that goes to _____ Limiting Reactant – is ____ completely in a ____ that goes to ______ Sandwich example

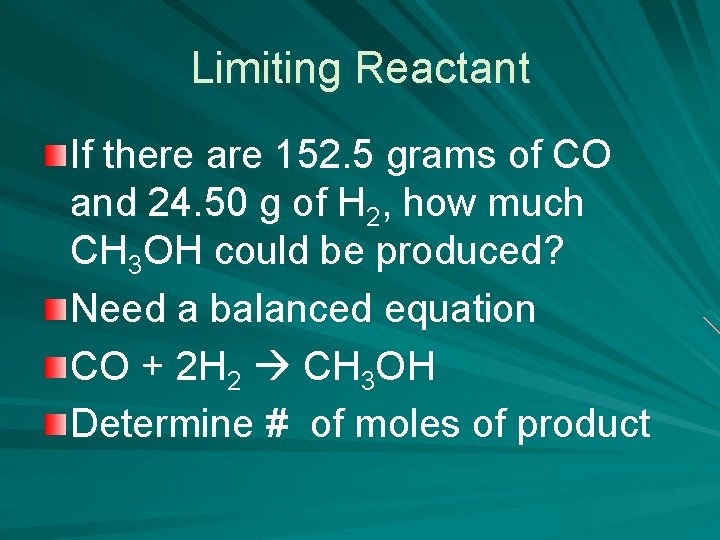
Limiting Reactant If there are 152. 5 grams of CO and 24. 50 g of H 2, how much CH 3 OH could be produced? Need a balanced equation CO + 2 H 2 CH 3 OH Determine # of moles of product

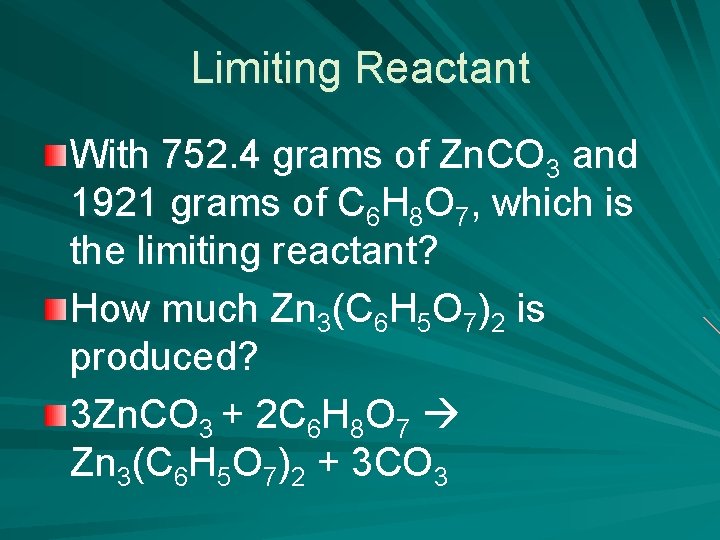
Limiting Reactant With 752. 4 grams of Zn. CO 3 and 1921 grams of C 6 H 8 O 7, which is the limiting reactant? How much Zn 3(C 6 H 5 O 7)2 is produced? 3 Zn. CO 3 + 2 C 6 H 8 O 7 Zn 3(C 6 H 5 O 7)2 + 3 CO 3

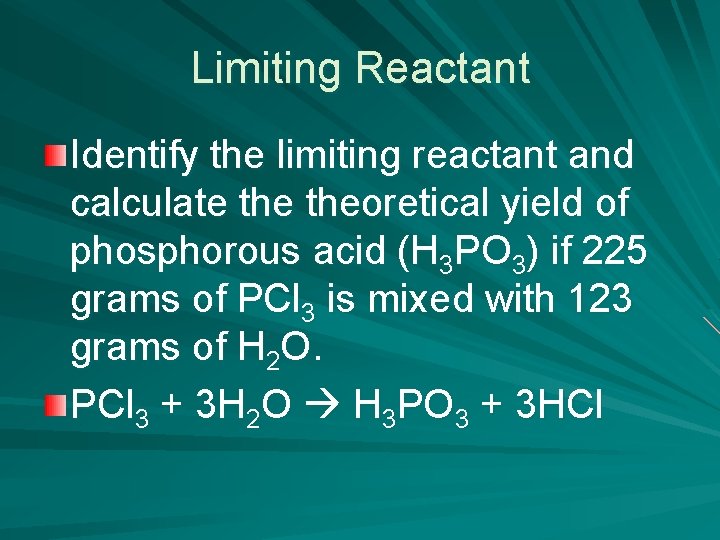
Limiting Reactant Identify the limiting reactant and calculate theoretical yield of phosphorous acid (H 3 PO 3) if 225 grams of PCl 3 is mixed with 123 grams of H 2 O. PCl 3 + 3 H 2 O H 3 PO 3 + 3 HCl

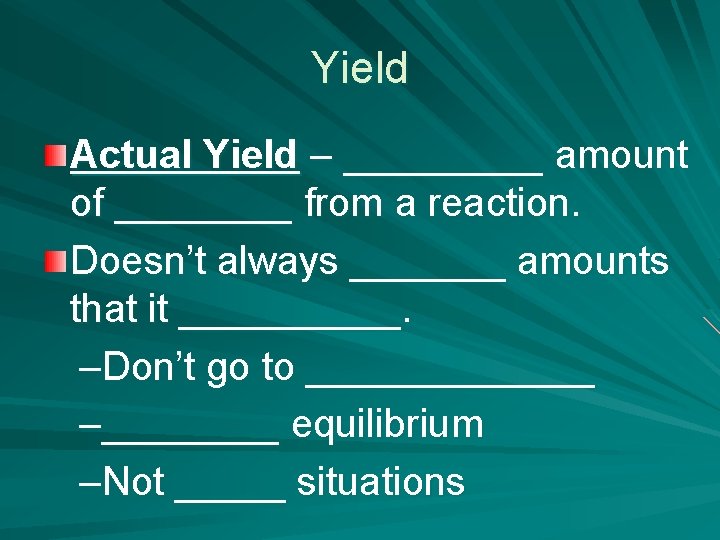
Yield Actual Yield – _____ amount of ____ from a reaction. Doesn’t always _______ amounts that it _____. –Don’t go to _______ –____ equilibrium –Not _____ situations

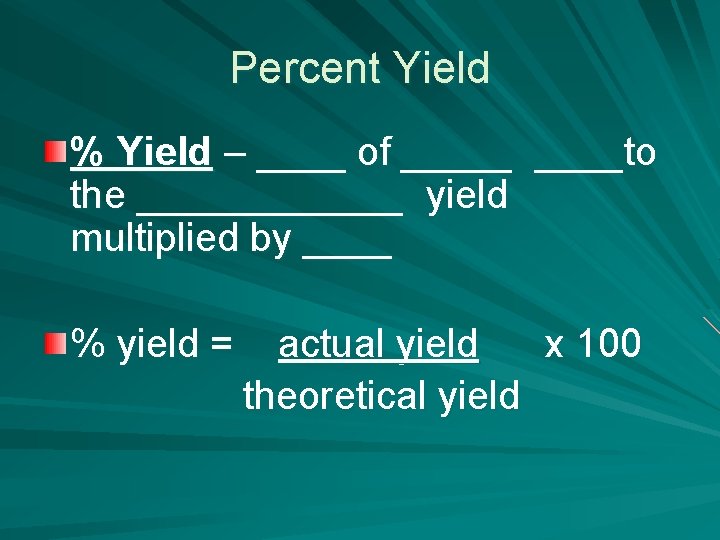
Percent Yield % Yield – ____ of _____to the ______ yield multiplied by ____ % yield = actual yield x 100 theoretical yield

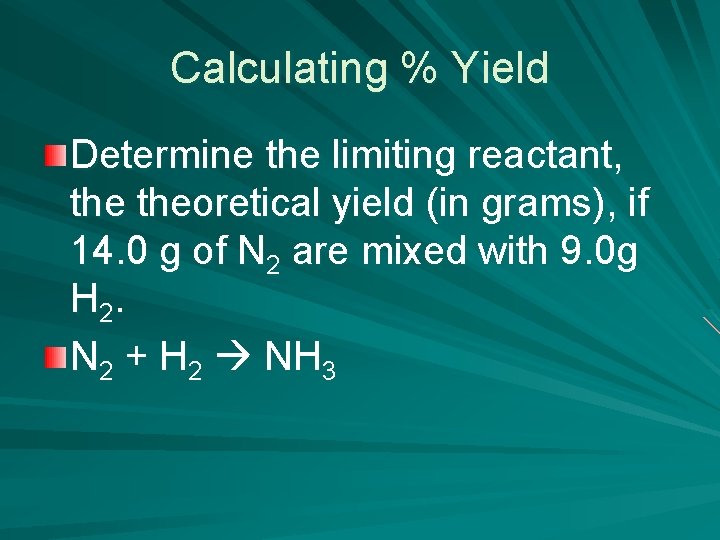
Calculating % Yield Determine the limiting reactant, theoretical yield (in grams), if 14. 0 g of N 2 are mixed with 9. 0 g H 2. N 2 + H 2 NH 3

Calculating % Yield Make sure the equation is balanced. Find limiting reactant first Then identify actual yield Then calculate % yield = Actual/Theoretical x 100

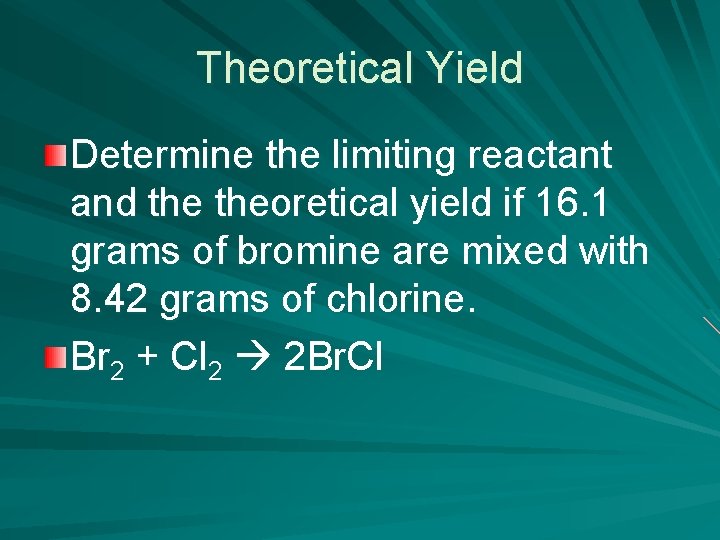
Theoretical Yield Determine the limiting reactant and theoretical yield if 16. 1 grams of bromine are mixed with 8. 42 grams of chlorine. Br 2 + Cl 2 2 Br. Cl

Stoichiometry Review 2 N 2 H 4 + (CH 3)2 N 2 H 2 + 3 N 2 O 4 6 N 2 + 2 CO 2 + 8 H 2 O The density of N 2 H 4 is 0. 982 g/m. L How many m. L of N 2 H 4 is needed to produce 20 grams of N 2?

Stoichiometry Review 2 N 2 H 4 + (CH 3)2 N 2 H 2 + 3 N 2 O 4 6 N 2 + 2 CO 2 + 8 H 2 O How many molecules of N 2 H 4 are needed to produce 50 grams of N 2?