Section 9 3 Limiting Reactants and Percent Yield

- Slides: 9

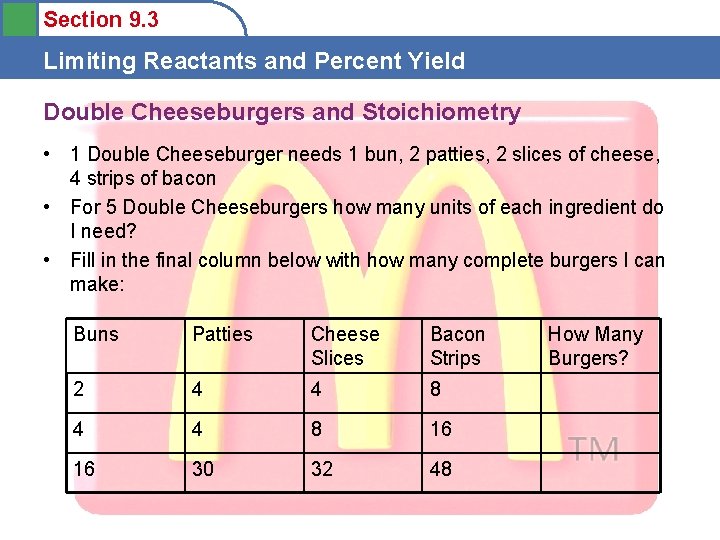
Section 9. 3 Limiting Reactants and Percent Yield Double Cheeseburgers and Stoichiometry • 1 Double Cheeseburger needs 1 bun, 2 patties, 2 slices of cheese, 4 strips of bacon • For 5 Double Cheeseburgers how many units of each ingredient do I need? • Fill in the final column below with how many complete burgers I can make: Buns Patties Cheese Slices Bacon Strips 2 4 4 8 16 16 30 32 48 How Many Burgers?

Section 9. 3 Limiting Reactants and Percent Yield Objectives 1. To understand the concept of limiting reactants 2. To learn to recognize the limiting reactant in a reaction 3. To learn to use the limiting reactant to do stoichiometric calculations 4. To learn to calculate percent yield

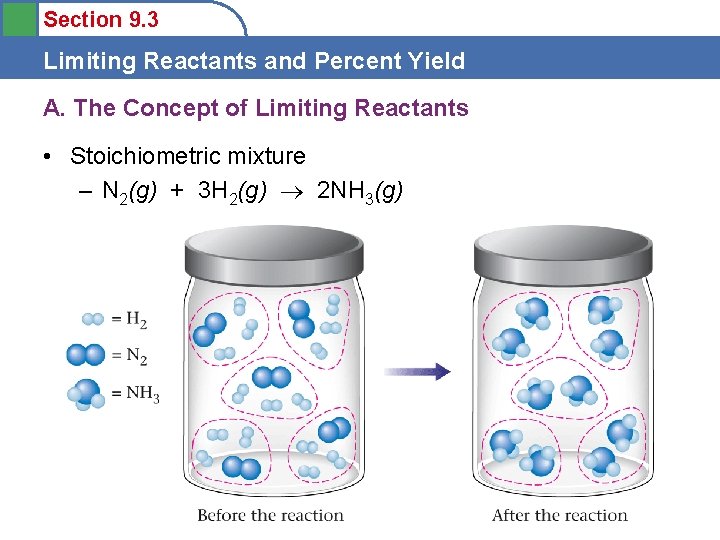
Section 9. 3 Limiting Reactants and Percent Yield A. The Concept of Limiting Reactants • Stoichiometric mixture – N 2(g) + 3 H 2(g) 2 NH 3(g)

Section 9. 3 Limiting Reactants and Percent Yield A. The Concept of Limiting Reactants • Limiting reactant mixture – N 2(g) + 3 H 2(g) 2 NH 3(g)

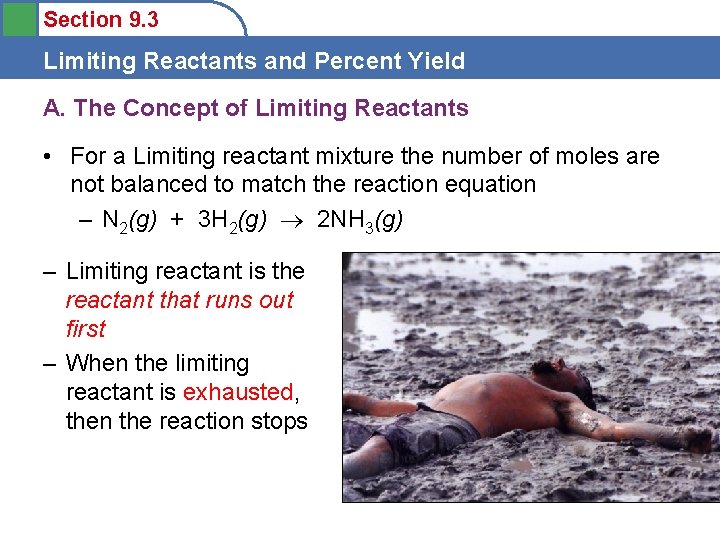
Section 9. 3 Limiting Reactants and Percent Yield A. The Concept of Limiting Reactants • For a Limiting reactant mixture the number of moles are not balanced to match the reaction equation – N 2(g) + 3 H 2(g) 2 NH 3(g) – Limiting reactant is the reactant that runs out first – When the limiting reactant is exhausted, then the reaction stops

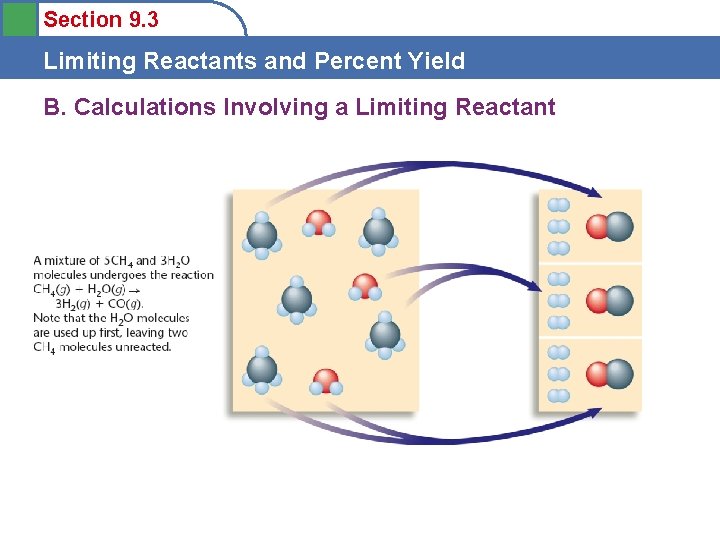
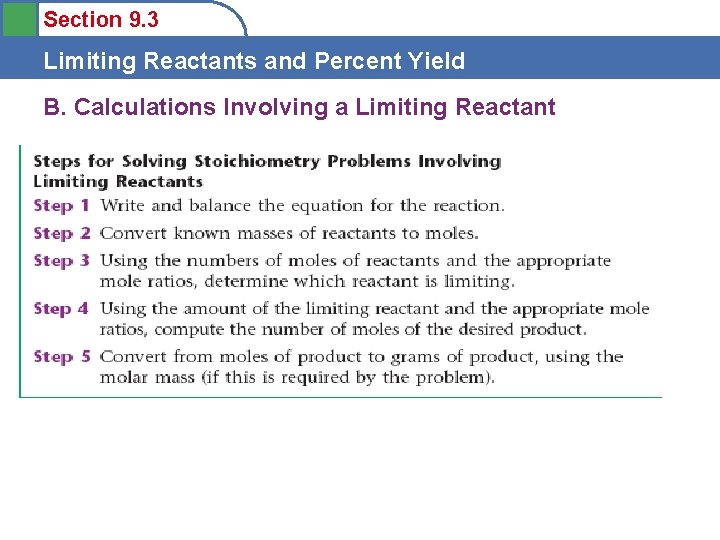
Section 9. 3 Limiting Reactants and Percent Yield B. Calculations Involving a Limiting Reactant

Section 9. 3 Limiting Reactants and Percent Yield B. Calculations Involving a Limiting Reactant

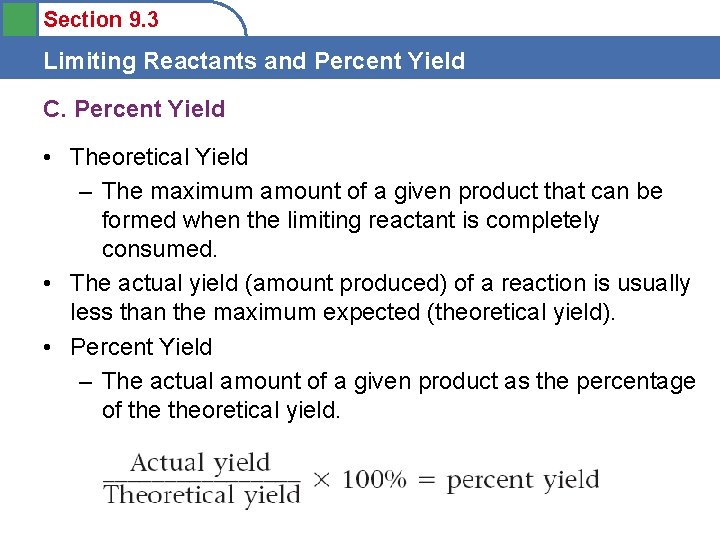
Section 9. 3 Limiting Reactants and Percent Yield C. Percent Yield • Theoretical Yield – The maximum amount of a given product that can be formed when the limiting reactant is completely consumed. • The actual yield (amount produced) of a reaction is usually less than the maximum expected (theoretical yield). • Percent Yield – The actual amount of a given product as the percentage of theoretical yield.

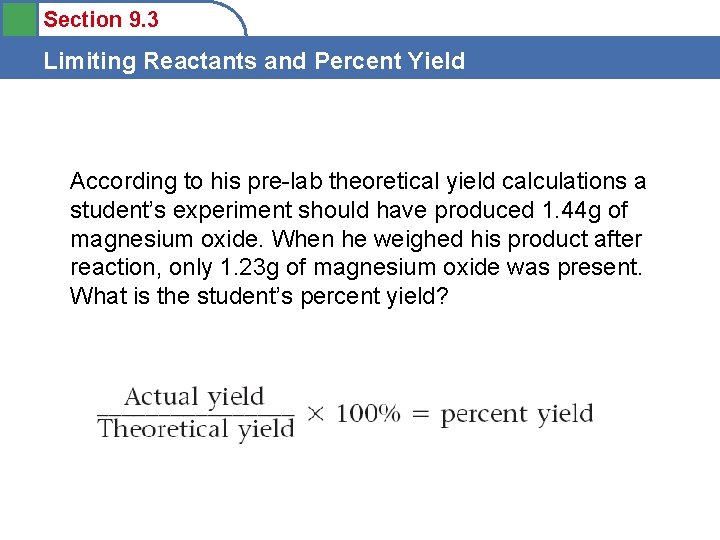
Section 9. 3 Limiting Reactants and Percent Yield According to his pre-lab theoretical yield calculations a student’s experiment should have produced 1. 44 g of magnesium oxide. When he weighed his product after reaction, only 1. 23 g of magnesium oxide was present. What is the student’s percent yield?