Limiting and Excess Reactants 1 MassMass conversions 2



- Slides: 10

Limiting and Excess Reactants 1. Mass-Mass conversions 2. Identifying limiting reactant 3. Calculation of % Yield April 27, 2015

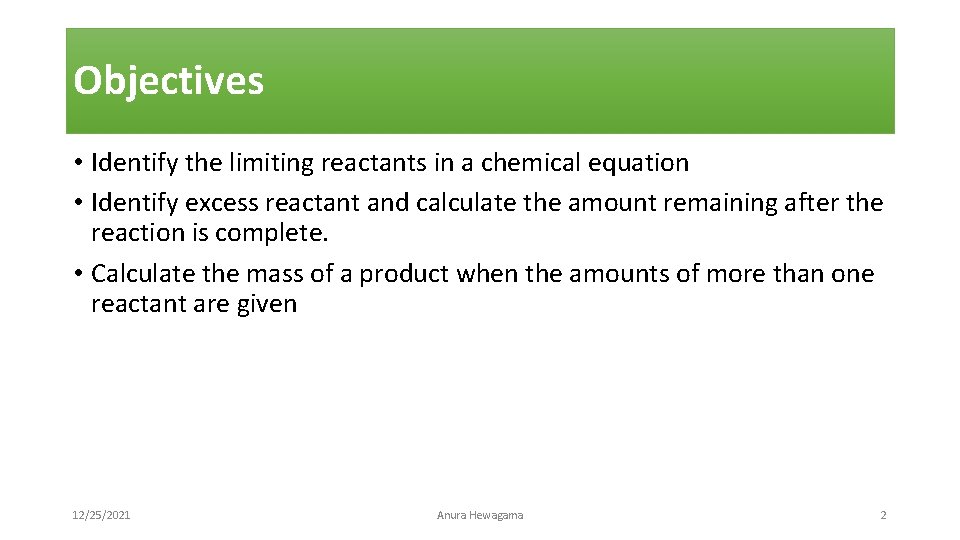
Objectives • Identify the limiting reactants in a chemical equation • Identify excess reactant and calculate the amount remaining after the reaction is complete. • Calculate the mass of a product when the amounts of more than one reactant are given 12/25/2021 Anura Hewagama 2

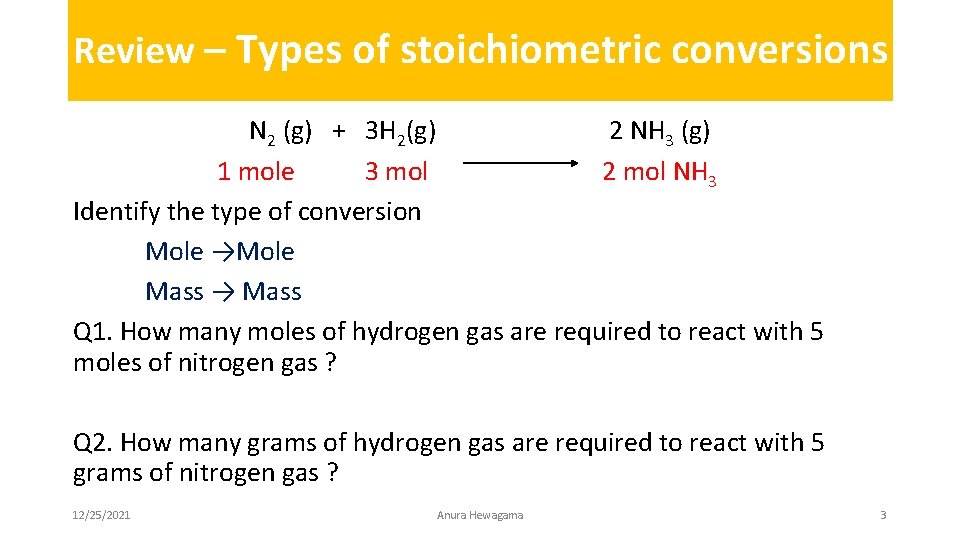
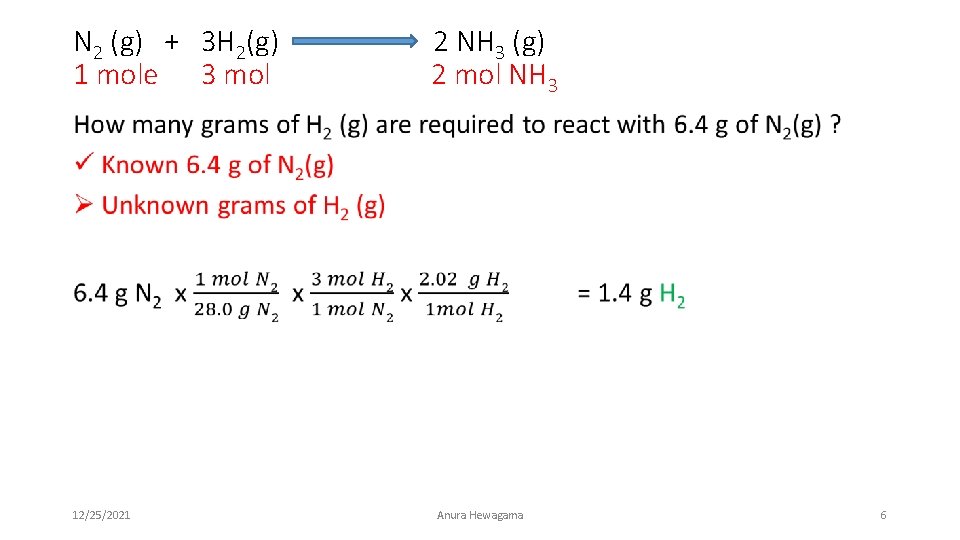
Review – Types of stoichiometric conversions N 2 (g) + 3 H 2(g) 2 NH 3 (g) 1 mole 3 mol 2 mol NH 3 Identify the type of conversion Mole →Mole Mass → Mass Q 1. How many moles of hydrogen gas are required to react with 5 moles of nitrogen gas ? Q 2. How many grams of hydrogen gas are required to react with 5 grams of nitrogen gas ? 12/25/2021 Anura Hewagama 3

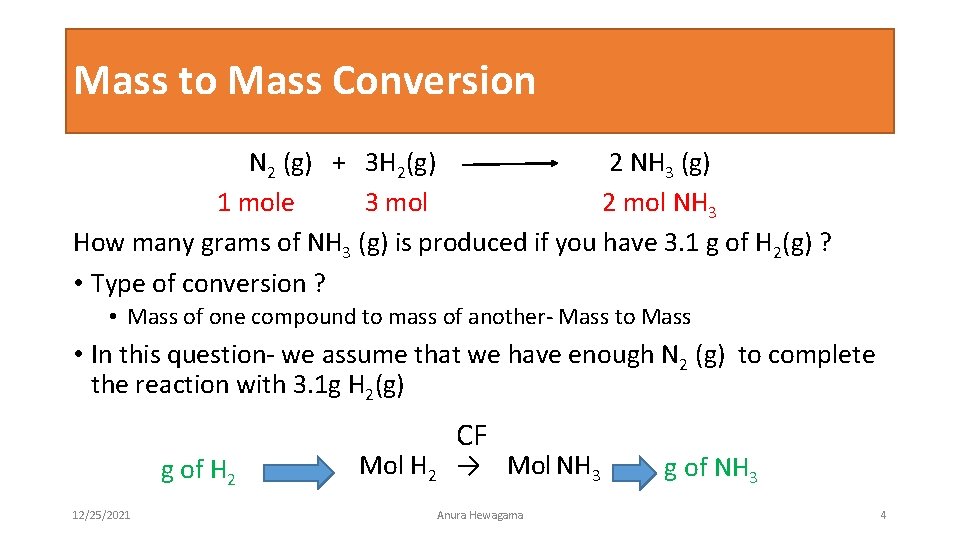
Mass to Mass Conversion N 2 (g) + 3 H 2(g) 2 NH 3 (g) 1 mole 3 mol 2 mol NH 3 How many grams of NH 3 (g) is produced if you have 3. 1 g of H 2(g) ? • Type of conversion ? • Mass of one compound to mass of another- Mass to Mass • In this question- we assume that we have enough N 2 (g) to complete the reaction with 3. 1 g H 2(g) g of H 2 12/25/2021 CF Mol H 2 → Mol NH 3 Anura Hewagama g of NH 3 4

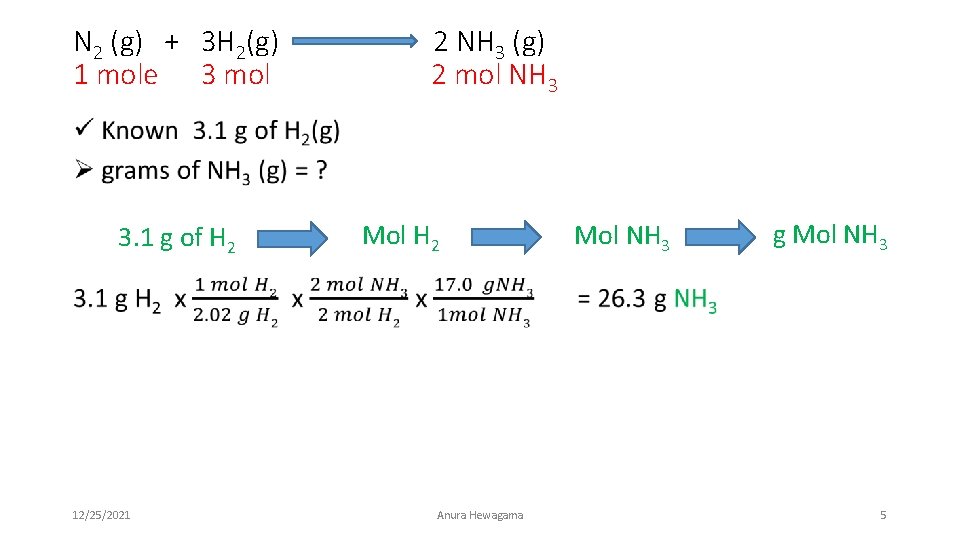
N 2 (g) + 3 H 2(g) 1 mole 3 mol 2 NH 3 (g) 2 mol NH 3 • 3. 1 g of H 2 12/25/2021 Mol H 2 Anura Hewagama Mol NH 3 g Mol NH 3 5

N • 2 (g) + 3 H 2(g) 1 mole 3 mol 12/25/2021 2 NH 3 (g) 2 mol NH 3 Anura Hewagama 6

Limiting Reactants In previous calculations we make several assumptions. v Reaction occurs to 100% efficiency (assume everything goes perfect) and therefore expect the maximum product is formed. (Theoretical Yield) v We have enough of the second reactant v There are no side reactions or unexpected occurrences v. In practice it is not possible to obtain a 100% perfect reaction. 12/25/2021 Anura Hewagama 7

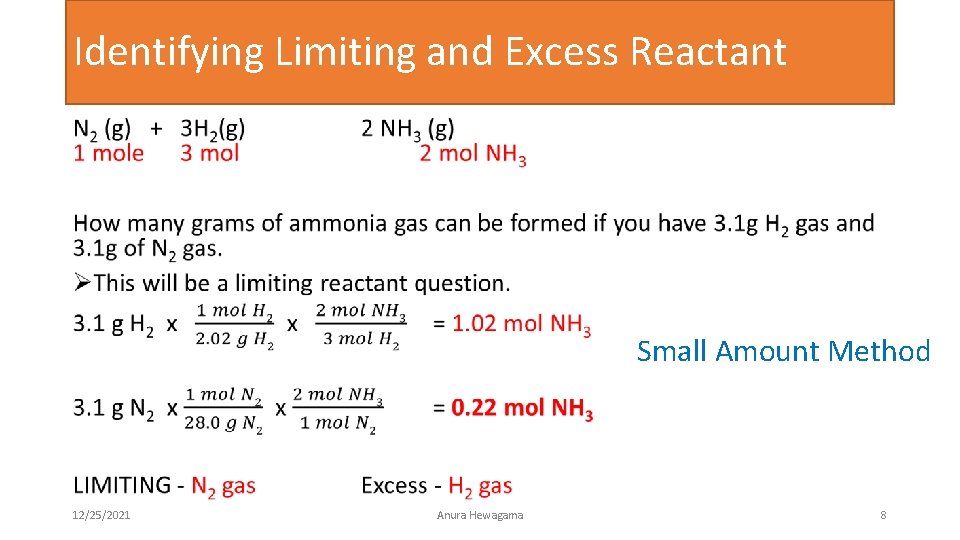
Identifying Limiting and Excess Reactant • Small Amount Method 12/25/2021 Anura Hewagama 8

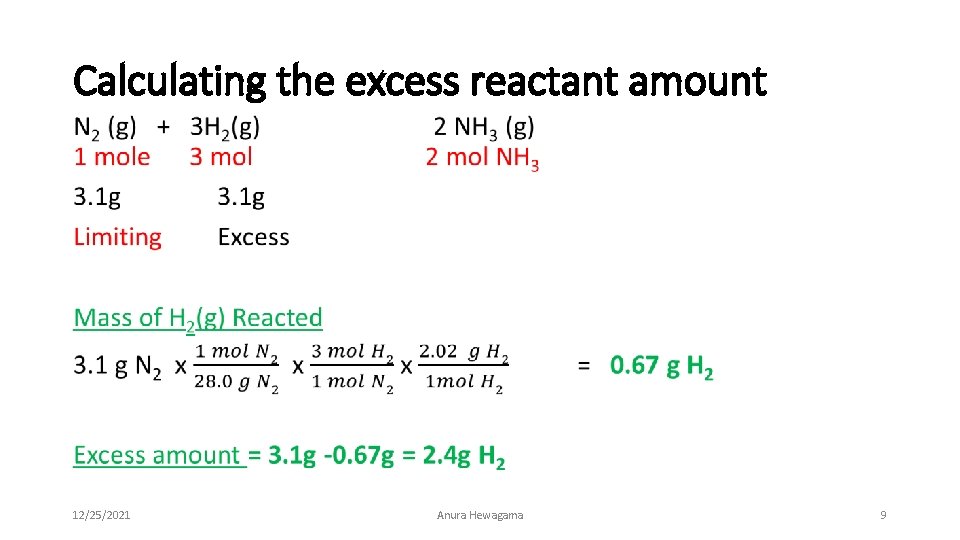
Calculating the excess reactant amount • 12/25/2021 Anura Hewagama 9

Book Chapter Problems Review example problem 11. 5, p 382 Practice Problems 23, 24 – p 383 12/25/2021 Anura Hewagama 10