Limiting Reactants Excess Limiting Reactant Calculations In many



- Slides: 6

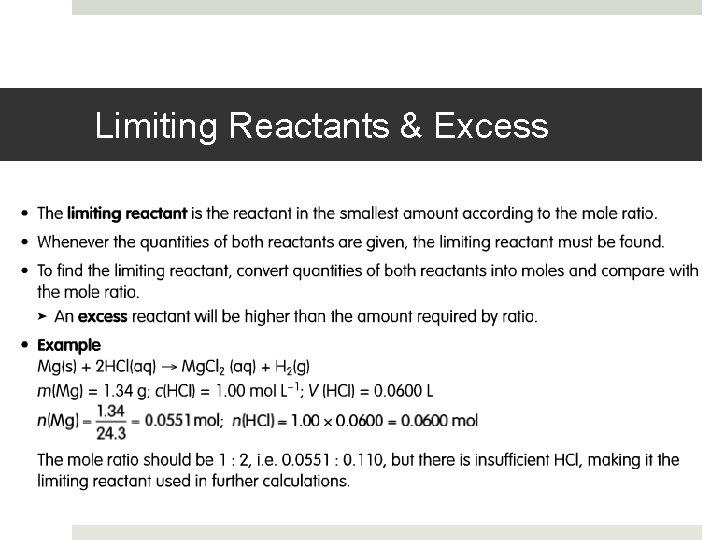
Limiting Reactants & Excess

Limiting Reactant Calculations In many chemical reactions an excess of one reactant is added to ensure complete reaction of another – generally more valuable – reactant. The reaction stops when one reactant is used up (the limiting reagent), even though some of the other substance is unreacted. The other reactant is said to be in excess (the excess reagent) – some of it remains when the reaction has finished In associated problems it is often necessary to determine which reactant is in excess before amounts of product can be determined. Again there are logical calculation techniques, which will lead to efficient solutions of such problems Chemical reactions may be considered to involved three stages: an initial stage where the reactants are added, a reacting stage where the reactants combine in the mole ration suggested by the equation, and a final stage where the reactions appear to be complete

Limiting Reactant Calculations An example A gaseous mixture of 25. 0 g of hydrogen gas and 100. 0 g of oxygen gas are mixed and ignited. The water produced is collected and weighed. What is the expected mass of water produced? Suggested answer 1. Tag the equation with the data supplied and the quantity you have to determine 2 H 2(g) + O 2(g) → 2 H 2 O(g) 25. 0 g 100. 0 g ? g

Limiting Reactant Calculations 2. Calculate the amount of each reactant. n(H 2) = m(H 2)/M(H 2) and = 25. 0 g / 2 x 1. 01 g mol-1 = 12. 4 mol n(O 2) = m(O 2)/M(O 2) = 100. 0 g / 2 x 16. 0 g mol-1 = 3. 13 mol 3. i) Work out which reactant is fully used (the other must be in excess). This requires comparison of initial amounts of reactants with the mole ratios suggested in the equation. 2 H 2(g) + O 2(g) → n(H 2) = 12. 4/2 = 6. 3 mol : n(O 2) = 3. 13 mol Which figure is larger? H 2 So H 2 is in excess and O 2 will be fully used. 2 H 2 O(g)

4. ii) Show that all the O 2 does react and find amounts present at end of reaction Limiting 2 H (g) Reactant + O (g) → Calculations 2 H O(g) 2 2 Initially 12. 4 mol 3. 13 mol Reacting 6. 2 mol 3. 13 mol 2 Present at the end 6. 26 mol H 2 n(H 2) = 12. 4 - 6. 2 mol = 6. 2 mol n(H 2 O) = 6. 26 mol N(O 2) = 0 mol (all has reacted!) 5. Complete the calculations m(H 2 O) = n(H 2 O) x M(H 2 O) = 6. 26 mol x 18. 02 g mol-1 = 113 g Therefore, 113 g of water is produced from this hydrogen & oxygen mixture.

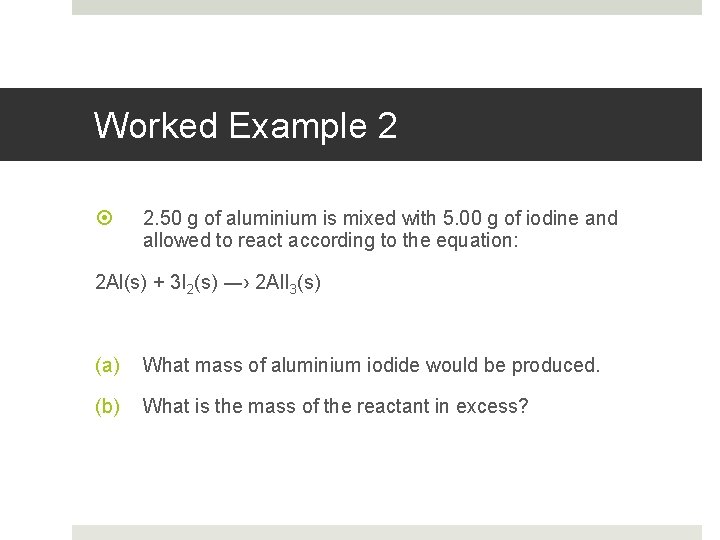
Worked Example 2 2. 50 g of aluminium is mixed with 5. 00 g of iodine and allowed to react according to the equation: 2 Al(s) + 3 I 2(s) ―› 2 Al. I 3(s) (a) What mass of aluminium iodide would be produced. (b) What is the mass of the reactant in excess?