Limiting Reactants and Excess What is the Limiting







- Slides: 12

Limiting Reactants and Excess • What is the Limiting Reagent (Reactant)? It is the substance in a chemical reaction that runs out first. • The limiting reactant (reagent) determines how much product you can make. • If you are given amounts of more than one reactant, determine how much product you can make with each of them. Whichever produces the LEAST amount of product is your limiting reagent.

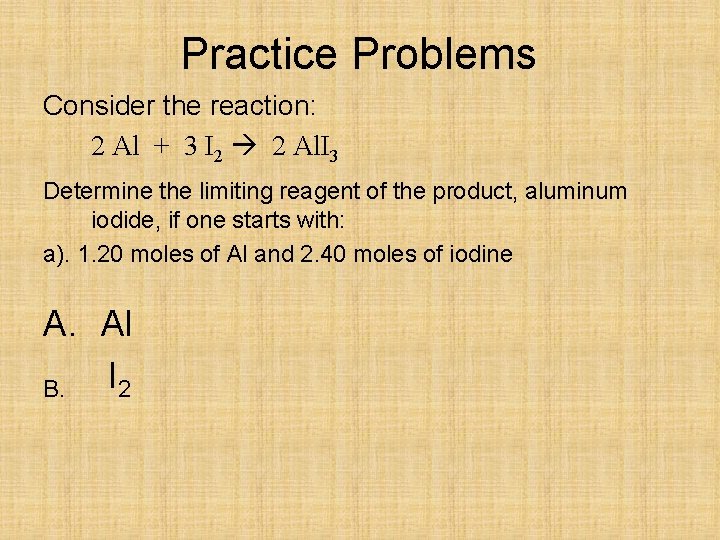
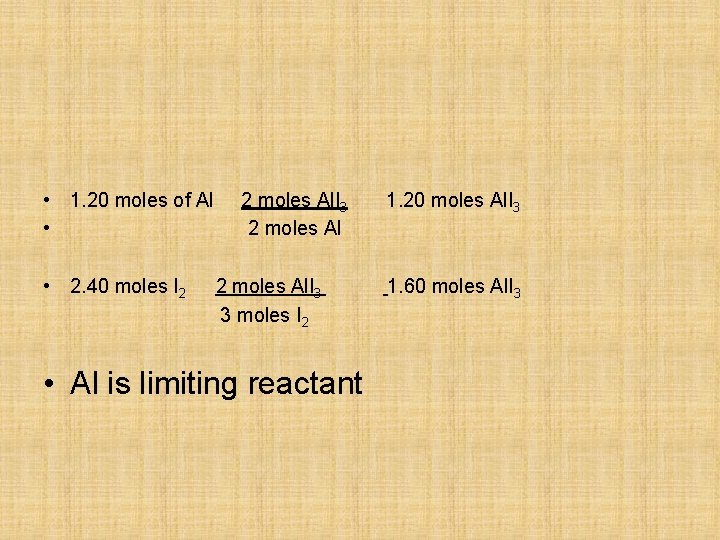
Practice Problems Consider the reaction: 2 Al + 3 I 2 2 Al. I 3 Determine the limiting reagent of the product, aluminum iodide, if one starts with: a). 1. 20 moles of Al and 2. 40 moles of iodine A. Al I 2 B.

• 1. 20 moles of Al • • 2. 40 moles I 2 2 moles Al. I 3 3 moles I 2 • Al is limiting reactant 1. 20 moles Al. I 3 1. 60 moles Al. I 3

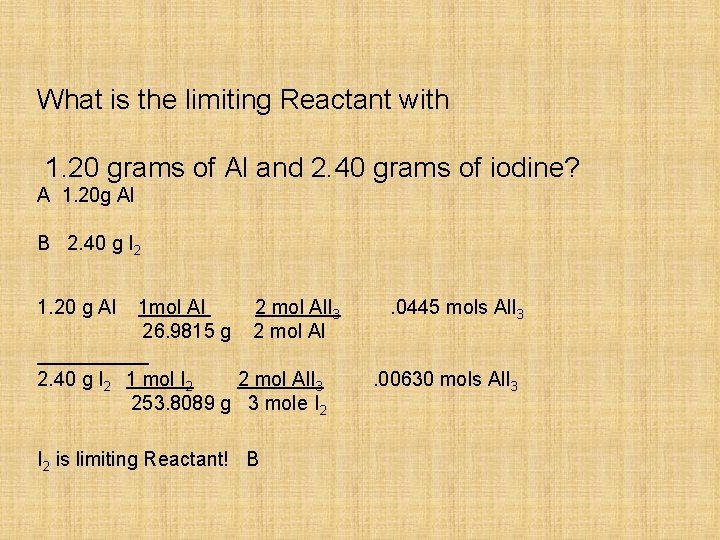
What is the limiting Reactant with 1. 20 grams of Al and 2. 40 grams of iodine? A 1. 20 g Al B 2. 40 g I 2 1. 20 g Al 1 mol Al 26. 9815 g 2 mol Al. I 3 2 mol Al 2. 40 g I 2 1 mol I 2 2 mol Al. I 3 253. 8089 g 3 mole I 2 is limiting Reactant! B . 0445 mols Al. I 3. 00630 mols Al. I 3

How many grams of excess reactant will remain? 2. 40 g I 2 1 mol I 2 2 mols Al 26. 9815 g 253. 8089 g 3 mole I 2 1 mol Al. 0. 170 g of Al reacted Started with 1. 20 g -0. 170 g reacted 1. 03 g in excess

15. 00 g aluminum sulfide and 12. 00 g of water react until the limiting reagent is used up. Here is the balanced equation for the reaction. Al 2 S 3 + 6 H 2 O 2 Al(OH)3 + 3 H 2 S a. ) What is the maximum mass of H 2 S which can be formed from these reagents ? A. 10. 21 g B. 11. 35 g 15. 00 g Al 2 S 3 1 mol Al 2 S 3 150. 16167 g 10. 21 g H 2 S 3 mols H 2 S 1 mol Al 2 S 3 34. 08 g 1 mol H 2 S 12. 00 g H 2 O 3 mols H 2 S 6 mol H 2 O 34. 08 g 1 mol H 2 S 11. 35 g H 2 S A. 10. 21 g 1 mol H 2 O 18. 008 g

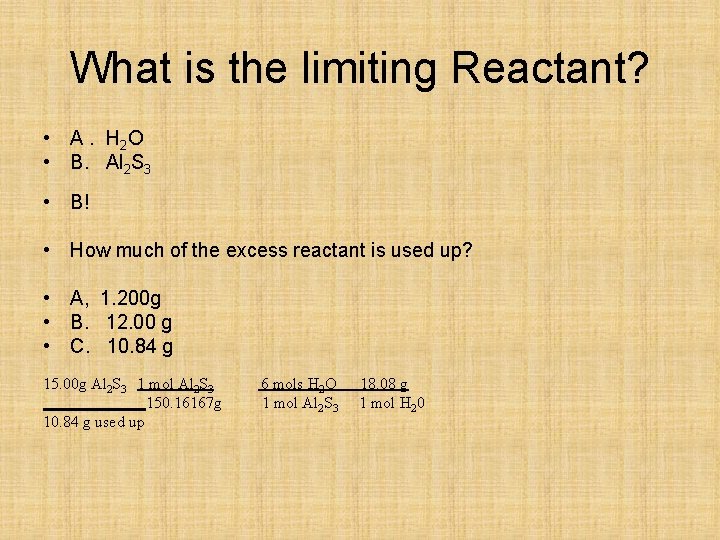
What is the limiting Reactant? • A. H 2 O • B. Al 2 S 3 • B! • How much of the excess reactant is used up? • A, 1. 200 g • B. 12. 00 g • C. 10. 84 g 15. 00 g Al 2 S 3 1 mol Al 2 S 3 150. 16167 g 10. 84 g used up 6 mols H 2 O 1 mol Al 2 S 3 18. 08 g 1 mol H 20

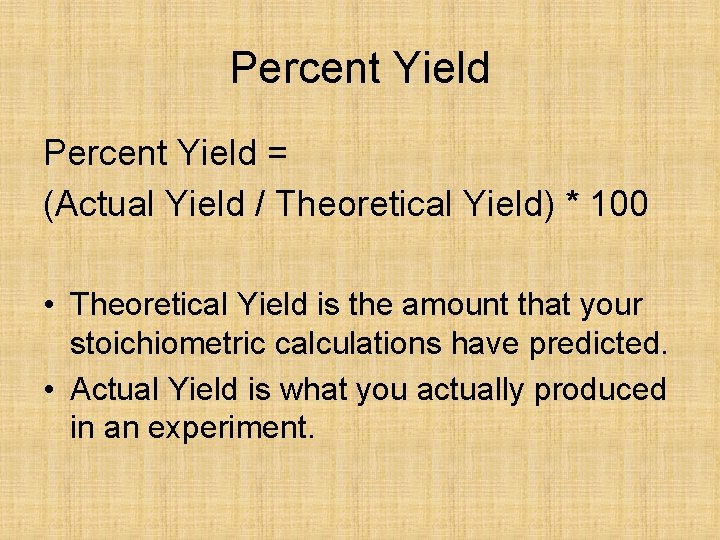
Percent Yield = (Actual Yield / Theoretical Yield) * 100 • Theoretical Yield is the amount that your stoichiometric calculations have predicted. • Actual Yield is what you actually produced in an experiment.

Example: In the reaction between nitrogen gas and hydrogen gas producing ammonia: a. ) If you start with 14 grams of N 2 and 6. 0 grams of H 2, what will be the limiting reagent and theoretical yield? b. ) If 10. grams of the product was formed, what would be the percent yield?

Stoichiometry with Solutions • Just like other problems but use Moles = Molarity X Liters to get moles, or Liters = Moles/Molarity to find the volume of a solution needed in a reaction.

How many grams of aluminum will be required to completely replace copper from a 208 m. L of a 0. 100 M solution of copper(II) chloride? 2 Al + 3 Cu. Cl 2 2 Al. Cl 3 + 3 Cu Answer = 0. 374 g Al

How many milliliters of a 3. 40 M Cu. SO 4 solution will be needed to react with 510. 0 g of Ag. Cl in the following reaction? Ag. Cl + Cu. SO 4 Ag 2 SO 4 + Cu. Cl 2 Answer = 523 m. L