Warm Up 1 2 3 4 5 What






























- Slides: 56


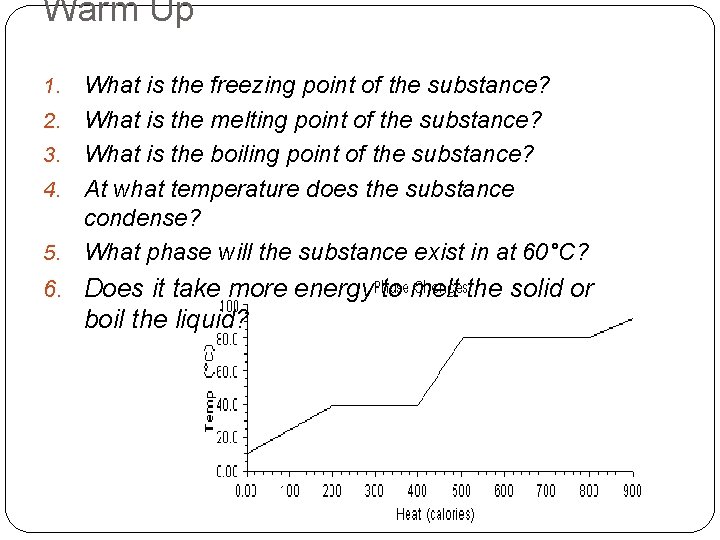
Warm Up 1. 2. 3. 4. 5. What is the freezing point of the substance? What is the melting point of the substance? What is the boiling point of the substance? At what temperature does the substance condense? What phase will the substance exist in at 60°C? 6. Does it take more energy to melt the solid or boil the liquid?

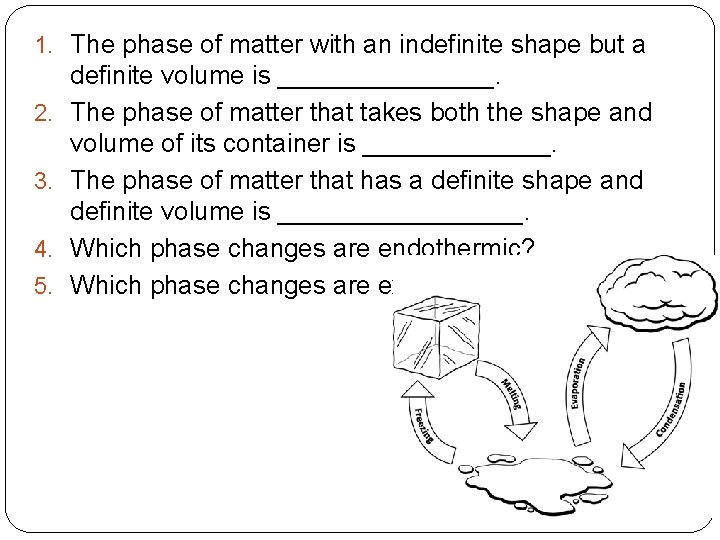
1. The phase of matter with an indefinite shape but a 2. 3. 4. 5. definite volume is ________. The phase of matter that takes both the shape and volume of its container is _______. The phase of matter that has a definite shape and definite volume is _________. Which phase changes are endothermic? Which phase changes are exothermic?

�If you were asked to describe an orange to someone who had never seen an orange, what would you tell the person? �Come up with 5 adjectives or descriptions you would give

Remember… �Matter: has mass and takes up space

Physical Properties �Physical property: can be observed without altering the chemical identity of a substance

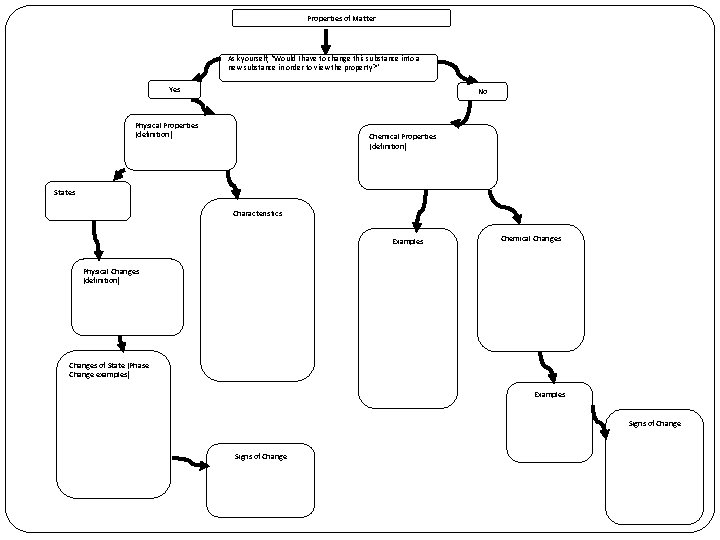
Properties of Matter Ask yourself, “Would I have to change this substance into a new substance in order to view the property? ” Yes No Physical Properties (definition) Chemical Properties (definition) States Characteristics Examples Chemical Changes Physical Changes (definition) Changes of State (Phase Change examples) Examples Signs of Change

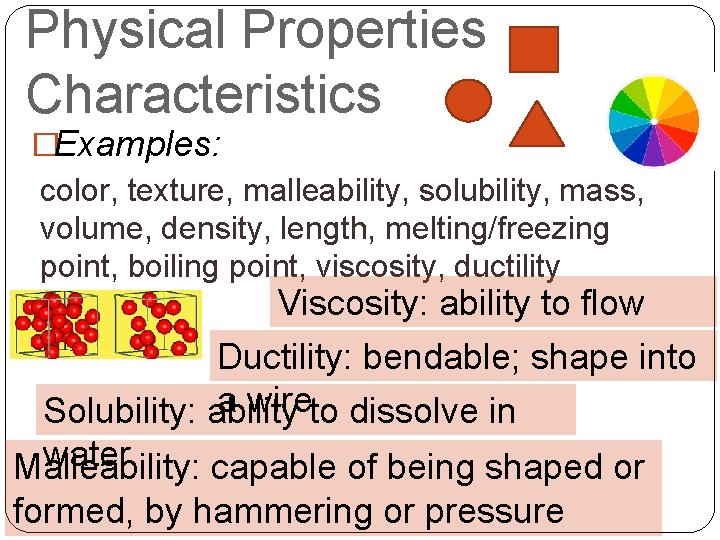
Physical Properties Characteristics �Examples: color, texture, malleability, solubility, mass, volume, density, length, melting/freezing point, boiling point, viscosity, ductility Viscosity: ability to flow Ductility: bendable; shape into a wire Solubility: ability to dissolve in water Malleability: capable of being shaped or formed, by hammering or pressure

Chemical Properties �Chemical property: describes the ability of a substance to change into one or more new chemical substances. �Can only be observed by changing the chemical identity of a substance

Chemical Properties �Examples: Toxicity, reactivity, flammability, combustibility, radioactivity, rotting, tarnishing, acidity, alkalinity

Chemical vs. Physical Properties �Ask yourself, “Would I have to change this substance into a new substance in order to view the property? ”

#1 �The boiling point of a certain alcohol is 75 degrees Celsius Physical

#2 �Copper forms green copper carbonate when in contact with moist air Chemical

#3 � Table salt dissolves in water Physical

#4 �Copper is a good conductor of heat and electricity Physical

#5 �Magnesium burns brightly when ignited Chemical

#6 �Iron is more dense than aluminum Physical

Physical vs. Chemical Changes

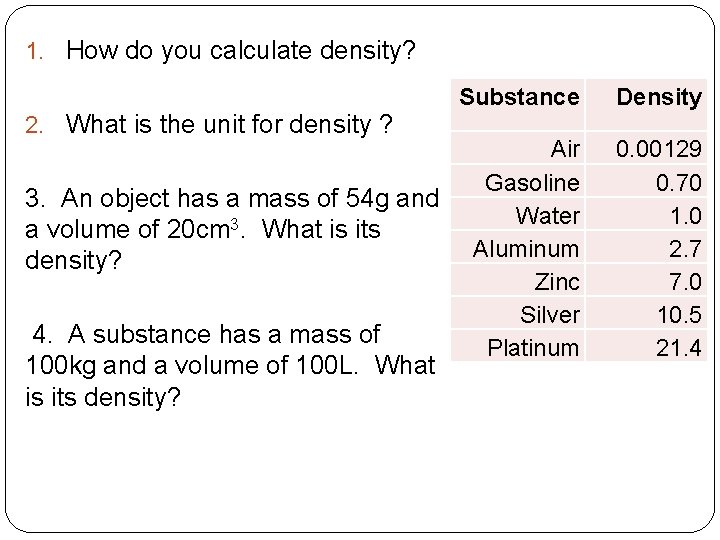
1. How do you calculate density? 2. What is the unit for density ? 3. An object has a mass of 54 g and a volume of 20 cm 3. What is its density? 4. A substance has a mass of 100 kg and a volume of 100 L. What is its density? Substance Density Air Gasoline Water Aluminum Zinc Silver Platinum 0. 00129 0. 70 1. 0 2. 7 7. 0 10. 5 21. 4

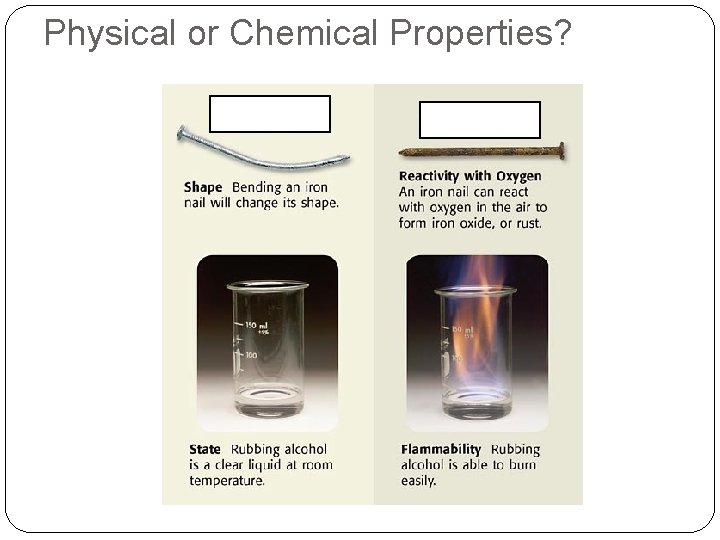
Physical or Chemical Properties?

1. Everything is made of 2. 3. 4. 5. 6. __________________. Chemicals can react to form new _____________. There is____ times as much hydrogen as there is oxygen in water. When you mix vinegar and baking soda it produces _________ gas. A pyrotechnic is the name of a person who uses or manufactures __________. Potassium can be found in ____________.

What’s the Difference? � Matter can change � Examples: Freeze, cut, burn, etc. � 2 types of changes 1. Physical changes 2. Chemical changes

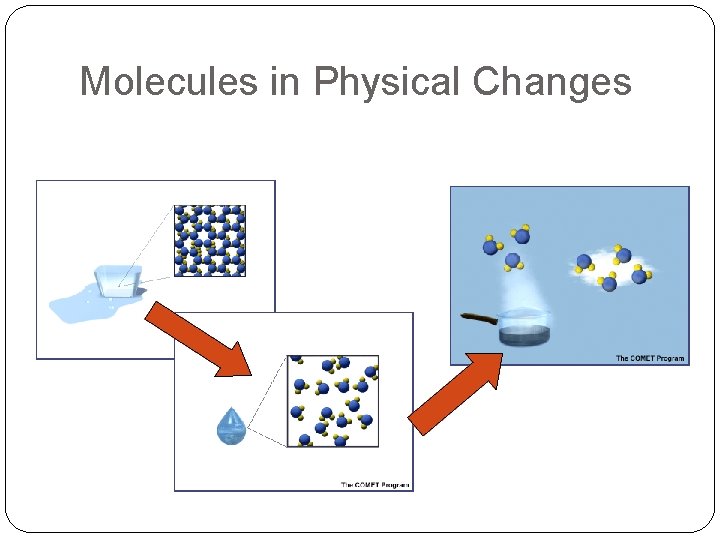
Physical Changes �Matter changes form WITHOUT becoming something new �You start and end with same thing (see below) �Examples: cutting, phase changes, etc.

Molecules in Physical Changes

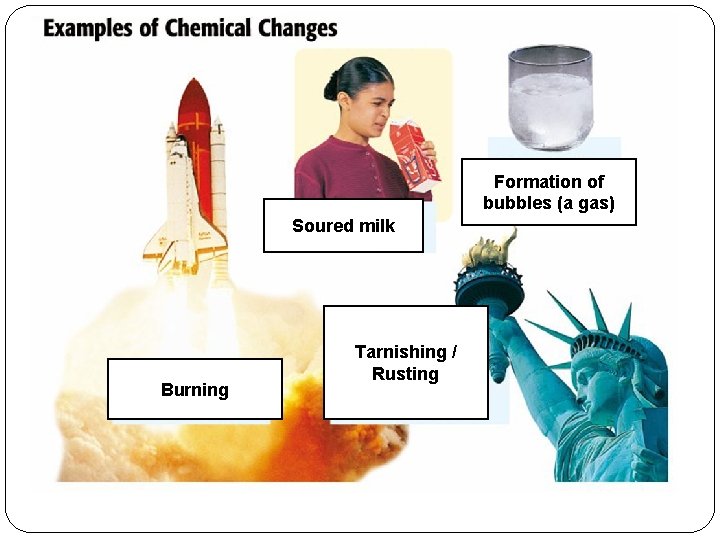
Chemical Changes �Identity of the matter changes, becomes NEW substance �Start and end with different things �Usually irreversible (can’t change back) �Examples: burning, rusting, milk going sour

Formation of bubbles (a gas) Soured milk Burning Tarnishing / Rusting

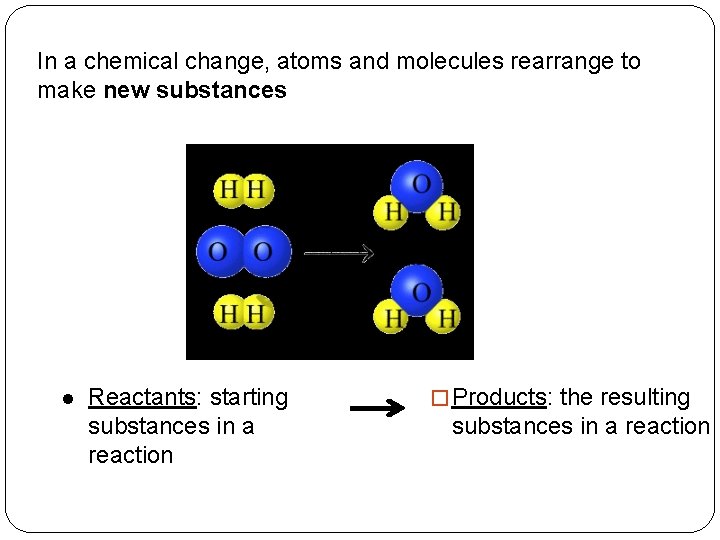
In a chemical change, atoms and molecules rearrange to make new substances l Reactants: starting substances in a reaction � Products: the resulting substances in a reaction

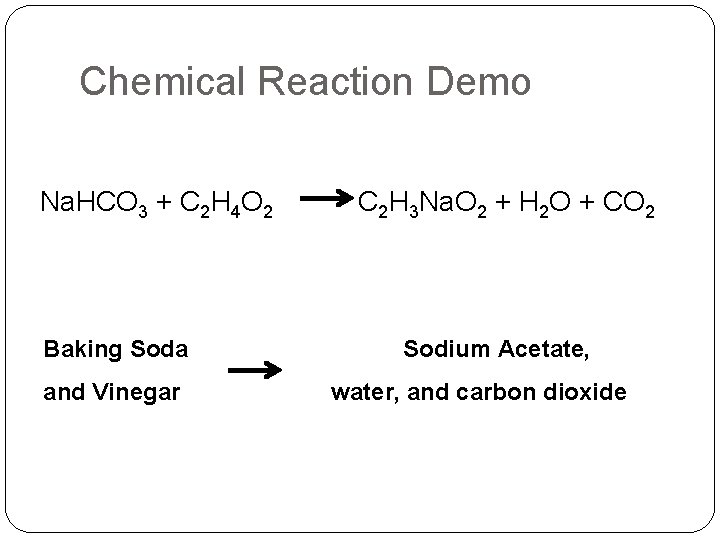
Chemical Reaction Demo Na. HCO 3 + C 2 H 4 O 2 Baking Soda and Vinegar C 2 H 3 Na. O 2 + H 2 O + CO 2 Sodium Acetate, water, and carbon dioxide

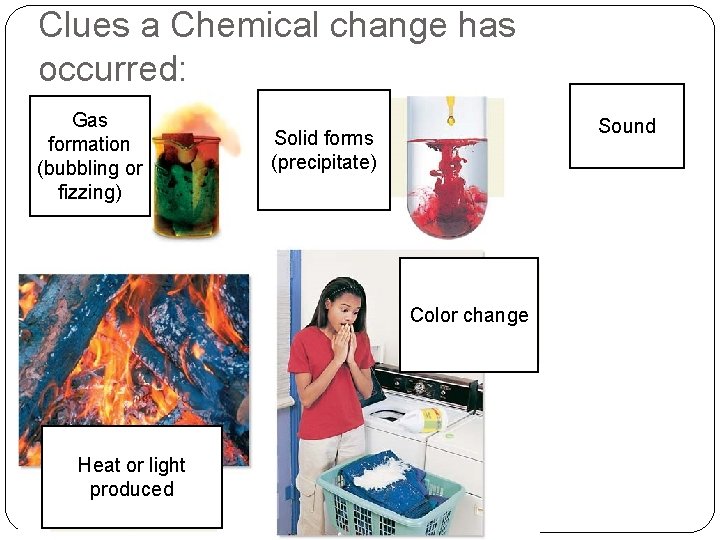
Signs of Change �Physical ◦ Size ◦ Shape �Chemical �Temperature change �Color change �Bubbles/fizzing- a gas is being formed �Smoke �Smell �Light produced �Sound �Precipitate formed- solid particles formed when two liquids are mixed

Clues a Chemical change has occurred: Gas formation (bubbling or fizzing) Sound Solid forms (precipitate) Color change Heat or light produced

Is it a chemical or physical change? �Sugar dissolving in tea • Physical Change

Is it a chemical or physical change? �Logs burning • Chemical Change

Is it a chemical or physical change? �Breaking water up by separating it into hydrogen and oxygen • Chemical Change

Is it a chemical or physical change? �Cutting paper • Physical Change

Is it a chemical or physical change? �Crushing an aspirin • Physical Change

Is it a chemical or physical change? �Metal rusting • Chemical Change

Is it a chemical or physical change? �An egg rotting • Chemical Change

Is it a chemical or physical change? �Melting ice • Physical Change

Is it a chemical or physical change? �An egg breaking • Physical Change

Is it a chemical or physical change? �A candle burning • Chemical Change

Is it a chemical or physical change? �Butter melting • Physical Change

Is it a chemical or physical change? �Water boils • Physical Change

Is it a chemical or physical change? �Baking bread • Chemical change

Is it a chemical or physical change? �Burning a match • Chemical Change

Is it a chemical or physical change? �Pancakes cooking • Chemical Change

Acids & Bases

What are Acids? �Acids are common �Some are dangerous and can burn your skin �Some are safe to eat and drink �Stomach acid helps digest food �Feel “squeaky” explosion

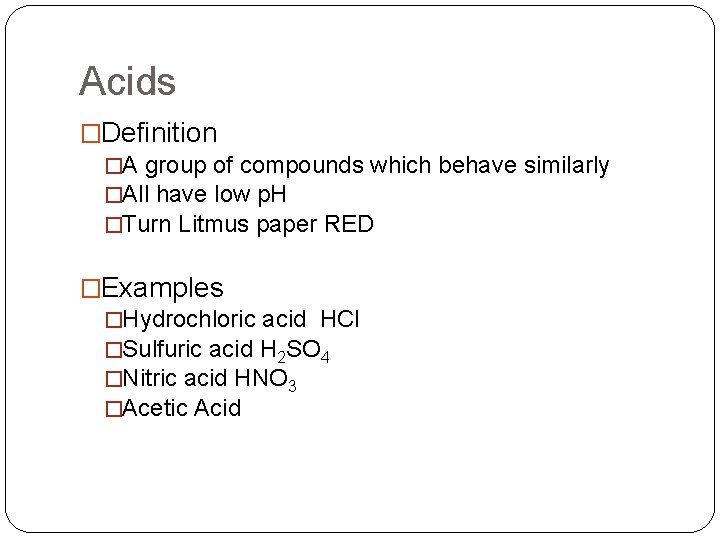
Acids �Definition �A group of compounds which behave similarly �All have low p. H �Turn Litmus paper RED �Examples �Hydrochloric acid HCl �Sulfuric acid H 2 SO 4 �Nitric acid HNO 3 �Acetic Acid

What are Bases (Alkalis)? �In our home we often use bases to clean things. Eg Bleach and toothpaste �Feel “slippery” �Some things are not acids or bases, we say that they are neutral. Eg Water

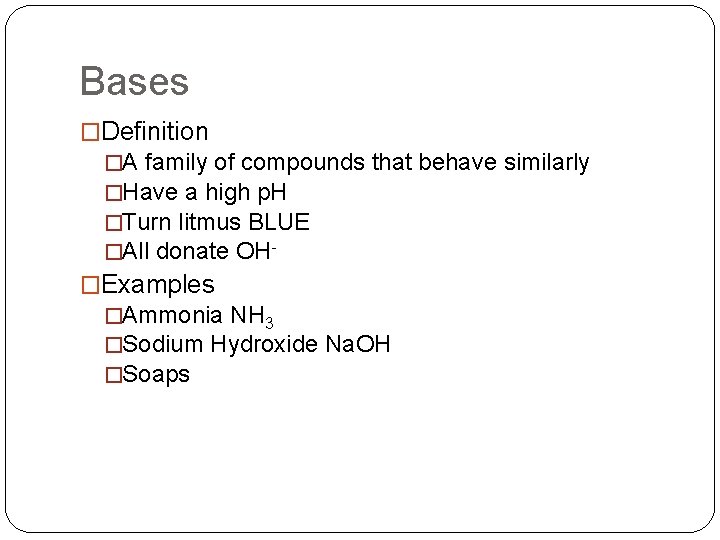
Bases �Definition �A family of compounds that behave similarly �Have a high p. H �Turn litmus BLUE �All donate OH- �Examples �Ammonia NH 3 �Sodium Hydroxide Na. OH �Soaps

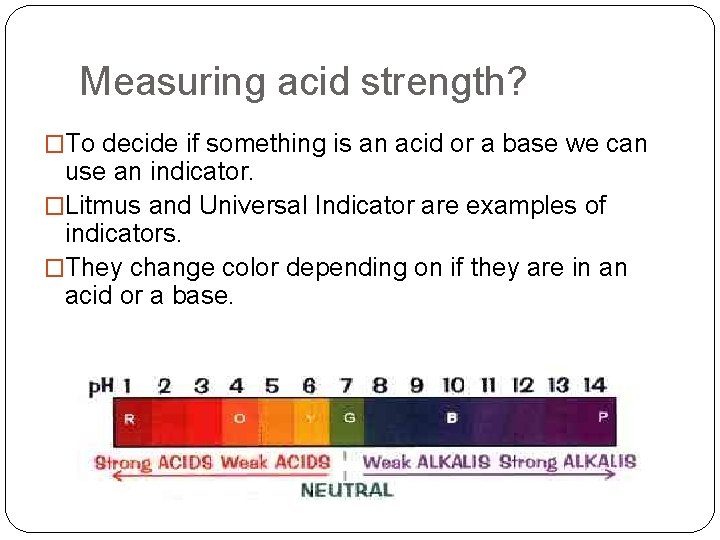
Measuring acid strength? �To decide if something is an acid or a base we can use an indicator. �Litmus and Universal Indicator are examples of indicators. �They change color depending on if they are in an acid or a base.

Working with Indicators �Red litmus turns BLUE in the presence of Bases �Blue litmus turns RED in the presence of acid �Acids and bases react together in a NEUTRALISATION reaction

Properties and Changes Stations �At each station you will complete practice for each of the concepts we learned. When you show me your work you can progress to the next station. You must get through each station before the end of the class period at least once.

Stations �Phase Change Diagrams �Density �Physical and Chemical Properties �Physical and Chemical Changes �Acids and Bases

Acids & Bases In your INB draw a T chart. Label one side acids and the other side bases. Based on the properties of the items & what you already know about acids and bases, classify the each as an acid or a base. Glue the acids on one side of a paper and the bases on the other. Explain why you classified each object as an acid or base.

Acids and Bases round 2 �http: //amrita. olabs. co. in/? sub=73&brch=3&sim=6 &cnt=25 �Complete the lab