Chemical reactions References Chemical equations Define a chemical

















- Slides: 51

Chemical reactions References:

Chemical equations • Define a chemical reaction • Chemical equation describes the reaction: reactants -------> products

Parts of a chemical reaction: Reactants - • Substances that interact with each other. • On the left side of the arrow in a chemical equation. Products - • New substances formed during a reaction. • On the right side of the arrow in at a chemical equation.

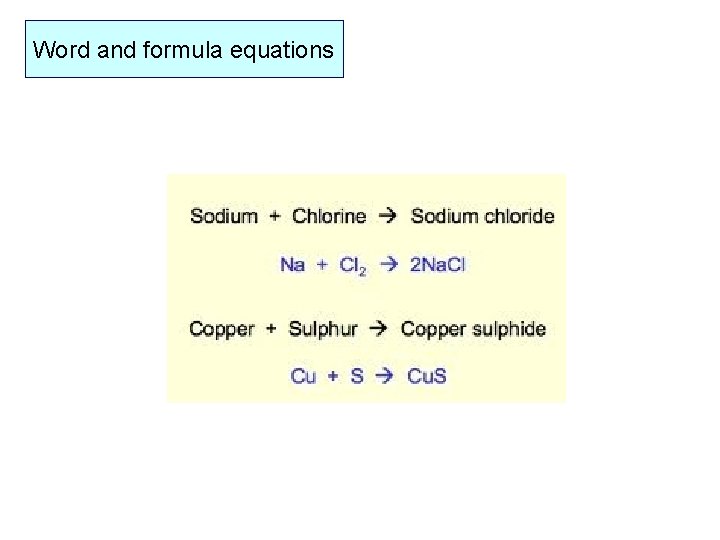
Word and formula equations

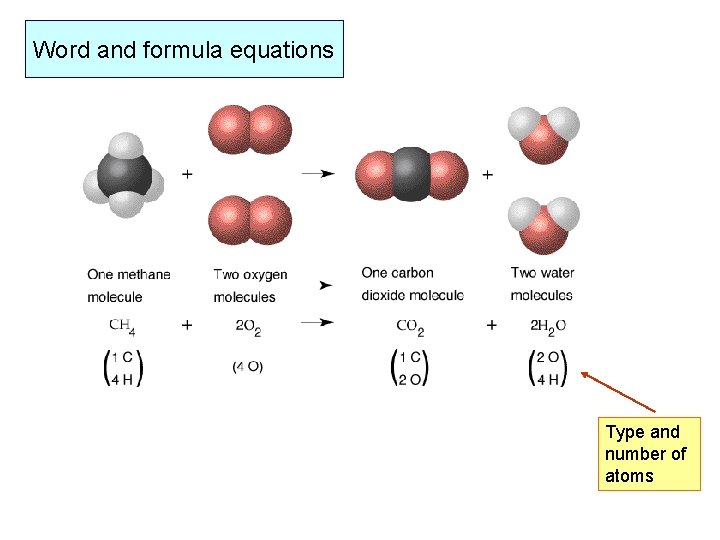
Word and formula equations Type and number of atoms

Steps involved in writing a 'balanced' equation for a chemical reaction: 1. Experimentally determine reactants and products: “Hydrogen reacts with oxygen to make water”

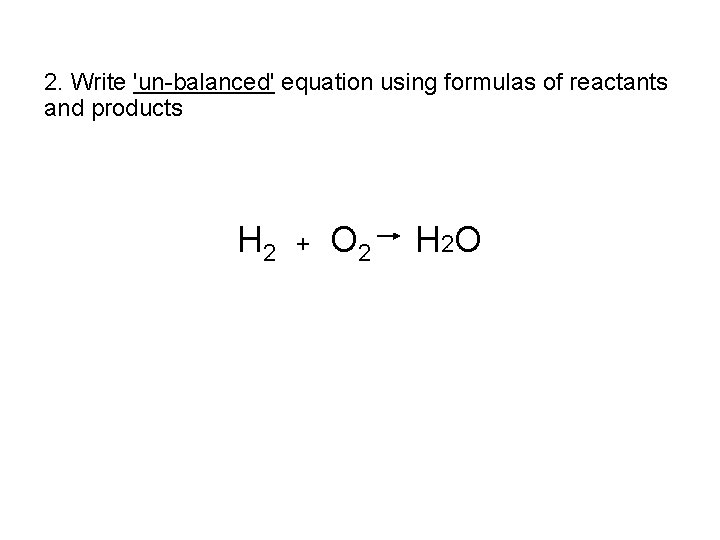
2. Write 'un-balanced' equation using formulas of reactants and products H 2 + O 2 H 2 O

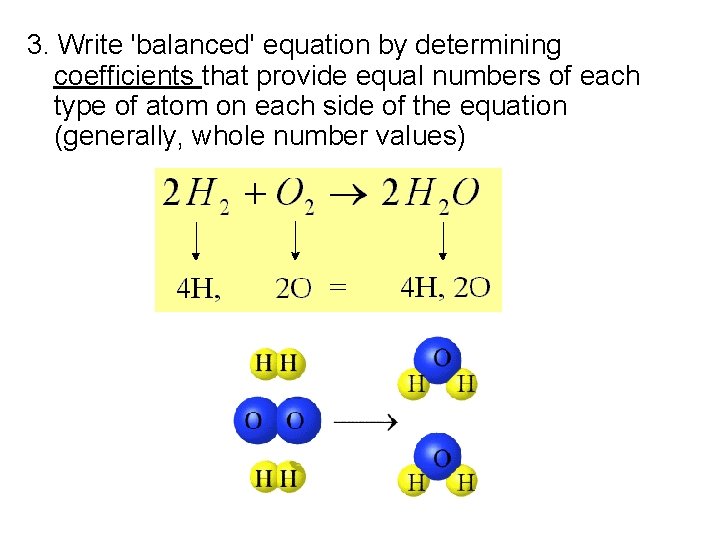
3. Write 'balanced' equation by determining coefficients that provide equal numbers of each type of atom on each side of the equation (generally, whole number values)

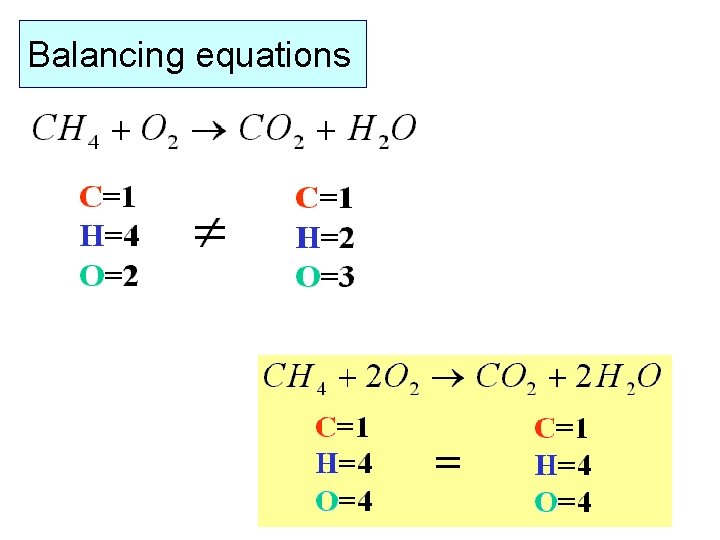
Balancing equations

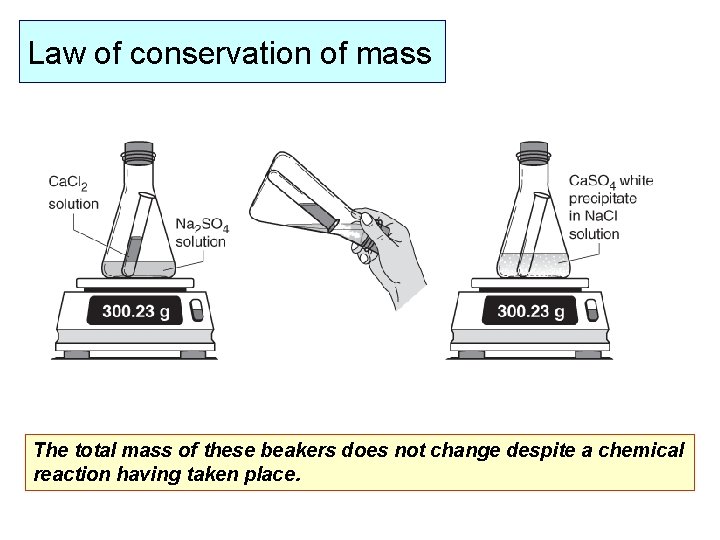
Law of conservation of mass The law

Law of conservation of mass The total mass of these beakers does not change despite a chemical reaction having taken place.

Antoine Lavoisier is also known as “The Father of Modern Chemistry”. He was a French nobleman prominent in the histories of chemistry and biology. He stated the first version of the Law of Conversion of mass, recognized and named oxygen in 1778 and also recognized and named hydrogen in 1783.

Skill builder p. 145 STM assignment p. 146 – make a ppt from 3 slides and present it

Pracs p. 148

Types of reactions

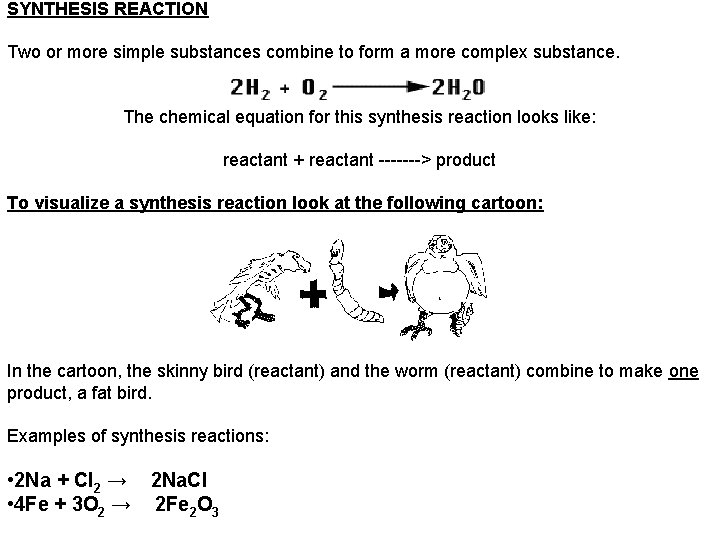
SYNTHESIS REACTION Two or more simple substances combine to form a more complex substance. The chemical equation for this synthesis reaction looks like: reactant + reactant -------> product To visualize a synthesis reaction look at the following cartoon: In the cartoon, the skinny bird (reactant) and the worm (reactant) combine to make one product, a fat bird. Examples of synthesis reactions: • 2 Na + Cl 2 → 2 Na. Cl • 4 Fe + 3 O 2 → 2 Fe 2 O 3

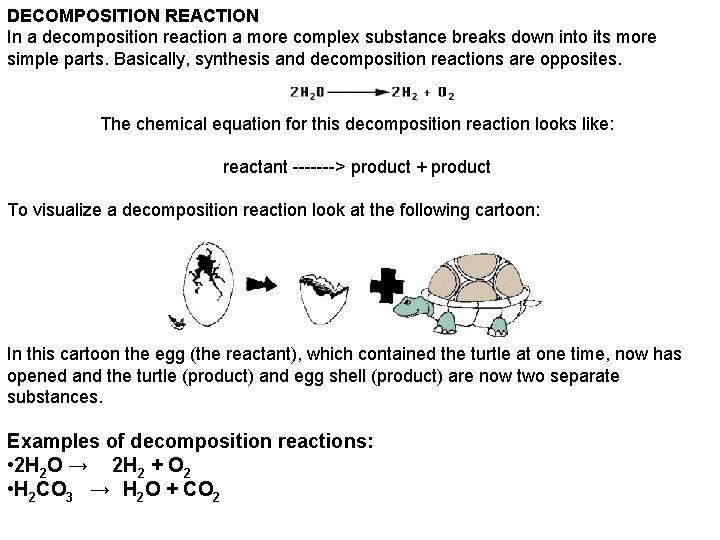
DECOMPOSITION REACTION In a decomposition reaction a more complex substance breaks down into its more simple parts. Basically, synthesis and decomposition reactions are opposites. The chemical equation for this decomposition reaction looks like: reactant -------> product + product To visualize a decomposition reaction look at the following cartoon: In this cartoon the egg (the reactant), which contained the turtle at one time, now has opened and the turtle (product) and egg shell (product) are now two separate substances. Examples of decomposition reactions: • 2 H 2 O → 2 H 2 + O 2 • H 2 CO 3 → H 2 O + CO 2

Raisin lava lamp • p. 150

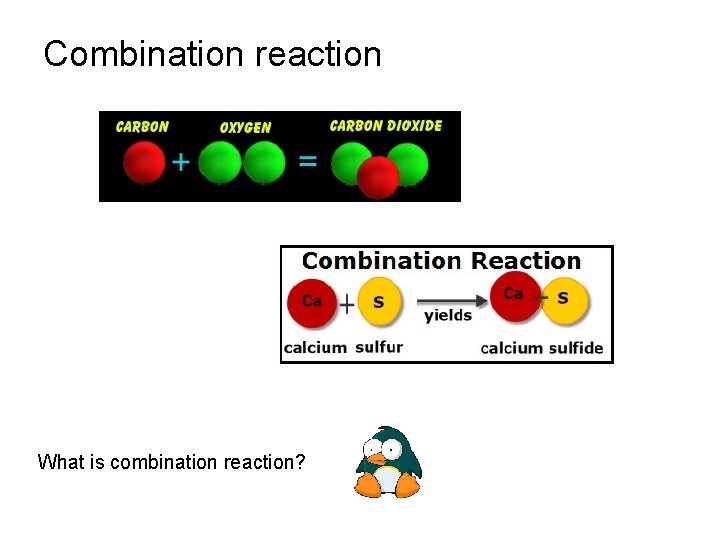
Combination reaction What is combination reaction?

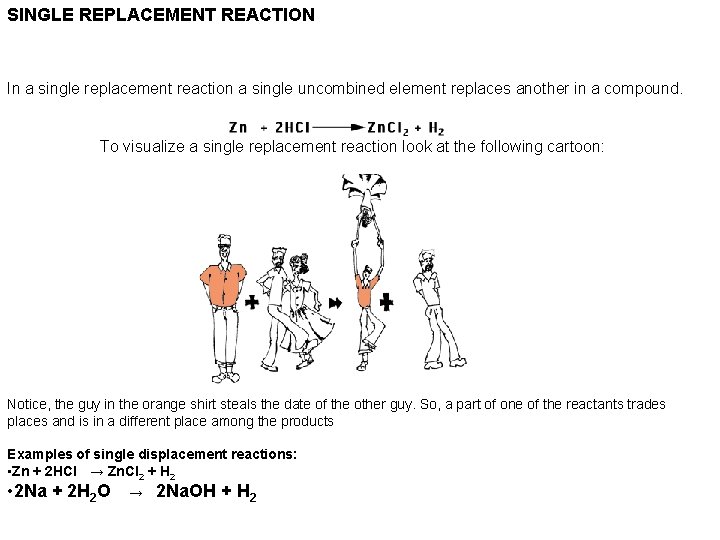
SINGLE REPLACEMENT REACTION In a single replacement reaction a single uncombined element replaces another in a compound. To visualize a single replacement reaction look at the following cartoon: Notice, the guy in the orange shirt steals the date of the other guy. So, a part of one of the reactants trades places and is in a different place among the products Examples of single displacement reactions: • Zn + 2 HCl → Zn. Cl 2 + H 2 • 2 Na + 2 H 2 O → 2 Na. OH + H 2

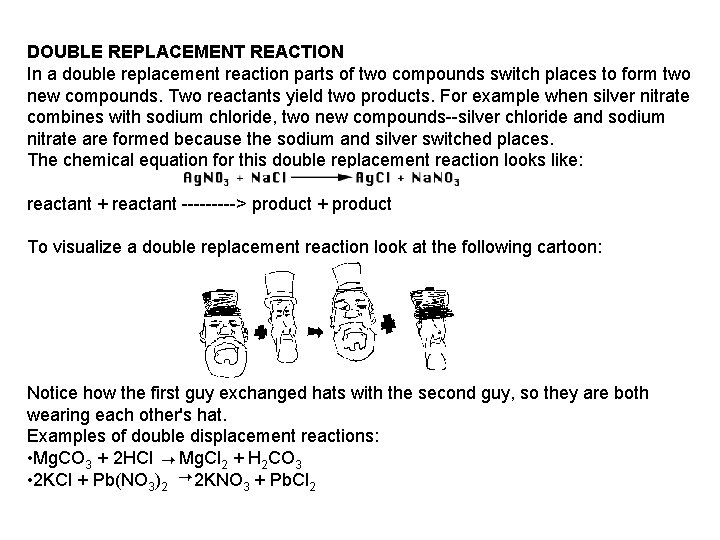
DOUBLE REPLACEMENT REACTION In a double replacement reaction parts of two compounds switch places to form two new compounds. Two reactants yield two products. For example when silver nitrate combines with sodium chloride, two new compounds--silver chloride and sodium nitrate are formed because the sodium and silver switched places. The chemical equation for this double replacement reaction looks like: reactant + reactant -----> product + product To visualize a double replacement reaction look at the following cartoon: Notice how the first guy exchanged hats with the second guy, so they are both wearing each other's hat. Examples of double displacement reactions: • Mg. CO 3 + 2 HCl Mg. Cl 2 + H 2 CO 3 • 2 KCl + Pb(NO 3)2 2 KNO 3 + Pb. Cl 2

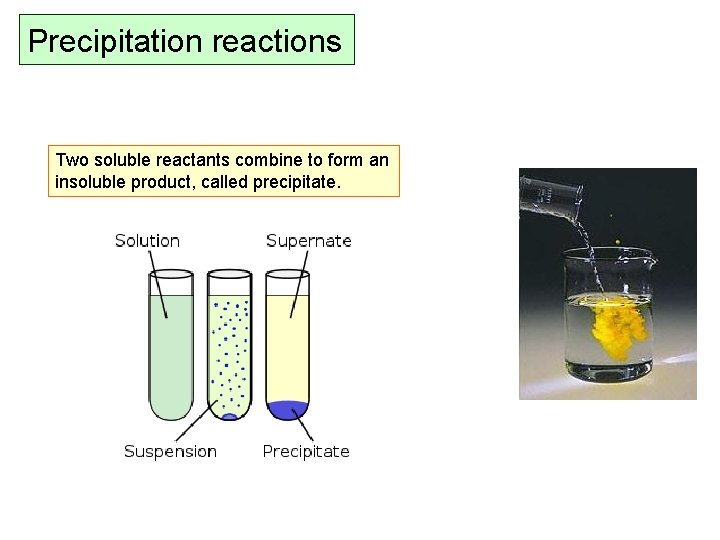
Precipitation reactions Two soluble reactants combine to form an insoluble product, called precipitate.

OPTION: Using text / tables p. 151 -153 make it prac lesson – base on p. 160 prac for summative prac

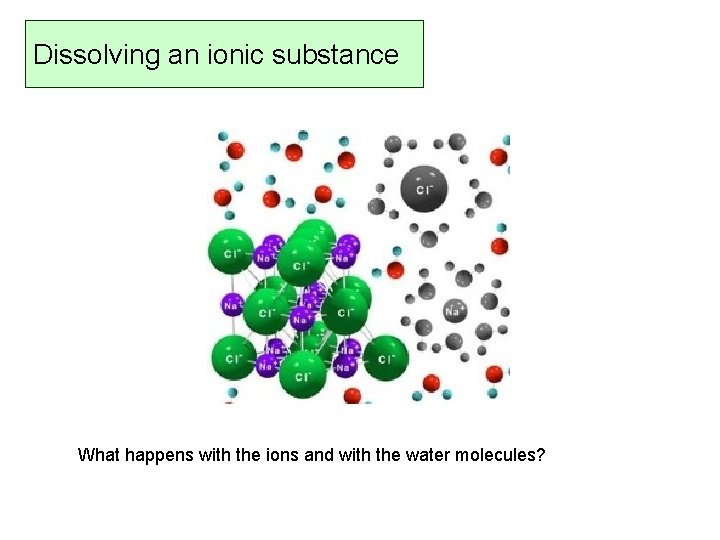
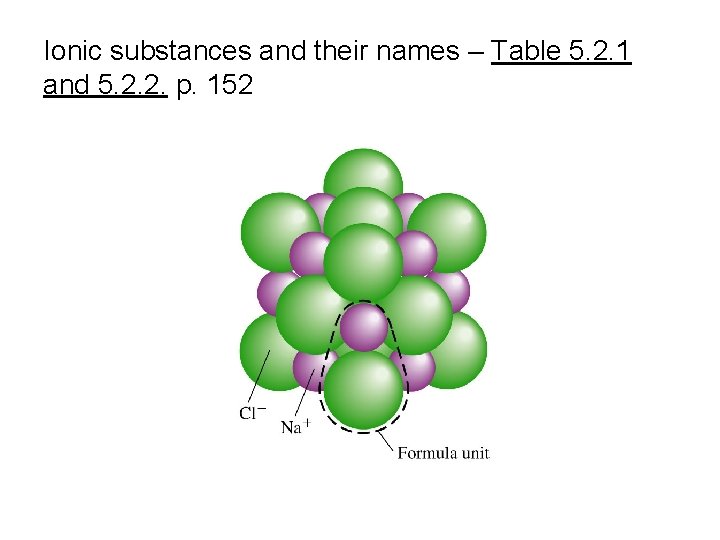
Dissolving an ionic substance What happens with the ions and with the water molecules?

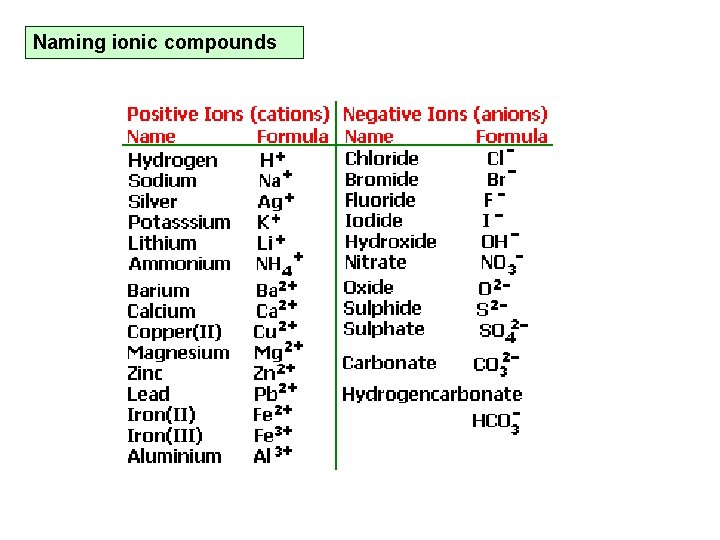
Ionic substances and their names – Table 5. 2. 1 and 5. 2. 2. p. 152

Naming ionic compounds

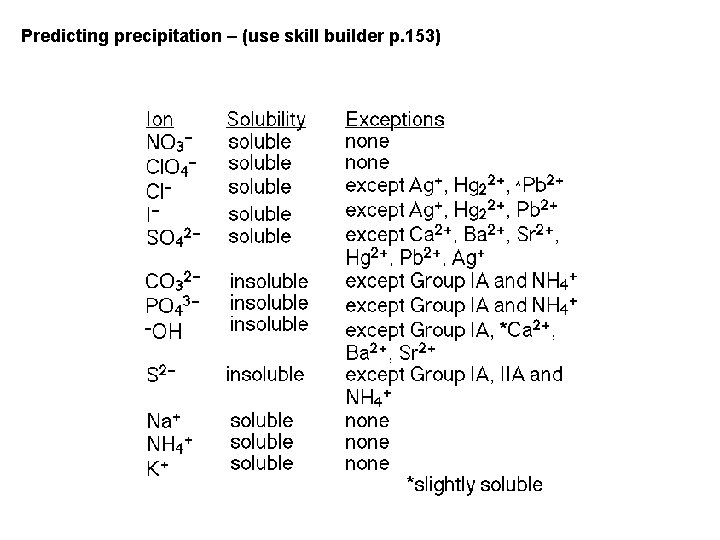
Predicting precipitation – (use skill builder p. 153)

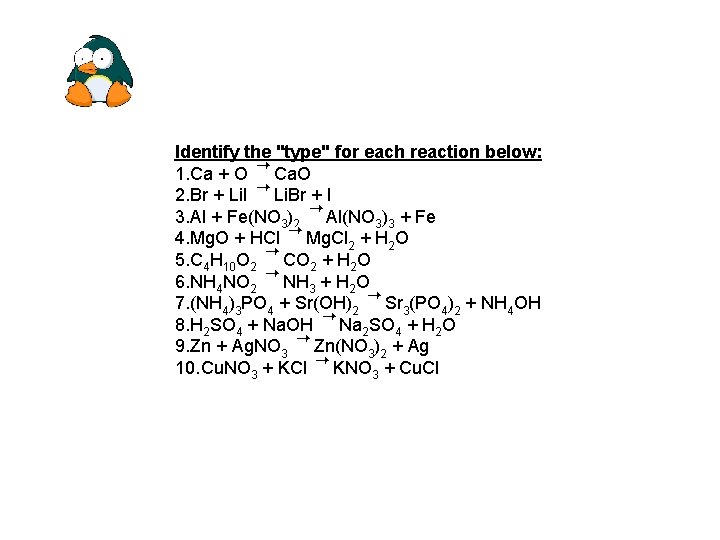
Identify the "type" for each reaction below: 1. Ca + O Ca. O 2. Br + Li. I Li. Br + I 3. Al + Fe(NO 3)2 Al(NO 3)3 + Fe 4. Mg. O + HCl Mg. Cl 2 + H 2 O 5. C 4 H 10 O 2 CO 2 + H 2 O 6. NH 4 NO 2 NH 3 + H 2 O 7. (NH 4)3 PO 4 + Sr(OH)2 Sr 3(PO 4)2 + NH 4 OH 8. H 2 SO 4 + Na. OH Na 2 SO 4 + H 2 O 9. Zn + Ag. NO 3 Zn(NO 3)2 + Ag 10. Cu. NO 3 + KCl KNO 3 + Cu. Cl

Oxidation and reduction reactions

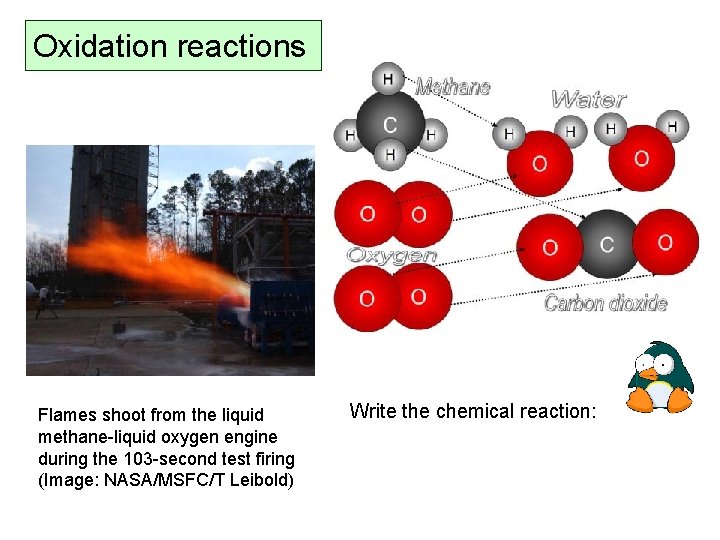
Oxidation reactions Flames shoot from the liquid methane-liquid oxygen engine during the 103 -second test firing (Image: NASA/MSFC/T Leibold) Write the chemical reaction:

Reduction reactions Copper oxide + carbon ? ? ? Write the chemical equation + explain what happens with oxygen

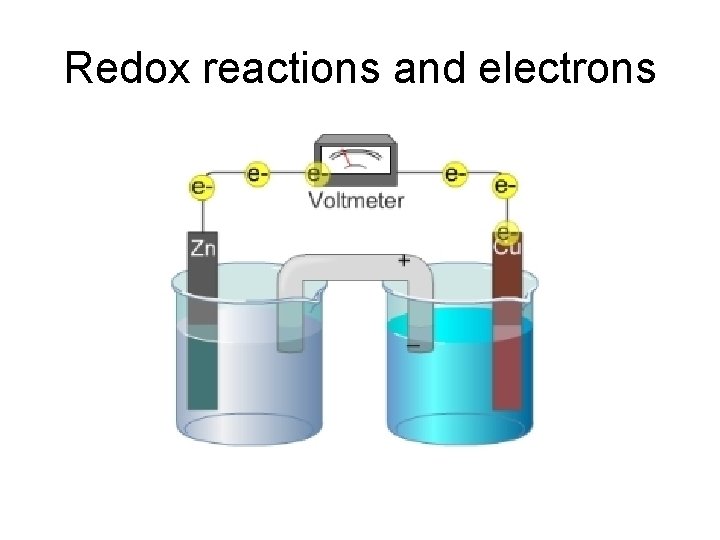
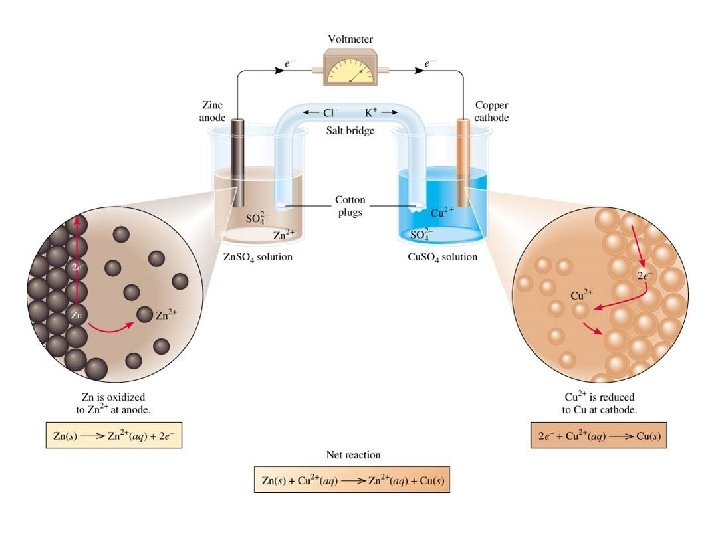
Redox reactions and electrons


http: //seniorphysics. com/chem/eei. html

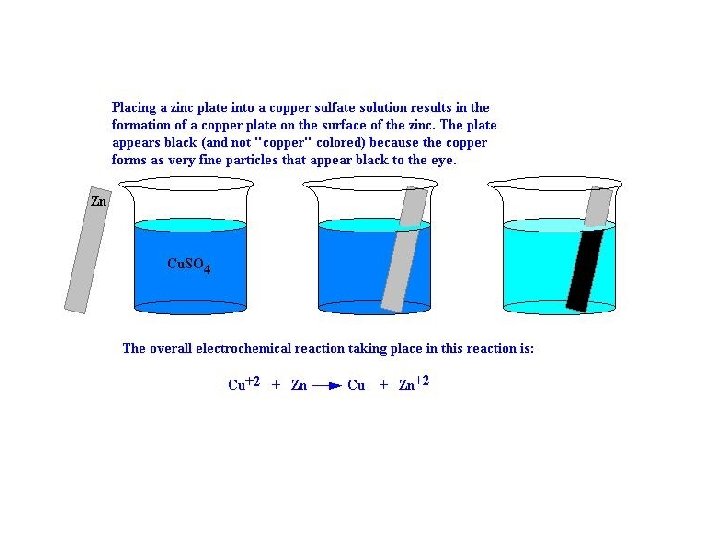
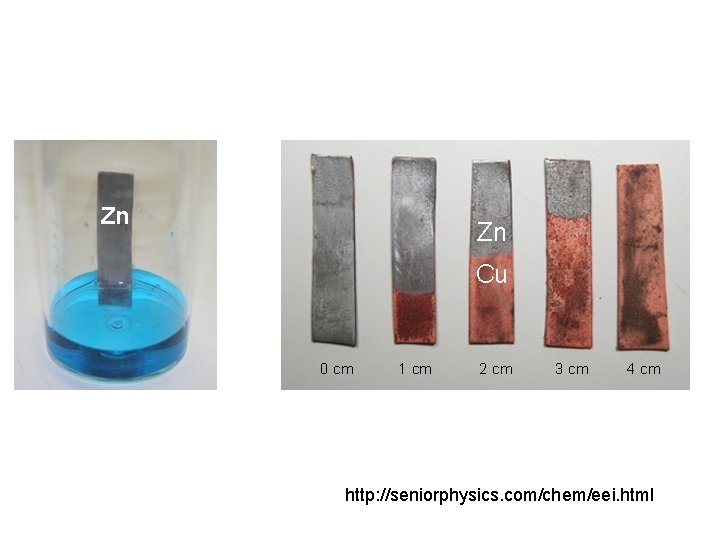
Make photos of the experiment, write the observations and explanation • After 1 minute the two solutions have different Cu 2+ concentrations shown by the different intensities of blue colour. This means more Zn has reacted in one than the other.

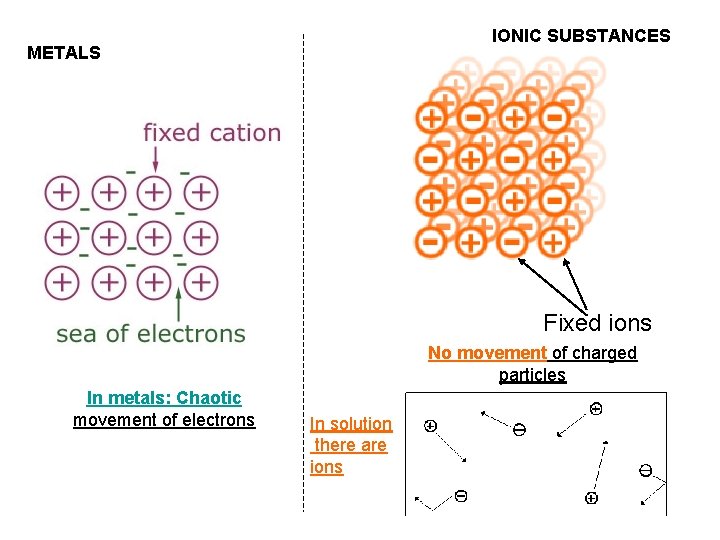
IONIC SUBSTANCES METALS Fixed ions No movement of charged particles In metals: Chaotic movement of electrons In solution there are ions

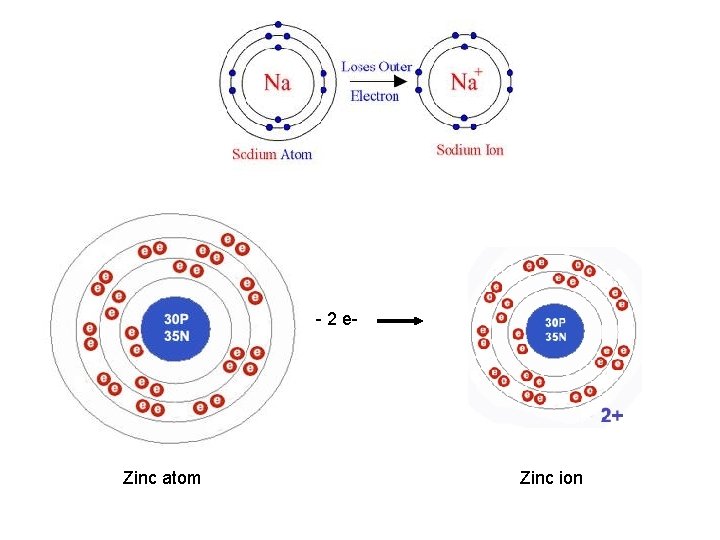
- 2 e- Zinc atom Zinc ion

Electricity • Need moving charges • Electrons in metals could move • Ions in solutions could move • THEREFORE, the metals and the solutions of the ionic substances can conduct electricity

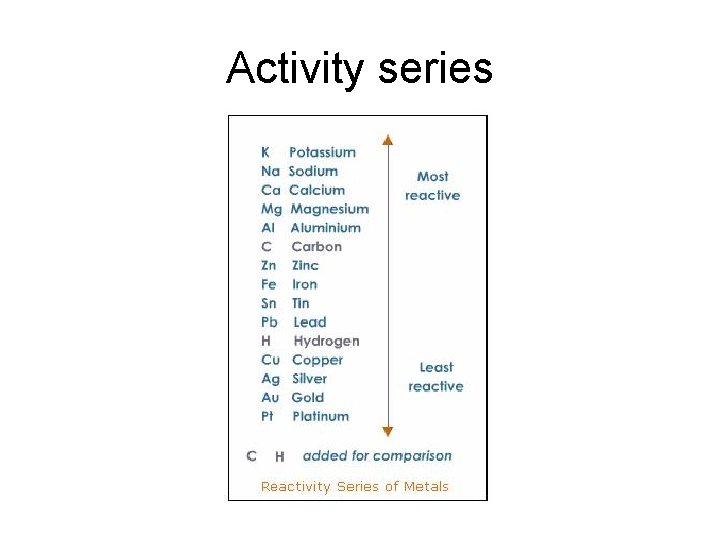
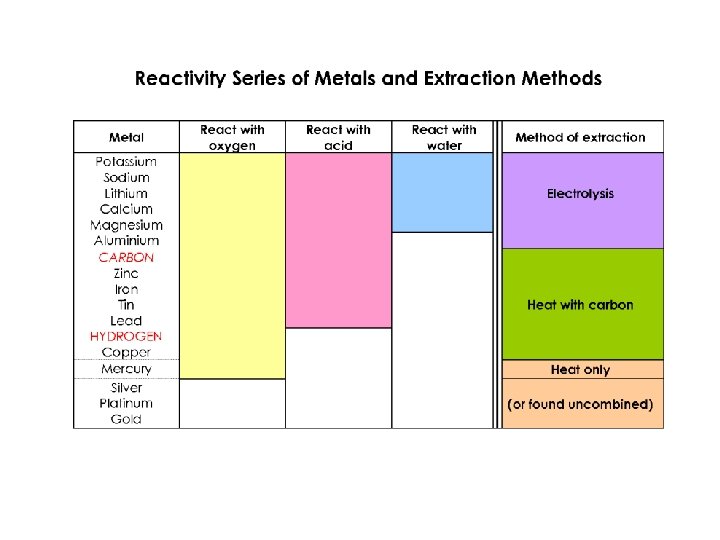
Metal displacement reactions. Activity series


Activity series


Extraction of metals Read p. 156 and write the important information

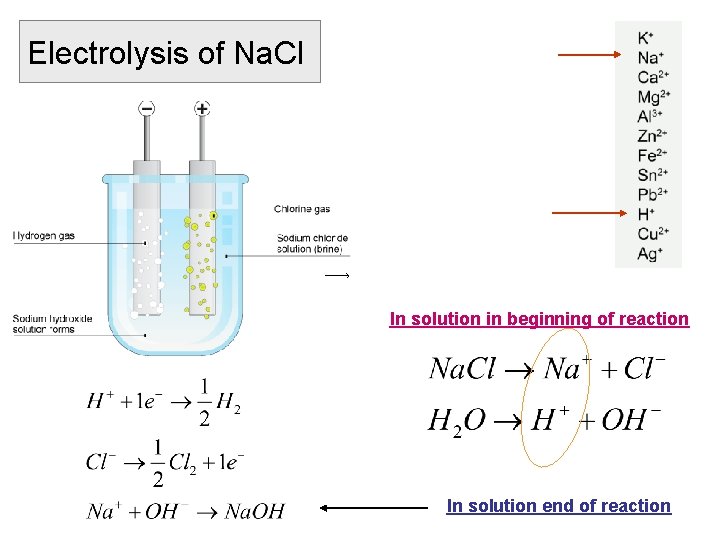
Electrolysis of Na. Cl In solution in beginning of reaction In solution end of reaction

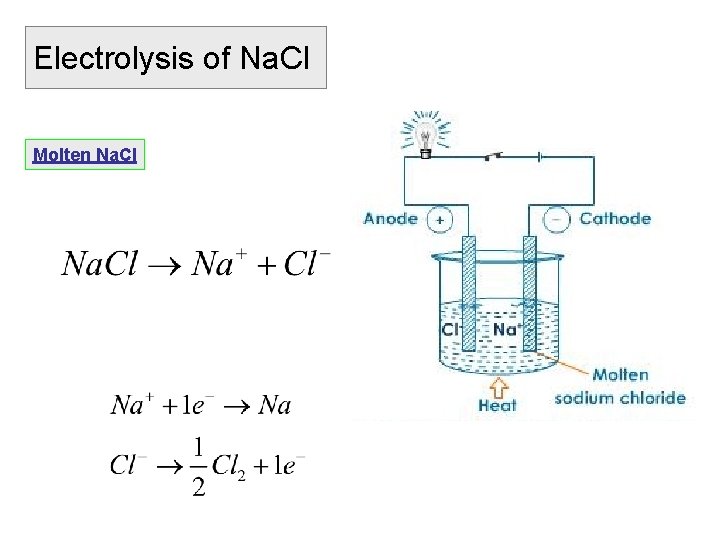
Electrolysis of Na. Cl Molten Na. Cl

Metal ores Less reactive metals are found uncombined in the earth's crust. Because of this the inactive metals were among the first to be discovered - gold is found in ancient tombs, the stone age was followed by the bronze age (copper). More reactive metals have to be extracted from ores. Ores are naturally-occurring compounds of metals.

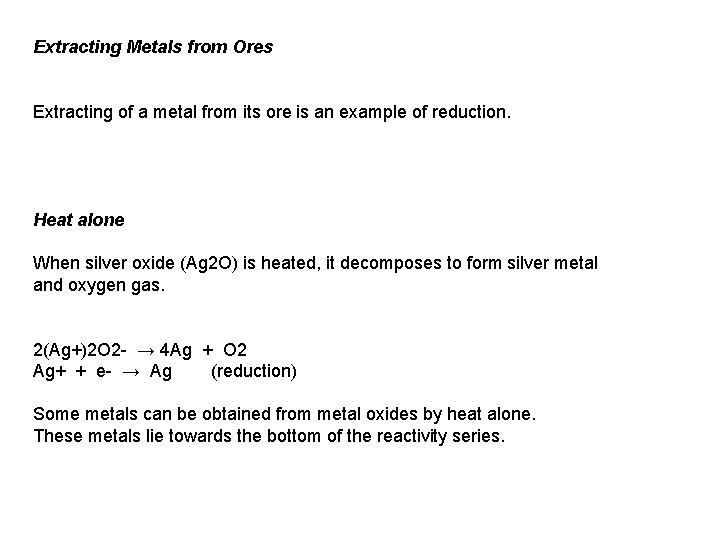
Extracting Metals from Ores Extracting of a metal from its ore is an example of reduction. Heat alone When silver oxide (Ag 2 O) is heated, it decomposes to form silver metal and oxygen gas. 2(Ag+)2 O 2 - → 4 Ag + O 2 Ag+ + e- → Ag (reduction) Some metals can be obtained from metal oxides by heat alone. These metals lie towards the bottom of the reactivity series.

Heating with some other substance Carbon Metals like copper or lead can be extracted from their oxides by heating with carbon powder. Cu 2+O 2 - + C → Cu + CO 2 Cu 2+ + 2 e- → Cu (reduction) Carbon monoxide Metals like copper, lead, iron and zinc are extracted from their oxides in a blast furnace (see later). Hydrogen Metals like copper and lead can be obtained from their oxides by heating in a stream of hydrogen gas Pb 2+O 2 - + H 2 → Pb + H 2 O Pb 2+ + 2 e- → Pb (reduction) These metals that can be extracted from ores by heating with another substance, lie in the middle of the reactivity series. Electricity Metals high up the reactivity series, like aluminium and sodium have to be extracted using heat and electricity. The metal gains electrons from the negative electrode. Al 3+ + 3 e- → Al (reduction)

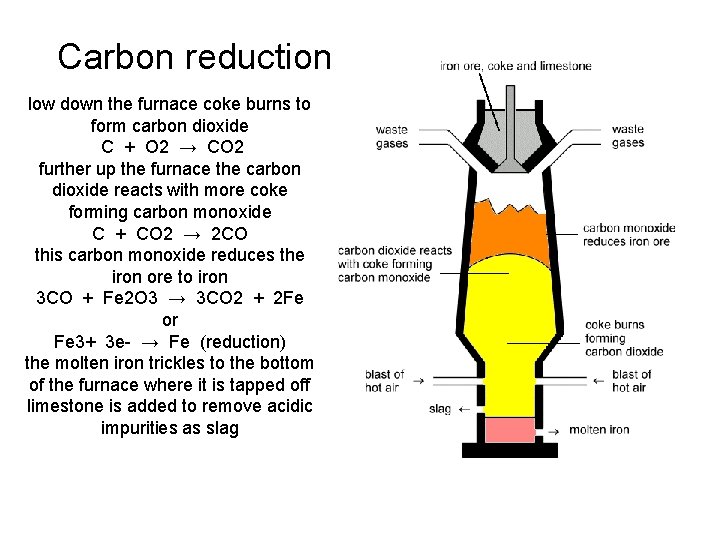
Carbon reduction low down the furnace coke burns to form carbon dioxide C + O 2 → CO 2 further up the furnace the carbon dioxide reacts with more coke forming carbon monoxide C + CO 2 → 2 CO this carbon monoxide reduces the iron ore to iron 3 CO + Fe 2 O 3 → 3 CO 2 + 2 Fe or Fe 3+ 3 e- → Fe (reduction) the molten iron trickles to the bottom of the furnace where it is tapped off limestone is added to remove acidic impurities as slag

Pracs p. 59 -161

5. 3. Rate of chemical reactions – only pracs