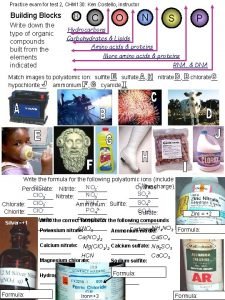
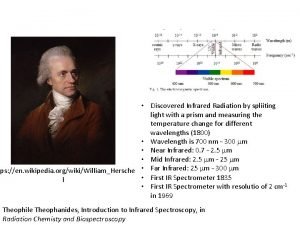
Chem II Wed 91416 Do Now Agenda Drop









































- Slides: 90

Chem II - Wed, 9/14/16 Do Now Agenda Drop off any study guides you want color coded ØStoich Pull out stoich HW ØCh 4 Homework See board ØLabish thing

Chapter 4 Chemical Reactions & Solution Stoich

Water l l Possesses the ability to dissolve many substances Draw the Lewis Dot Diagram now! 3

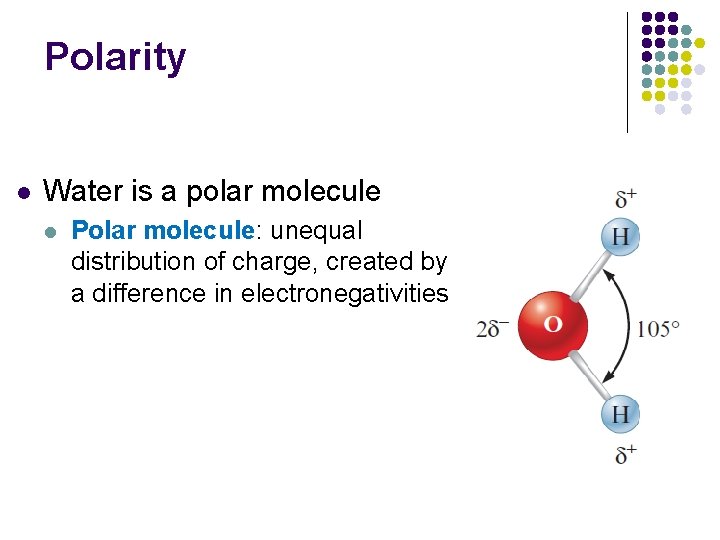
Polarity l Water is a polar molecule l Polar molecule: unequal distribution of charge, created by a difference in electronegativities

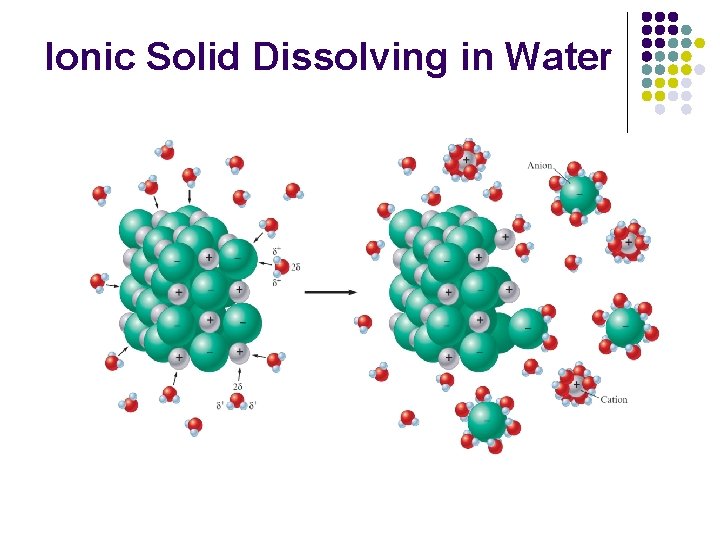
Ionic Solid Dissolving in Water

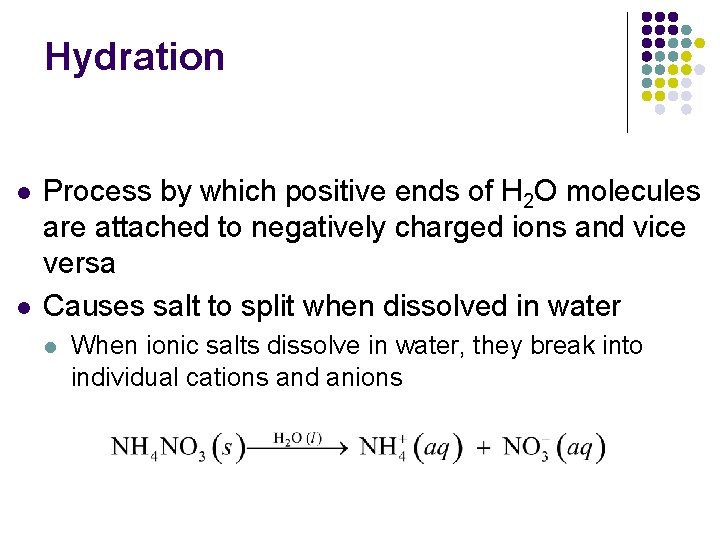
Hydration l l Process by which positive ends of H 2 O molecules are attached to negatively charged ions and vice versa Causes salt to split when dissolved in water l When ionic salts dissolve in water, they break into individual cations and anions

Concept of Solubility l Solubility of ionic substances in water varies depending on: l l l The attraction among ions The attraction of ions for water molecules Polar and ionic substances > nonpolar substances

Parts of a Solution l l Solute: Substance being dissolved Solvent: The dissolving medium l Example - Water Copyright © Cengage Learning. All rights reserved 8

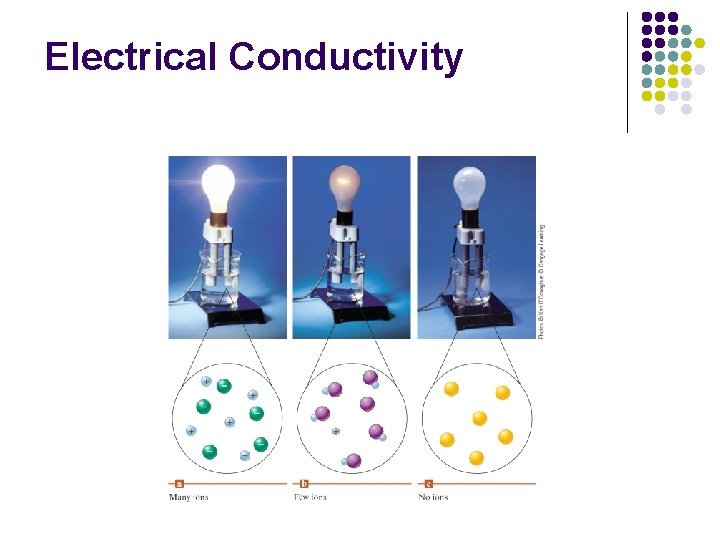
Types of Solutes l Strong electrolytes: complete dissociation, high electrical conductivity l l Weak electrolytes: partial dissociation, moderate electrical conductivity l l Example – Na. Cl, strong acid, strong base Example - Acetic acid, weak base Nonelectrolytes: no dissociation, no electrical conductivity l Example - Sugar 9

Electrical Conductivity

Svante Arrhenius l Postulated that the extent to which a solution can conduct an electric current directly depends on the number of ions present

Soluble Salts l Salts such as Na. Cl contain an array of cations and anions l Disintegrate and undergo hydration when the salt dissolves

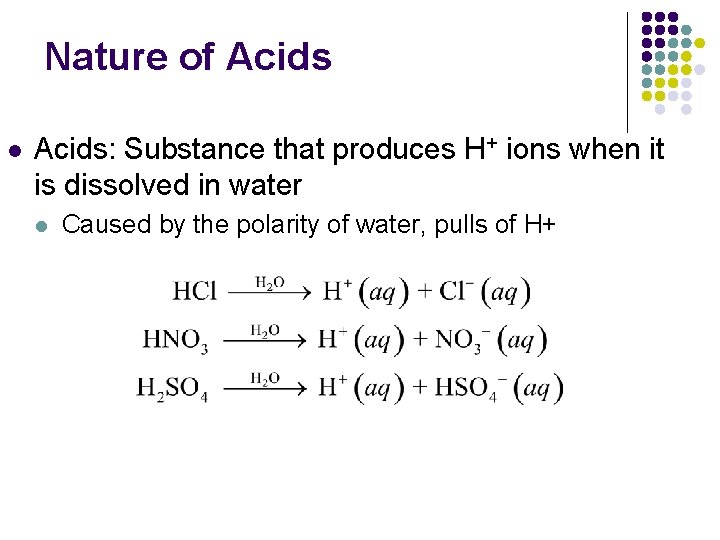
Nature of Acids l Acids: Substance that produces H+ ions when it is dissolved in water l Caused by the polarity of water, pulls of H+

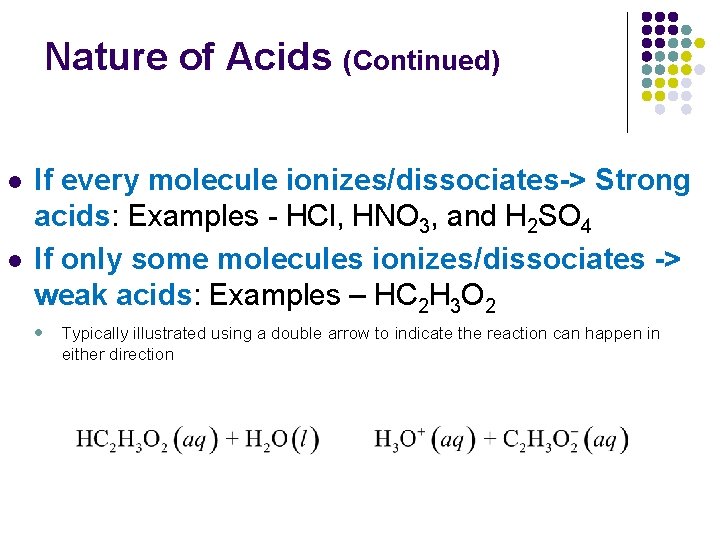
Nature of Acids (Continued) l l If every molecule ionizes/dissociates-> Strong acids: Examples - HCl, HNO 3, and H 2 SO 4 If only some molecules ionizes/dissociates -> weak acids: Examples – HC 2 H 3 O 2 l Typically illustrated using a double arrow to indicate the reaction can happen in either direction

Bases l l Soluble ionic compounds that contain the OH– (hydroxide) ion When dissolved in water, cations and OH– ions separate and move independently Strong and weak exist based on same criteria as acids Example l Dissolving potassium hydroxide in water

Nonelectrolytes l Substances that dissolve in water but do not produce any ions l l Leads to absence of electrical conductivity Examples l l Ethanol (C 2 H 5 OH) Table sugar (sucrose, C 12 H 22 O 11)

Chemical Reactions of Solutions l l Occur when two solutions are mixed To perform stoichiometric calculations, one must know: l The nature of the reaction l l Depends on the exact forms taken by the chemicals when dissolved The amounts of chemicals present in the solutions l Expressed as concentrations Copyright © Cengage Learning. All rights reserved 17


Chem II - Fri, 9/16/16 Do Now PT and calculator for quiz Homework See board Agenda ØStoich ØCh 4 ØLabish thing

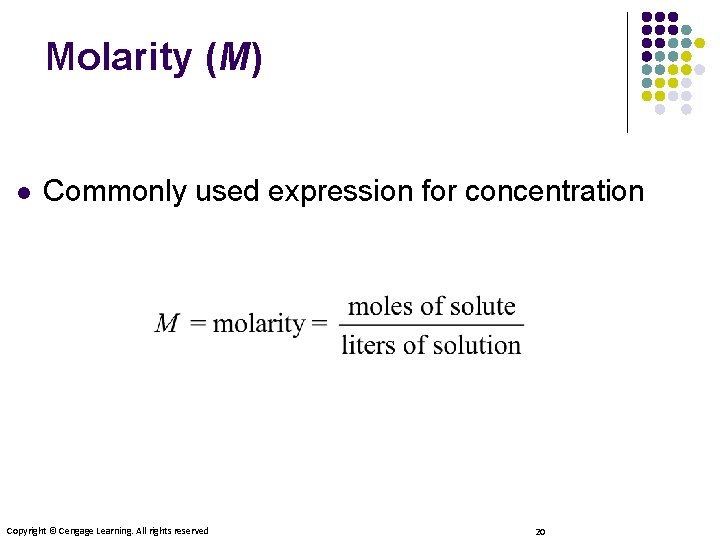
Molarity (M) l Commonly used expression for concentration Copyright © Cengage Learning. All rights reserved 20

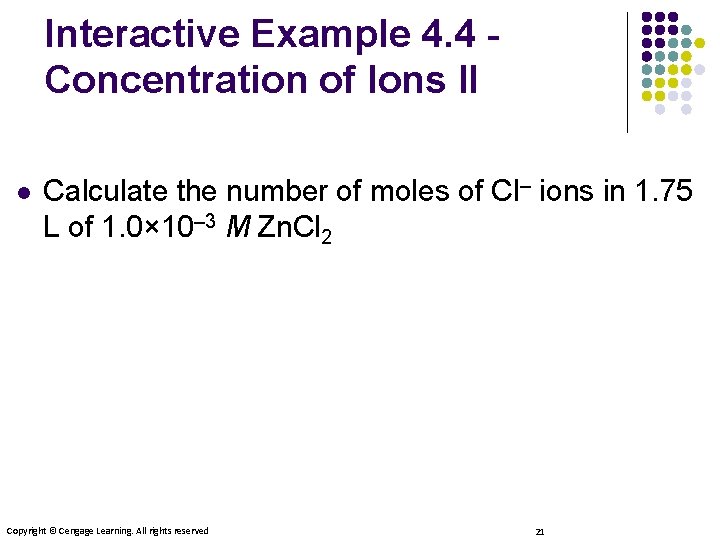
Interactive Example 4. 4 Concentration of Ions II l Calculate the number of moles of Cl– ions in 1. 75 L of 1. 0× 10– 3 M Zn. Cl 2 Copyright © Cengage Learning. All rights reserved 21

Interactive Example 4. 4 Solution l Where are we going? l l What do we know? l l l To find the moles of Cl– ion in the solution 1. 0× 10– 3 M Zn. Cl 2 1. 75 L What information is needed to find moles of Cl–? l Balanced equation for dissolving Zn. Cl 2 Copyright © Cengage Learning. All rights reserved 22

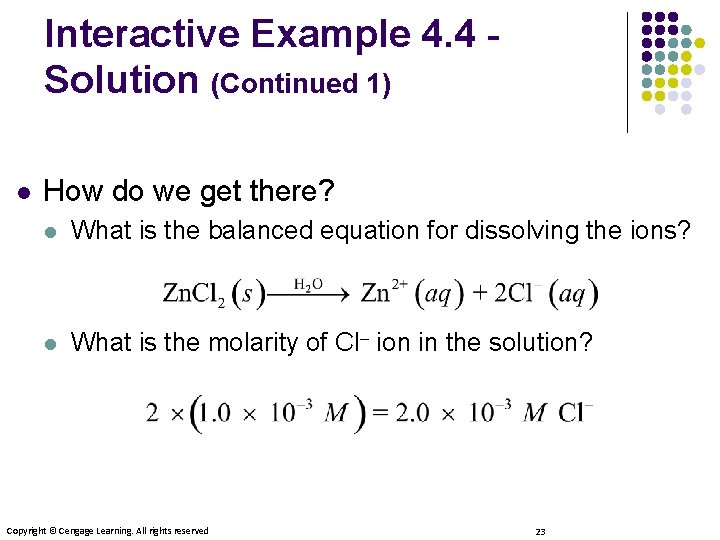
Interactive Example 4. 4 Solution (Continued 1) l How do we get there? l What is the balanced equation for dissolving the ions? l What is the molarity of Cl– ion in the solution? Copyright © Cengage Learning. All rights reserved 23

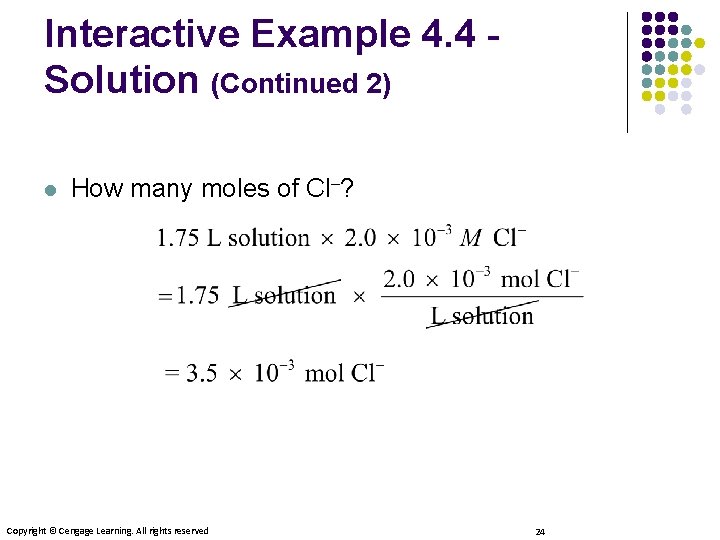
Interactive Example 4. 4 Solution (Continued 2) l How many moles of Cl–? Copyright © Cengage Learning. All rights reserved 24

Standard Solution l l Solution whose concentration is accurately known Process of preparation l l l Place a weighed amount of the solute into a volumetric flask, and add a small amount of water Dissolve the solid by swirling the flask Add more water until the level of the solution reaches the mark etched on the flask l Mix the solution by inverting the flask several times

Prepare a standard solution

Dilution l l Process of adding water to a concentrated (stock) solution to achieve the molarity desired for a particular solution Since only water is added to accomplish dilution: Copyright © Cengage Learning. All rights reserved 27

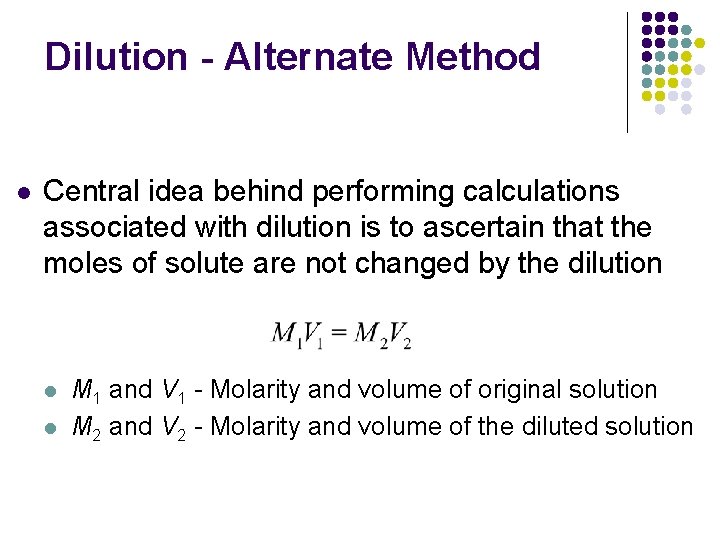
Dilution - Alternate Method l Central idea behind performing calculations associated with dilution is to ascertain that the moles of solute are not changed by the dilution l l M 1 and V 1 - Molarity and volume of original solution M 2 and V 2 - Molarity and volume of the diluted solution

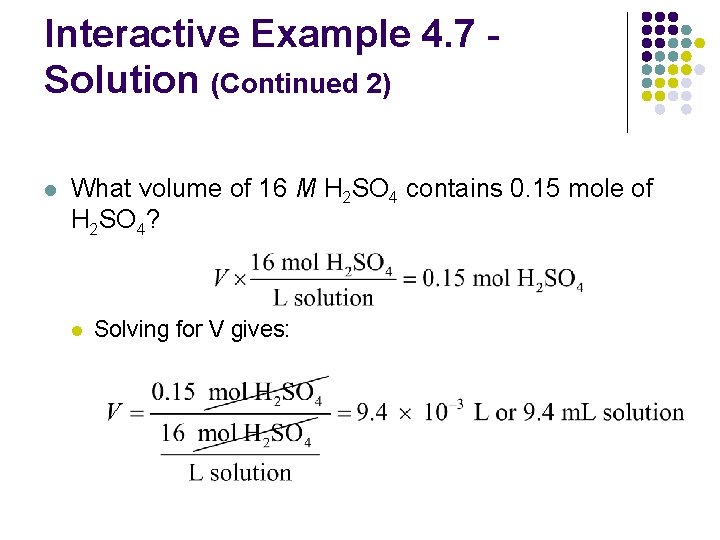
Interactive Example 4. 7 Concentration and Volume l What volume of 16 M sulfuric acid must be used to prepare 1. 5 L of a 0. 10 -M H 2 SO 4 solution?

Interactive Example 4. 7 Solution l Where are we going? l l To find the volume of H 2 SO 4 required to prepare the solution What do we know? l l 1. 5 L of 0. 10 M H 2 SO 4 is required We have 16 M H 2 SO 4

Interactive Example 4. 7 Solution (Continued 1) l What information do we need to find the volume of H 2 SO 4? l l Moles of H 2 SO 4 in the required solution How do we get there? l What are the moles of H 2 SO 4 required?

Interactive Example 4. 7 Solution (Continued 2) l What volume of 16 M H 2 SO 4 contains 0. 15 mole of H 2 SO 4? l Solving for V gives:

Interactive Example 4. 7 Solution (Continued 3) l Conclusion l l To make 1. 5 L of 0. 10 M H 2 SO 4 using 16 M H 2 SO 4, we must take 9. 4 m. L of the concentrated acid and dilute it with water to 1. 5 L The correct way to do this is to add the 9. 4 m. L of acid to about 1 L of distilled water and then dilute to 1. 5 L by adding more water


Types of Solution Reactions Precipitation reactions Acid–base reactions Oxidation– reduction reactions Copyright © Cengage Learning. All rights reserved 35

Precipitation Reaction l l Precipitate – insoluble solid that is formed in precipitation reaction DEMO! Copyright © Cengage Learning. All rights reserved 36

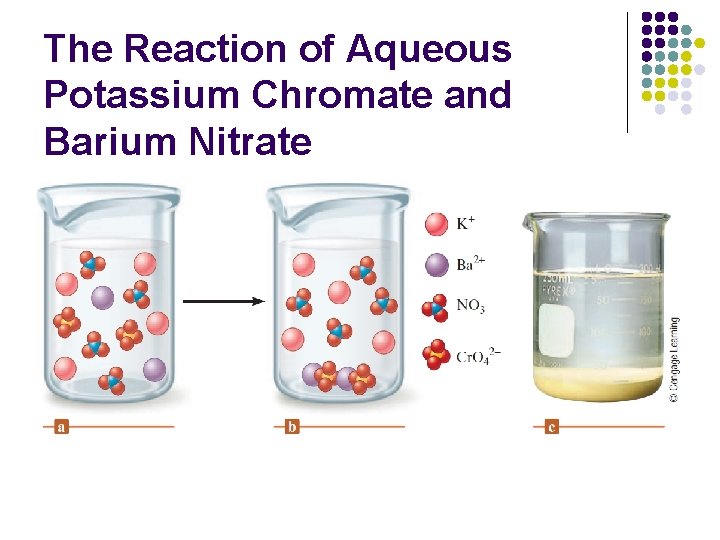
The Reaction of Aqueous Potassium Chromate and Barium Nitrate

Simple Rules for the Solubility of Salts in Water

But who wants that… l l Here’s a song!!!!!!! SONG

Types of Equations Used to Represent Reactions in Solution l POGIL Copyright © Cengage Learning. All rights reserved 40

Interactive Example 4. 11 Determining the Mass of Product Formed II l When aqueous solutions of Na 2 SO 4 and Pb(NO 3)2 are mixed, Pb. SO 4 precipitates l Calculate the mass of Pb. SO 4 formed when 1. 25 L of 0. 0500 M Pb(NO 3)2 and 2. 00 L of 0. 0250 M Na 2 SO 4 are mixed

Interactive Example 4. 11 Solution l Where are we going? l l What do we know? l l l To find the mass of solid Pb. SO 4 formed 1. 25 L of 0. 0500 M Pb(NO 3)2 and 2. 00 L of 0. 0250 M Na 2 SO 4 Chemical reaction - What information do we need? l The limiting reactant

Interactive Example 4. 11 Solution (Continued 1) l How do we get there? l What are the ions present in the combined solution? Na+ SO 42– Pb 2+ NO 3– l l Reaction - Since Na. NO 3 is soluble and Pb. SO 4 is insoluble, solid Pb. SO 4 will form What is the balanced net ionic equation for the reaction?

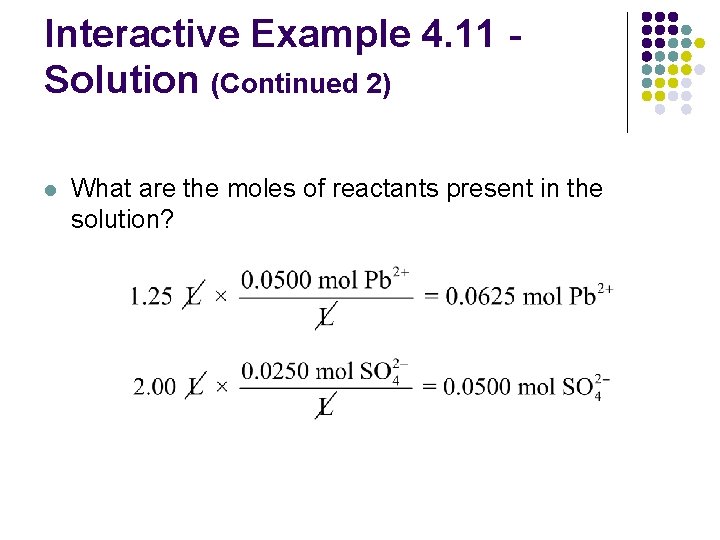
Interactive Example 4. 11 Solution (Continued 2) l What are the moles of reactants present in the solution?

Interactive Example 4. 11 Solution (Continued 3) l Which reactant is limiting? l l Since Pb 2+ and SO 42– react in a 1: 1 ratio, the amount of SO 42– will be limiting (0. 0500 mol SO 42– is less than 0. 0625 mole of Pb 2+) What number of moles of Pb. SO 4 will be formed? l Since SO 42– is limiting, only 0. 0500 mole of solid Pb. SO 4 will be formed

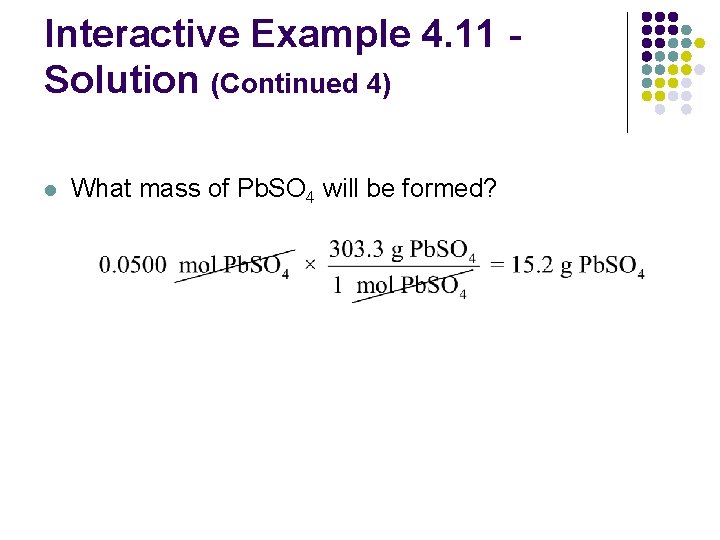
Interactive Example 4. 11 Solution (Continued 4) l What mass of Pb. SO 4 will be formed?

Definitions - Acid, Base, and Neutralization Reaction l Brønsted–Lowry definitions for acids and bases l l l Acid: Proton donor Base: Proton acceptor Neutralization reaction: General name given to acid–base reactions l An acid is neutralized when enough base reacts exactly with it in a solution Copyright © Cengage Learning. All rights reserved 47

Problem-Solving Strategy Performing Calculations for Acid–Base Reactions 1. List the species present in the combined solution before any reaction occurs l 2. 3. Decide what reaction will occur Write the balanced net ionic equation for the reaction Calculate moles of reactants l For reactions in solution, use the volumes of the original solutions and their molarities Copyright © Cengage Learning. All rights reserved 48

Problem-Solving Strategy Performing Calculations for Acid–Base Reactions (Continued) 4. 5. 6. Determine the limiting reactant where appropriate Calculate the moles of the required reactant or product Convert to grams or volume (of solution), as required Copyright © Cengage Learning. All rights reserved 49

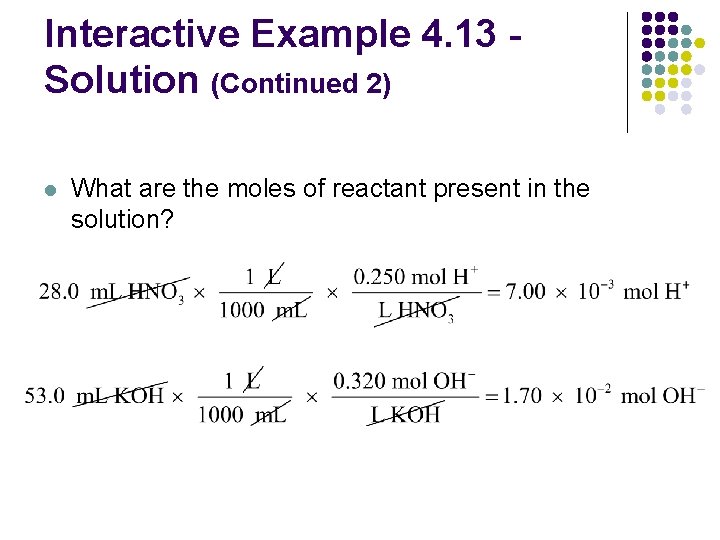
Interactive Example 4. 13 Neutralization Reactions II l In a certain experiment, 28. 0 m. L of 0. 250 M HNO 3 and 53. 0 m. L of 0. 320 M KOH are mixed l What is the concentration of H+ or OH– ions in excess after the reaction goes to completion?

Interactive Example 4. 13 Solution l Where are we going? l l To find the concentration of H+ or OH– in excess after the reaction is complete What do we know? l l l 28. 0 m. L of 0. 250 M HNO 3 53. 0 m. L of 0. 320 M KOH Chemical reaction

Interactive Example 4. 13 Solution (Continued 1) l How do we get there? l What are the ions present in the combined solution? H+ NO 3– K+ OH– l What is the balanced net ionic equation for the reaction?

Interactive Example 4. 13 Solution (Continued 2) l What are the moles of reactant present in the solution?

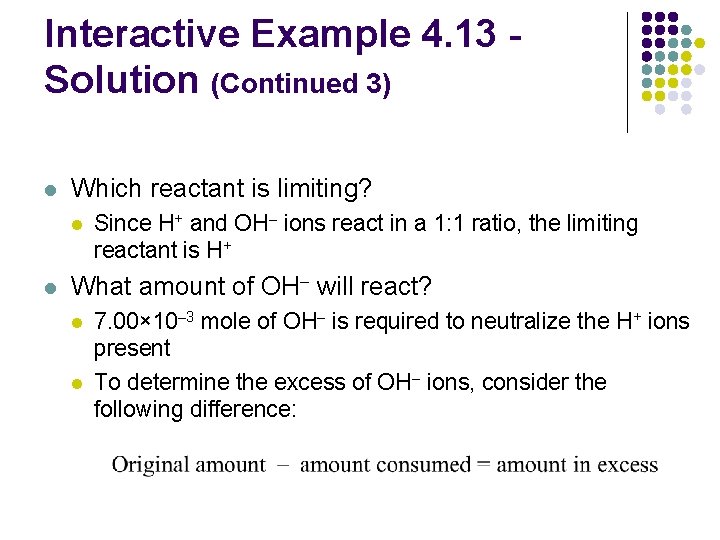
Interactive Example 4. 13 Solution (Continued 3) l Which reactant is limiting? l l Since H+ and OH– ions react in a 1: 1 ratio, the limiting reactant is H+ What amount of OH– will react? l l 7. 00× 10– 3 mole of OH– is required to neutralize the H+ ions present To determine the excess of OH– ions, consider the following difference:

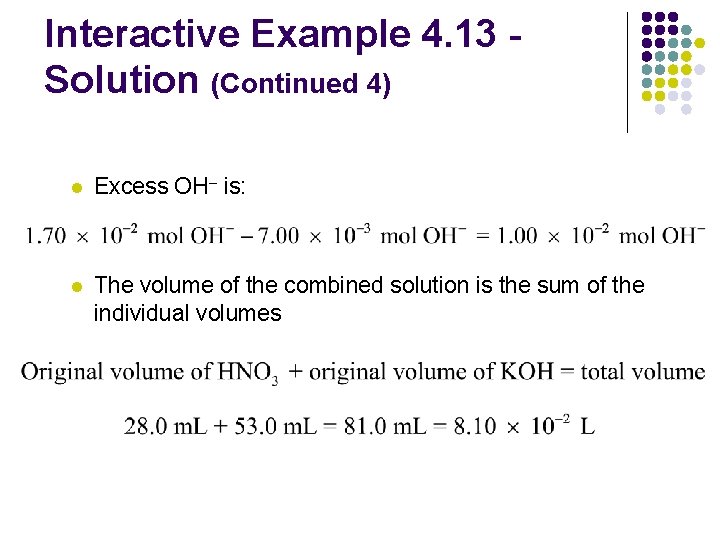
Interactive Example 4. 13 Solution (Continued 4) l Excess OH– is: l The volume of the combined solution is the sum of the individual volumes

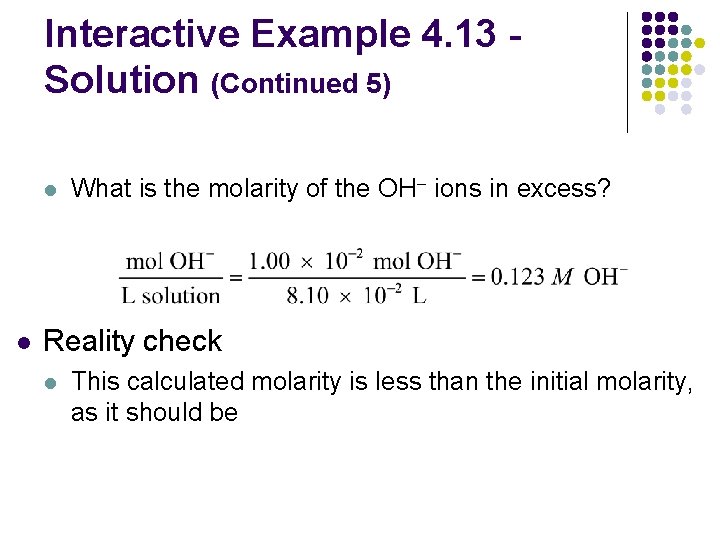
Interactive Example 4. 13 Solution (Continued 5) l l What is the molarity of the OH– ions in excess? Reality check l This calculated molarity is less than the initial molarity, as it should be

Volumetric Analysis l Technique used for ascertaining the amount of a certain substance by doing a titration l Titration: Delivery of a titrant into an analyte l l Titrant - Solution of known concentration Analyte - Solution containing the substance being analyzed Copyright © Cengage Learning. All rights reserved 57

Acid–Base Titrations l Equivalence (stoichiometric) point: Marks the point in titration where enough titrant has been added to react exactly with the analyte l Indicator: Substance added at the beginning of the titration l l Changes color at the equivalence point Endpoint: Point where the indicator actually changes color Copyright © Cengage Learning. All rights reserved 58

Requirements for a Successful Titration l l l Exact reaction between titrant and analyte must be known and must be rapid Equivalence point must be accurately marked Volume of titrant that is needed to reach the equivalence point must be accurately known

Indicator Used in Acid–Base Titrations l Phenolphthalein l l l Colorless in an acidic solution Pink in a basic solution When an acid is titrated with a base, the indicator remains colorless until after the acid is consumed and the first drop of excess base is added

Figure 4. 18 - Titration of an Acid with a Base

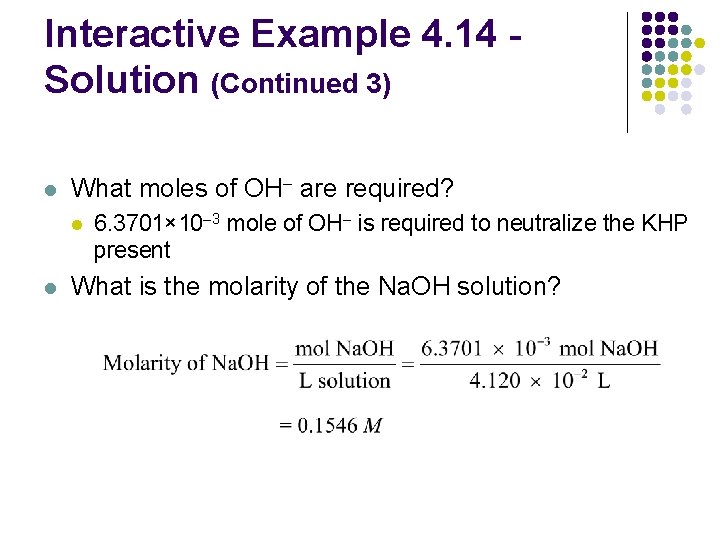
Interactive Example 4. 14 Neutralization Titration l A student carries out an experiment to standardize (determine the exact concentration of) a sodium hydroxide solution l To do this, the student weighs out a 1. 3009 -g sample of potassium hydrogen phthalate (KHC 8 H 4 O 4, often abbreviated KHP) l KHP (molar mass 204. 22 g/mol) has one acidic hydrogen

Interactive Example 4. 14 Neutralization Titration (Continued) l The student dissolves the KHP in distilled water, adds phenolphthalein as an indicator, and titrates the resulting solution with the sodium hydroxide solution to the phenolphthalein endpoint l l The difference between the final and initial buret readings indicates that 41. 20 m. L of the sodium hydroxide solution is required to react exactly with the 1. 3009 g KHP Calculate the concentration of the sodium hydroxide solution

Interactive Example 4. 14 Solution l Where are we going? l l To find the concentration of Na. OH solution What do we know? l l l 1. 3009 g KHC 8 H 4 O 4 (KHP), molar mass (204. 22 g/mol) 41. 20 m. L Na. OH solution to neutralize KHP The chemical reaction

Interactive Example 4. 14 Solution (Continued 1) l How do we get there? l What are the ions present in the combined solution? K+ HC 8 H 4 O 4– Na+ OH– l What is the balanced net ionic equation for the reaction?

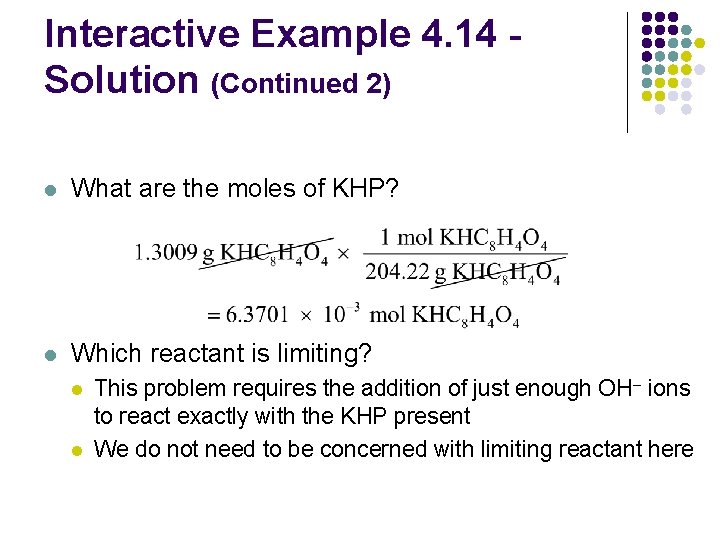
Interactive Example 4. 14 Solution (Continued 2) l What are the moles of KHP? l Which reactant is limiting? l l This problem requires the addition of just enough OH– ions to react exactly with the KHP present We do not need to be concerned with limiting reactant here

Interactive Example 4. 14 Solution (Continued 3) l What moles of OH– are required? l l 6. 3701× 10– 3 mole of OH– is required to neutralize the KHP present What is the molarity of the Na. OH solution?

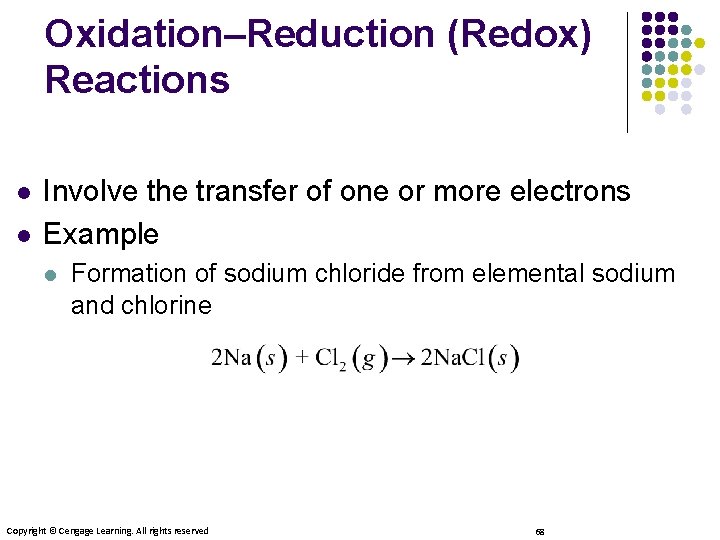
Oxidation–Reduction (Redox) Reactions l l Involve the transfer of one or more electrons Example l Formation of sodium chloride from elemental sodium and chlorine Copyright © Cengage Learning. All rights reserved 68

Oxidation States (Oxidation Numbers) l For atoms in covalent compounds, the oxidation state refers to imaginary charges that atoms would have if: l l Shared electrons were equally divided between identical atoms bonded to each other In different atoms, the shared electrons were all assigned to the atom in each bond that has greater electron affinity

Oxidation States (Oxidation Numbers) (Continued) l l In ionic compounds that contain monatomic ions, the oxidation states of the ions are equal to the ion charges For electrically neutral compounds, the sum of oxidation states must be zero

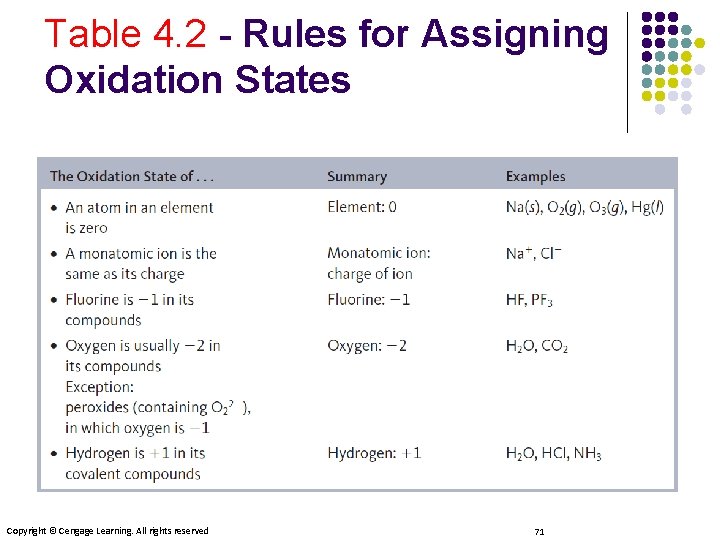
Table 4. 2 - Rules for Assigning Oxidation States Copyright © Cengage Learning. All rights reserved 71

Interactive Example 4. 16 Assigning Oxidation States l Assign oxidation states to all atoms in the following: a. b. CO 2 NO 3–

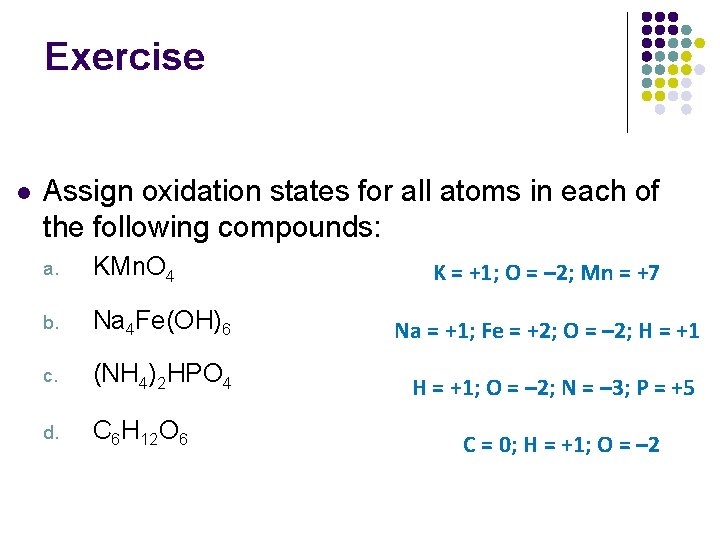
Exercise l Assign oxidation states for all atoms in each of the following compounds: a. KMn. O 4 b. Na 4 Fe(OH)6 Na = +1; Fe = +2; O = – 2; H = +1 c. (NH 4)2 HPO 4 H = +1; O = – 2; N = – 3; P = +5 d. C 6 H 12 O 6 K = +1; O = – 2; Mn = +7 C = 0; H = +1; O = – 2

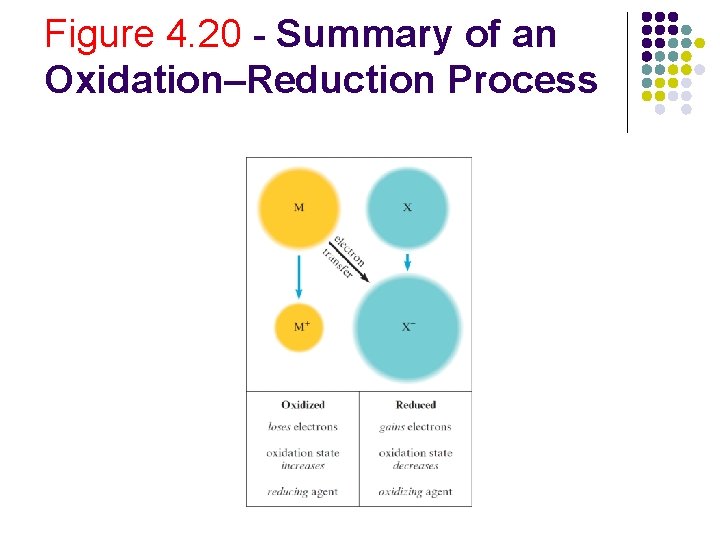
Terminologies l Oxidation: Increase in oxidation state l l Reduction: Decrease in oxidation state l l l Characterized by electron loss Characterized by electron gain Reducing agent: Electron donor Oxidizing agent: Electron acceptor

Figure 4. 20 - Summary of an Oxidation–Reduction Process

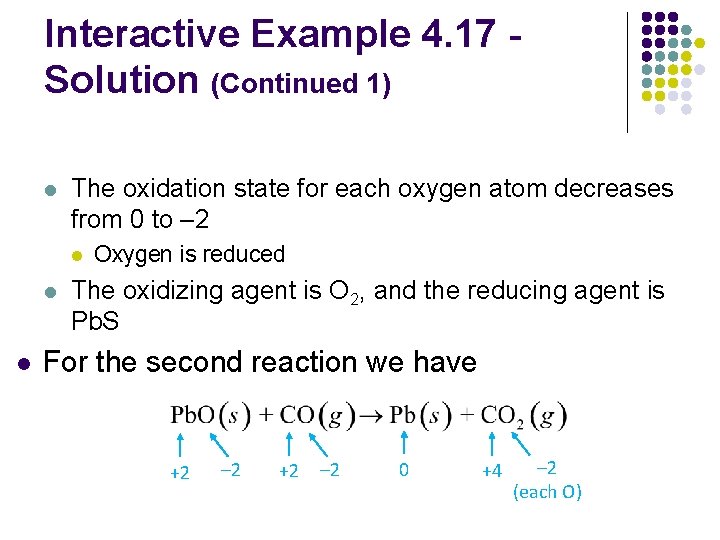
Interactive Example 4. 17 Oxidation–Reduction Reactions l Metallurgy, the process of producing a metal from its ore, always involves oxidation–reduction reactions l In the metallurgy of galena (Pb. S), the principal leadcontaining ore, the first step is the conversion of lead sulfide to its oxide (a process called roasting):

Interactive Example 4. 17 Oxidation–Reduction Reactions (Continued) l l The oxide is then treated with carbon monoxide to produce the free metal: For each reaction, identify the atoms that are oxidized and reduced, and specify the oxidizing and reducing agents

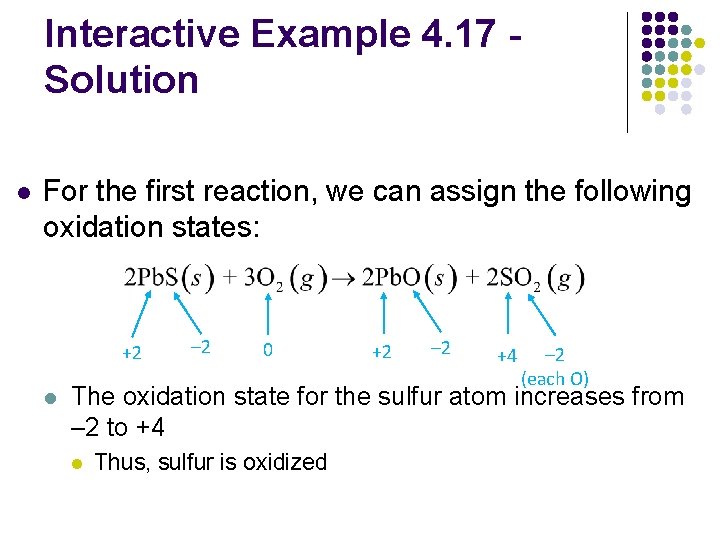
Interactive Example 4. 17 Solution l For the first reaction, we can assign the following oxidation states: +2 l – 2 0 +2 – 2 +4 – 2 (each O) The oxidation state for the sulfur atom increases from – 2 to +4 l Thus, sulfur is oxidized

Interactive Example 4. 17 Solution (Continued 1) l The oxidation state for each oxygen atom decreases from 0 to – 2 l l l Oxygen is reduced The oxidizing agent is O 2, and the reducing agent is Pb. S For the second reaction we have +2 – 2 0 +4 – 2 (each O)

Interactive Example 4. 17 Solution (Continued 2) l l Lead is reduced (its oxidation state decreases from +2 to 0), and carbon is oxidized (its oxidation state increases from +2 to +4) Pb. O is the oxidizing agent, and CO is the reducing agent

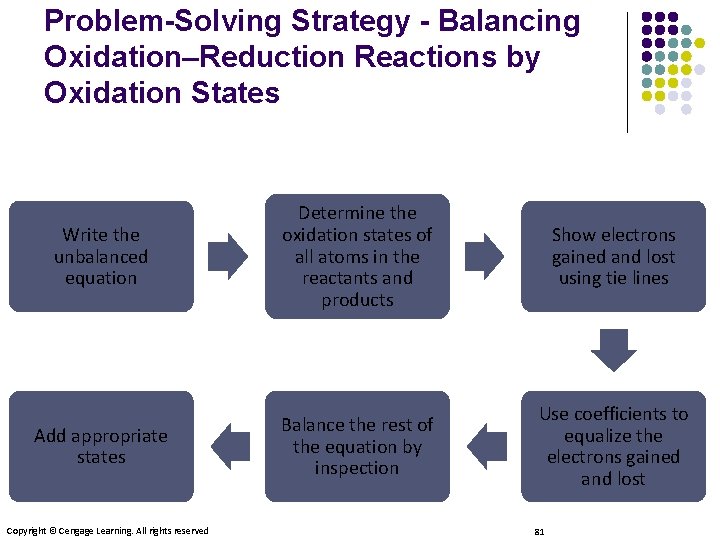
Problem-Solving Strategy - Balancing Oxidation–Reduction Reactions by Oxidation States Write the unbalanced equation Determine the oxidation states of all atoms in the reactants and products Show electrons gained and lost using tie lines Add appropriate states Balance the rest of the equation by inspection Use coefficients to equalize the electrons gained and lost Copyright © Cengage Learning. All rights reserved 81

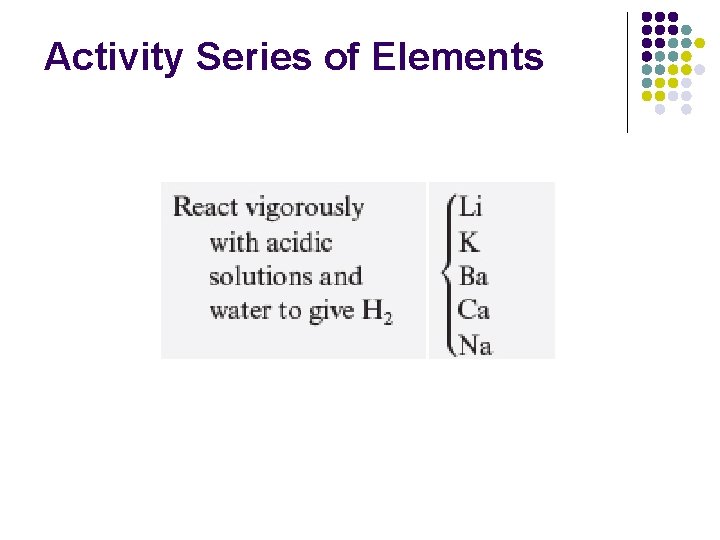
Activity Series of Elements

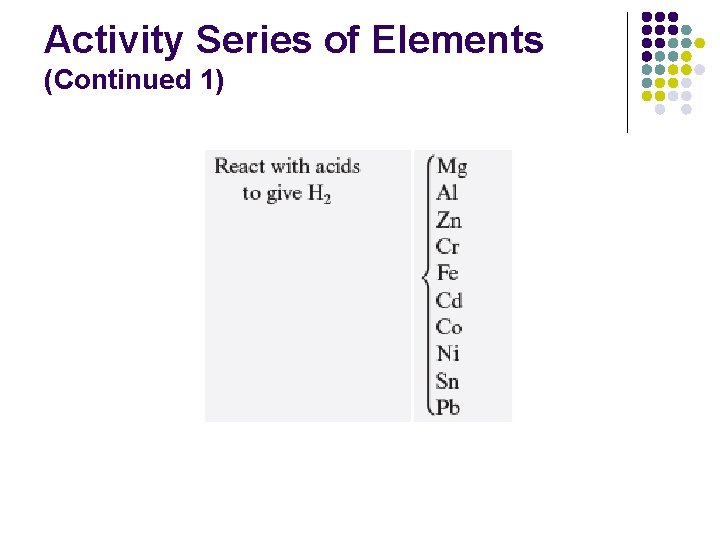
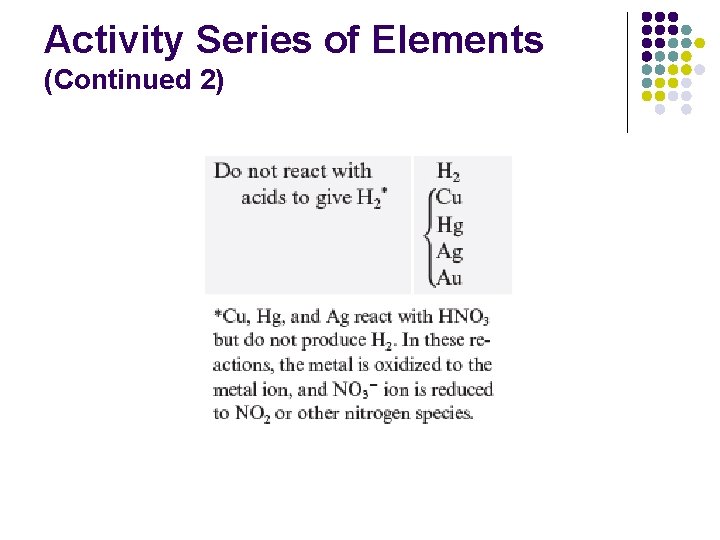
Activity Series of Elements (Continued 1)

Activity Series of Elements (Continued 2)

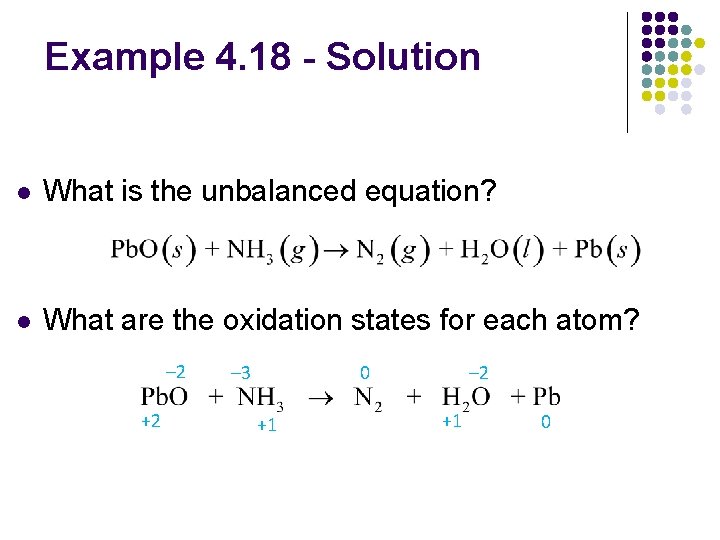
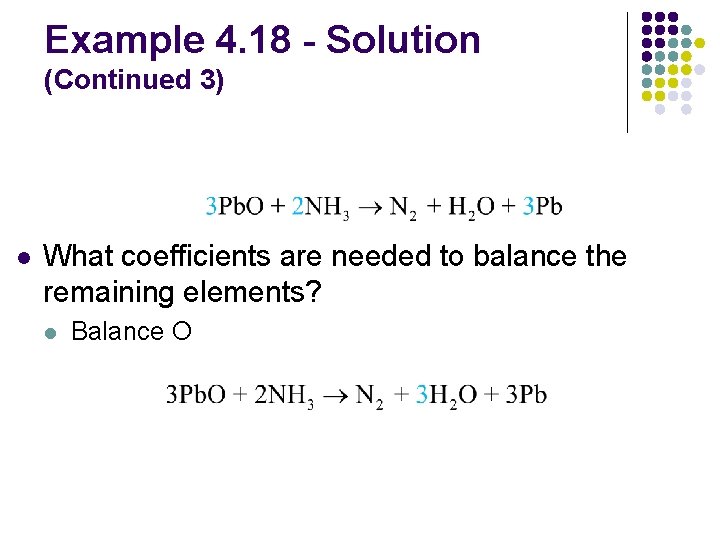
Example 4. 18 - Balancing Oxidation–Reduction Reactions l Balance the reaction between solid lead(II) oxide and ammonia gas to produce nitrogen gas, liquid water, and solid lead

Example 4. 18 - Solution l What is the unbalanced equation? l What are the oxidation states for each atom? – 2 +2 – 3 0 +1 – 2 +1 0

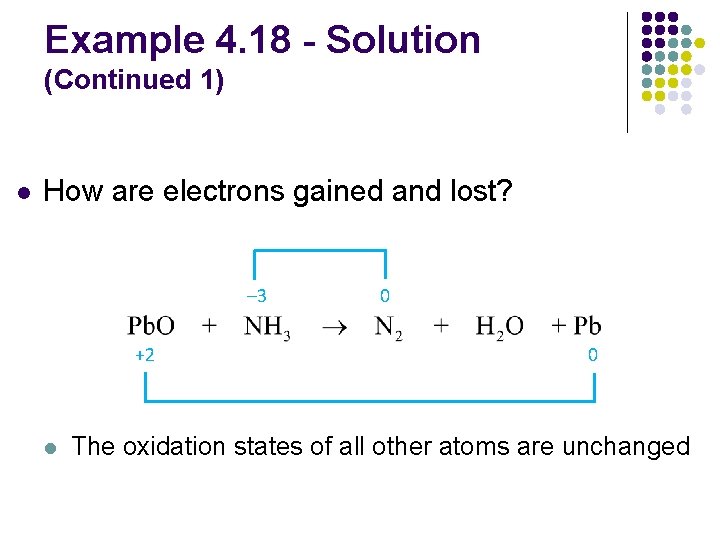
Example 4. 18 - Solution (Continued 1) l How are electrons gained and lost? 3 e– lost (each atom) – 3 0 0 +2 2 e– gained l The oxidation states of all other atoms are unchanged

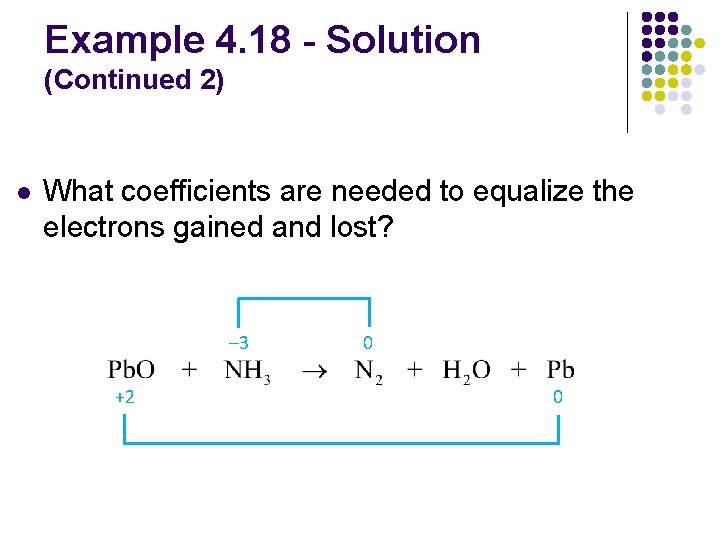
Example 4. 18 - Solution (Continued 2) l What coefficients are needed to equalize the electrons gained and lost? 3 e– lost (each atom) – 3 multiply by 2 0 0 +2 2 e– gained multiply by 3

Example 4. 18 - Solution (Continued 3) l What coefficients are needed to balance the remaining elements? l Balance O

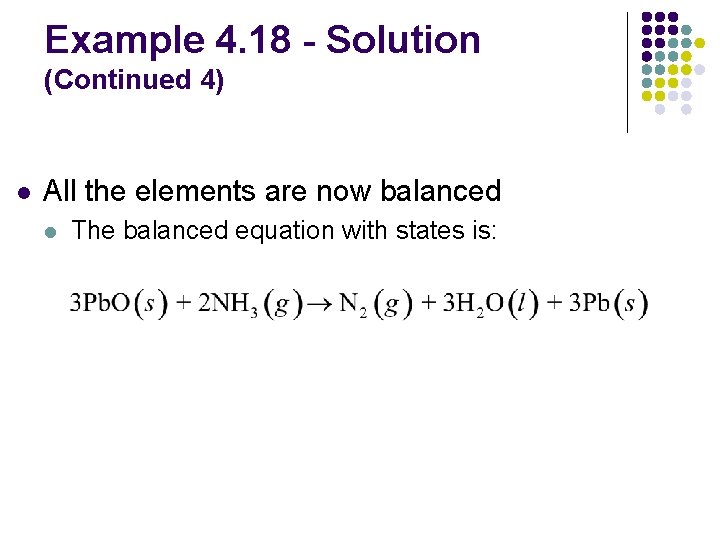
Example 4. 18 - Solution (Continued 4) l All the elements are now balanced l The balanced equation with states is:
 Agenda sistemica y agenda institucional
Agenda sistemica y agenda institucional Now i see it now you don't
Now i see it now you don't Chem.fsu.edu
Chem.fsu.edu Astex viewer
Astex viewer Hkale chem
Hkale chem Chem lab
Chem lab Electrochemistry ap chem
Electrochemistry ap chem How do significant figures work
How do significant figures work Hazard pay chemistry law
Hazard pay chemistry law Bó đỏ gai
Bó đỏ gai Msj chem topic 5
Msj chem topic 5 Chm 130 chapter 12 practice problems answer key
Chm 130 chapter 12 practice problems answer key Chem 494
Chem 494 Kinetics ap chemistry
Kinetics ap chemistry Herpesviridae
Herpesviridae Chem commun impact factor
Chem commun impact factor Amy gottfried umich
Amy gottfried umich Gen chem
Gen chem Chem libretext
Chem libretext Chem
Chem Chem 30 data booklet
Chem 30 data booklet Cpa chem
Cpa chem Ap chem spontaneity entropy and free energy
Ap chem spontaneity entropy and free energy Beer's law
Beer's law Chem quiz.net
Chem quiz.net Chem 101
Chem 101 Ka and kb
Ka and kb Chem
Chem Iannone chem moodle
Iannone chem moodle Olive oil ior
Olive oil ior Chem
Chem Chem
Chem Molecular polarity
Molecular polarity Static chemical equilibrium
Static chemical equilibrium Types of equations chem worksheet 10-3
Types of equations chem worksheet 10-3 Gen chem review for ochem
Gen chem review for ochem Ice table chemistry
Ice table chemistry Chem
Chem Photoelectron spectroscopy pogil
Photoelectron spectroscopy pogil Meth eth prop but pent table
Meth eth prop but pent table Organometallic
Organometallic Metylocyklopentan
Metylocyklopentan Chempro 100i
Chempro 100i Tera chem
Tera chem Chem 409
Chem 409 Brinclhof
Brinclhof Clavulator
Clavulator Chem 200
Chem 200 Chem
Chem Model chem lab
Model chem lab Chemsheets as 1047 worked answers
Chemsheets as 1047 worked answers 2017 ap chem exam
2017 ap chem exam Chem 1020
Chem 1020 Chem 4 kids.com
Chem 4 kids.com Specific heat chem worksheet 16-1
Specific heat chem worksheet 16-1 Ap chemistry unit 7
Ap chemistry unit 7 Science olympiad san diego
Science olympiad san diego Chem feed
Chem feed Chem
Chem Chem 433
Chem 433 Chem libretext
Chem libretext Dyno chem
Dyno chem Chem
Chem Chem 109
Chem 109 Chem pharma impex
Chem pharma impex Crystalline or amorphous
Crystalline or amorphous Ap chemistry big idea 6 review answers
Ap chemistry big idea 6 review answers Principles of kmt
Principles of kmt Chem
Chem Chem
Chem Chem 253
Chem 253 Chem
Chem Lei luo build
Lei luo build Chem 301 gas law simulator
Chem 301 gas law simulator Chem moles
Chem moles Chem 150
Chem 150 Ap chem equilibrium
Ap chem equilibrium Pub chem
Pub chem Chem ufl
Chem ufl Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Shapes of molecules in chemistry
Shapes of molecules in chemistry Imf chem
Imf chem Geminal coupling
Geminal coupling Spectroscopy ap chem
Spectroscopy ap chem Chem 4 kids.com
Chem 4 kids.com Gen chem cheat sheet
Gen chem cheat sheet E chem portal
E chem portal Chem
Chem Chem
Chem Gen chem
Gen chem Chem
Chem