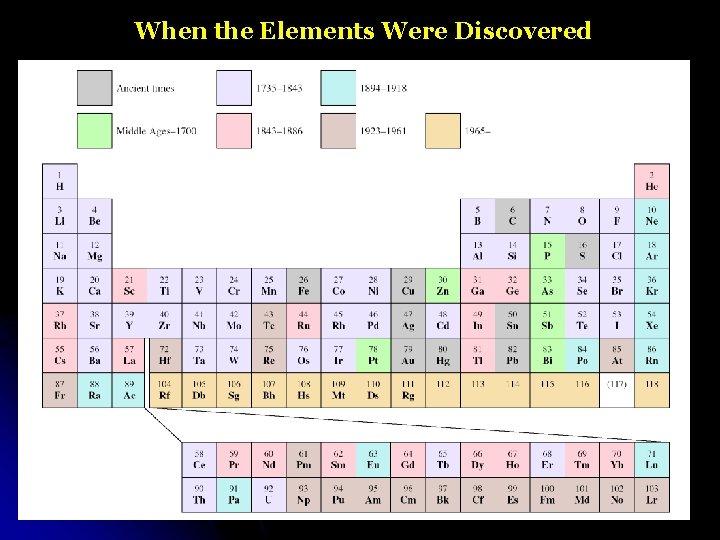
CHAPTER 5 When the Elements Were Discovered 4
![Electron Configurations of Cations and Anions Of Representative Elements Na: [Ne]3 s 1 Na+ Electron Configurations of Cations and Anions Of Representative Elements Na: [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image_h2/b8d83364f1a8340074d535a78d53f886/image-6.jpg)















- Slides: 47

CHAPTER 5

When the Elements Were Discovered

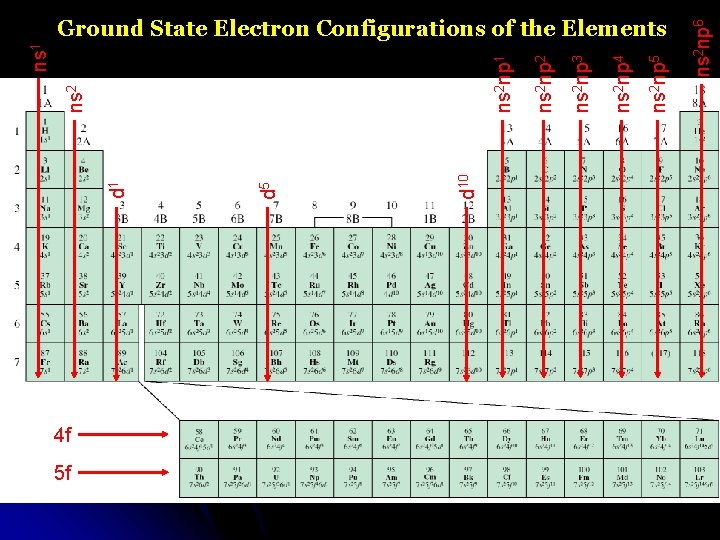
4 f 5 f d 10 d 5 d 1 ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 ns 2 np 1 ns 1 Ground State Electron Configurations of the Elements

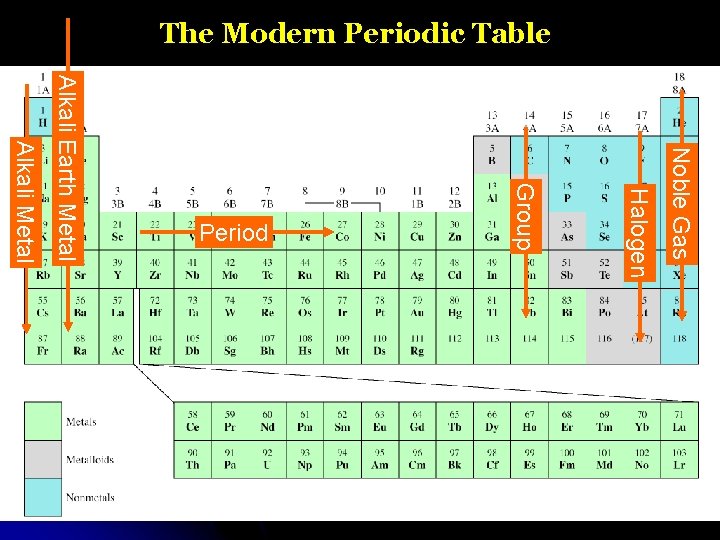
The Modern Periodic Table Noble Gas Halogen Group Alkali Metal Alkali Earth Metal Period

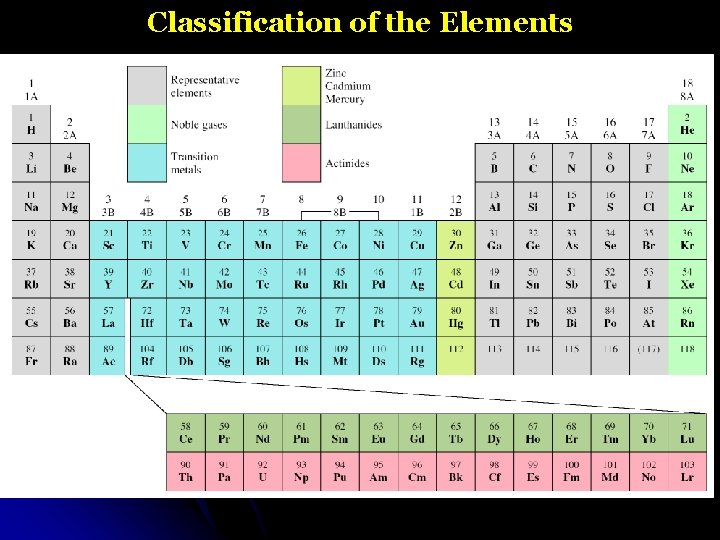
Classification of the Elements
![Electron Configurations of Cations and Anions Of Representative Elements Na Ne3 s 1 Na Electron Configurations of Cations and Anions Of Representative Elements Na: [Ne]3 s 1 Na+](https://slidetodoc.com/presentation_image_h2/b8d83364f1a8340074d535a78d53f886/image-6.jpg)
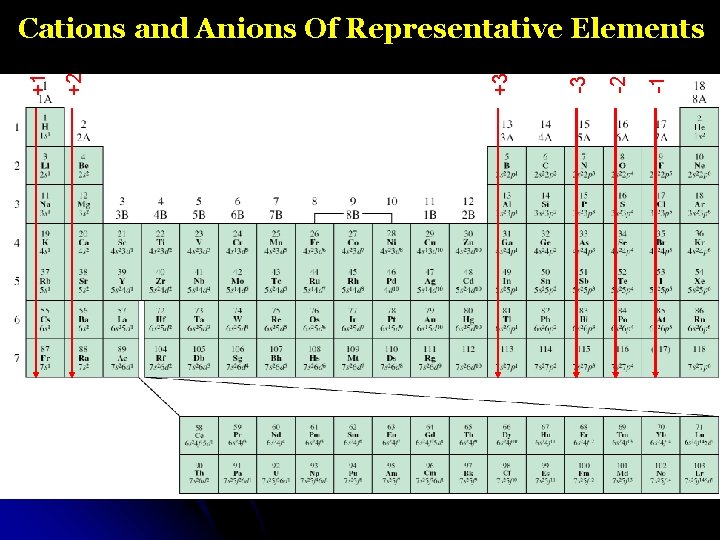
Electron Configurations of Cations and Anions Of Representative Elements Na: [Ne]3 s 1 Na+ [Ne] Ca: [Ar]4 s 2 Ca 2+ [Ar] Al: [Ne]3 s 23 p 1 Al 3+ [Ne] H: 1 s 1 Atoms lose electrons so that cation has a noble-gas outer electron configuration. H- 1 s 2 or [He] Atoms gain electrons so - 1 s 22 p 6 or [Ne] 22 s 22 p 5 F: 1 s F that anion has a noblegas outer electron O: 1 s 22 p 4 O 2 - 1 s 22 p 6 or [Ne] configuration. N: 1 s 22 p 3 N 3 - 1 s 22 p 6 or [Ne]

-1 -2 -3 +3 +2 +1 Cations and Anions Of Representative Elements

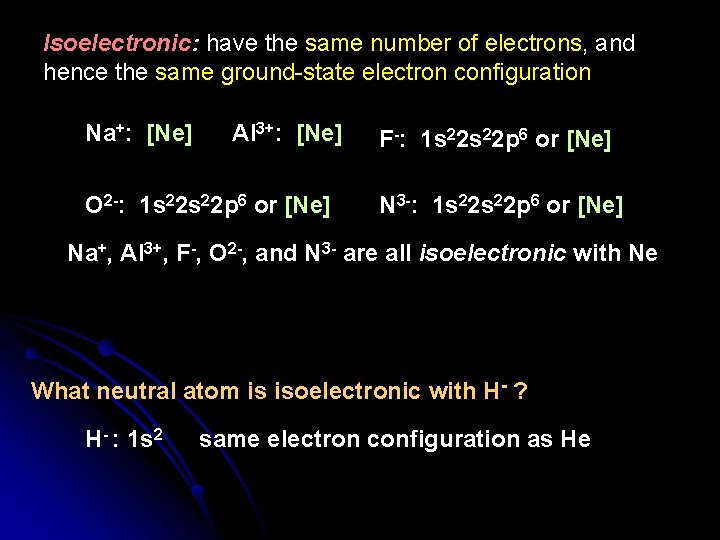
Isoelectronic: have the same number of electrons, and hence the same ground-state electron configuration Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] N 3 -: 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne What neutral atom is isoelectronic with H- ? H- : 1 s 2 same electron configuration as He

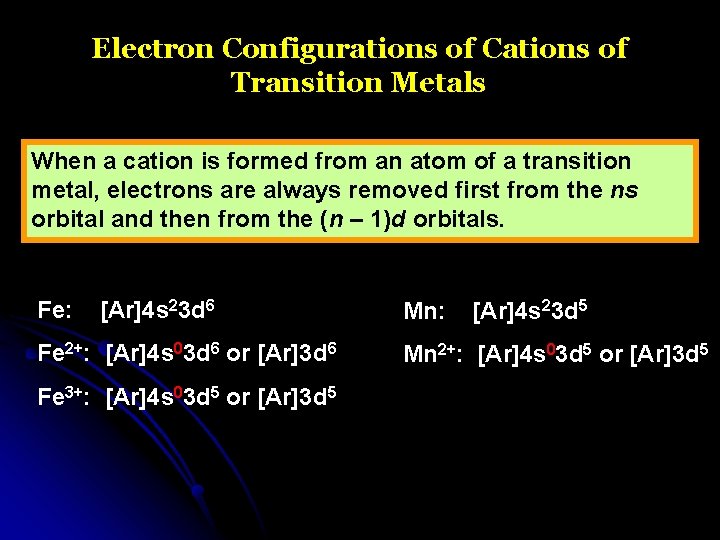
Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. Fe: [Ar]4 s 23 d 6 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Mn: [Ar]4 s 23 d 5 Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5

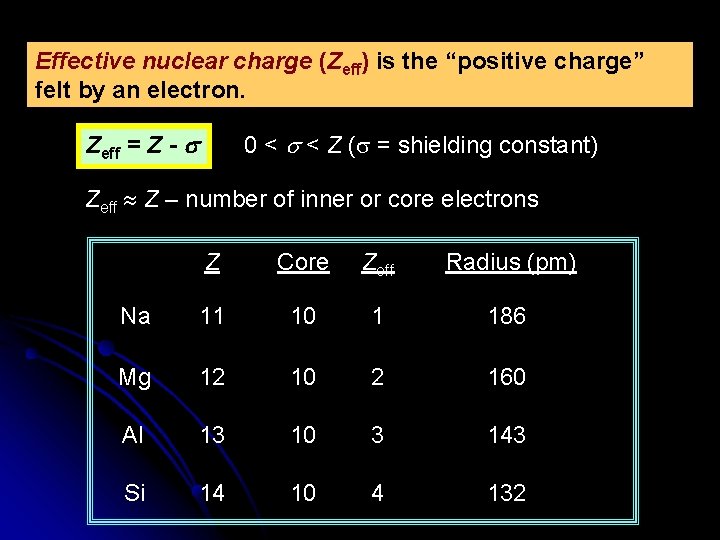
Effective nuclear charge (Zeff) is the “positive charge” felt by an electron. Zeff = Z - s 0 < s < Z (s = shielding constant) Zeff Z – number of inner or core electrons Z Core Zeff Radius (pm) Na 11 10 1 186 Mg 12 10 2 160 Al 13 10 3 143 Si 14 10 4 132

Effective Nuclear Charge (Zeff) increasing Zeff

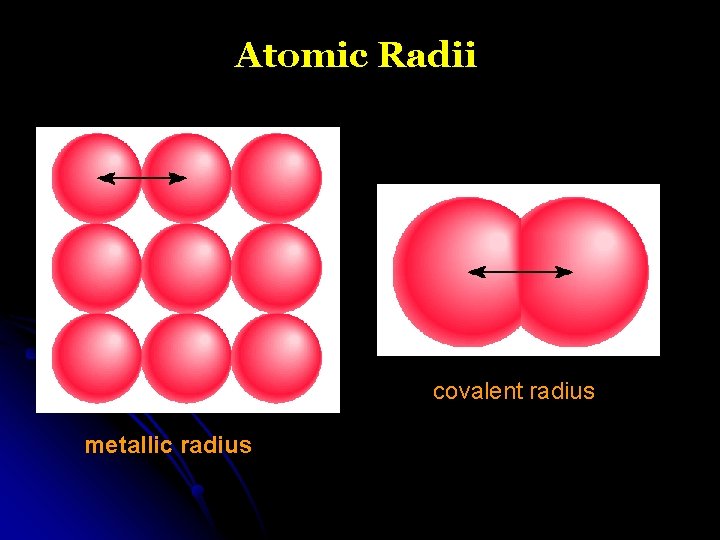
Atomic Radii covalent radius metallic radius


increasing atomic radius Decreasing atomic radius

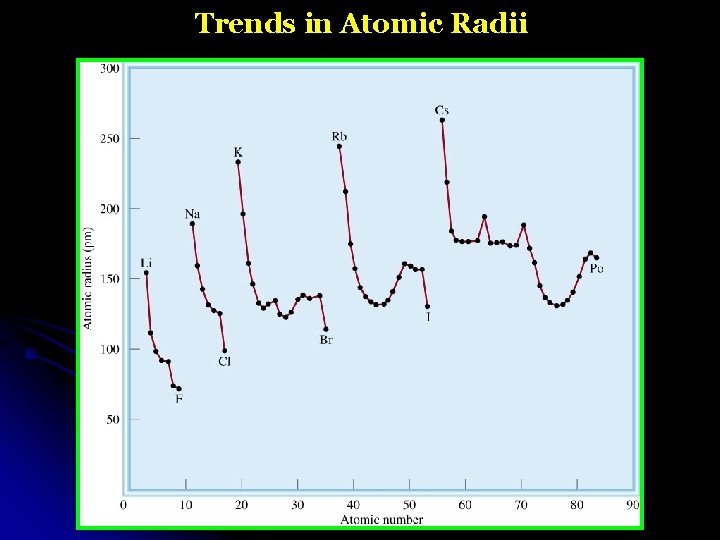
Trends in Atomic Radii

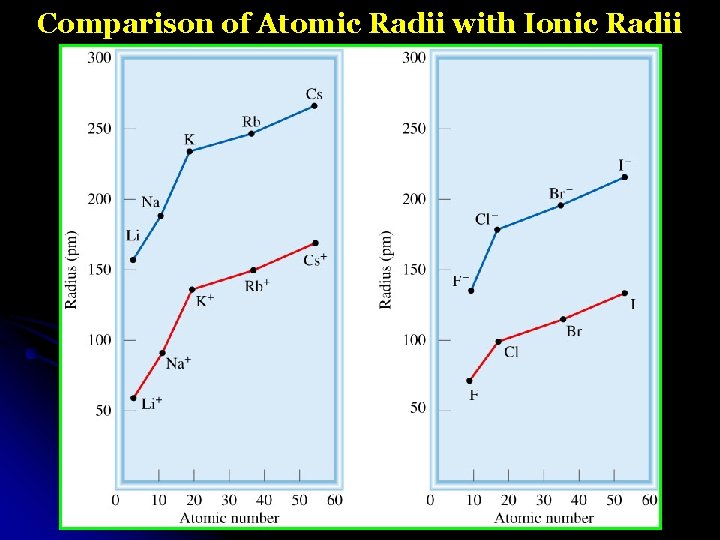
Comparison of Atomic Radii with Ionic Radii

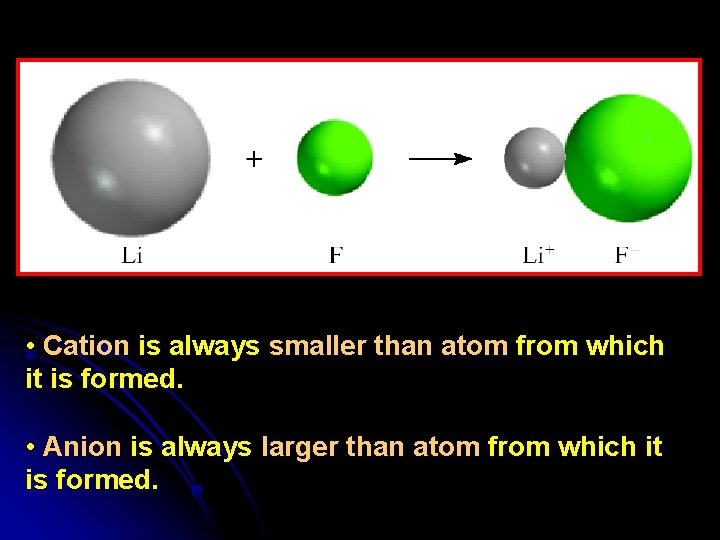
• Cation is always smaller than atom from which it is formed. • Anion is always larger than atom from which it is formed.

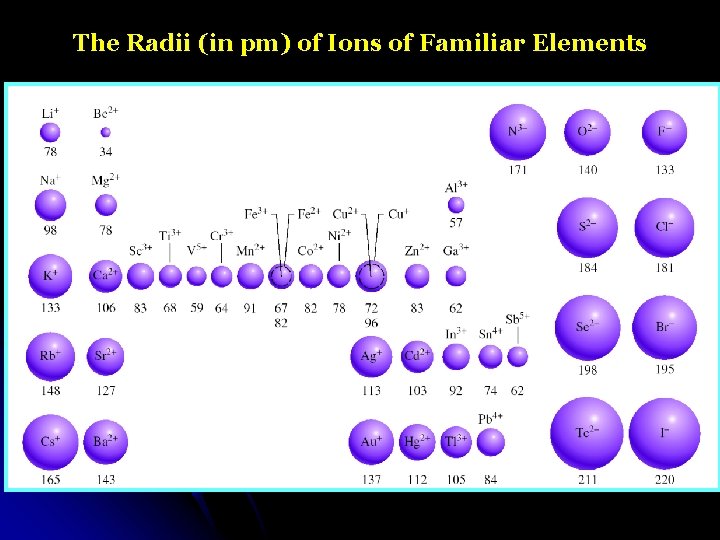
The Radii (in pm) of Ions of Familiar Elements



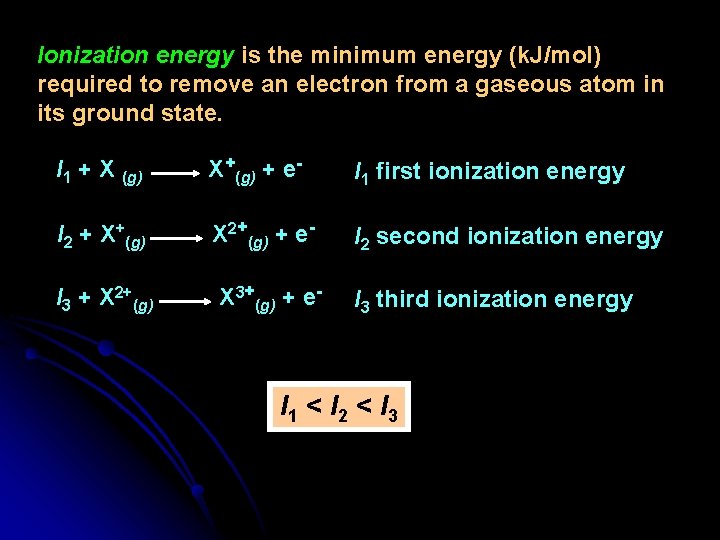
Ionization energy is the minimum energy (k. J/mol) required to remove an electron from a gaseous atom in its ground state. I 1 + X (g) X+(g) + e- I 1 first ionization energy I 2 + X+(g) X 2+(g) + e- I 2 second ionization energy I 3 + X 2+(g) X 3+(g) + e- I 3 third ionization energy I 1 < I 2 < I 3


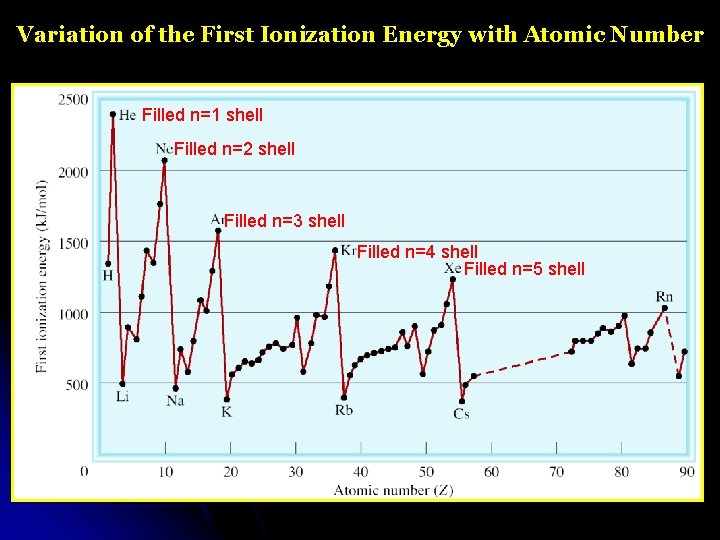
Variation of the First Ionization Energy with Atomic Number Filled n=1 shell Filled n=2 shell Filled n=3 shell Filled n=4 shell Filled n=5 shell

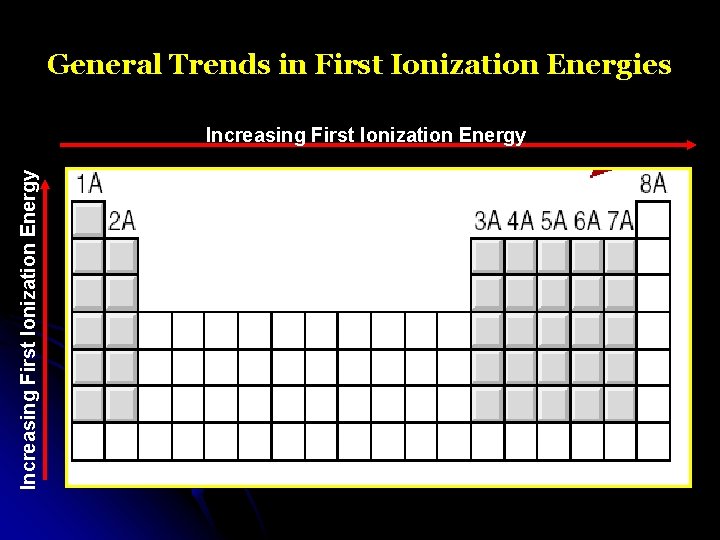
General Trends in First Ionization Energies Increasing First Ionization Energy


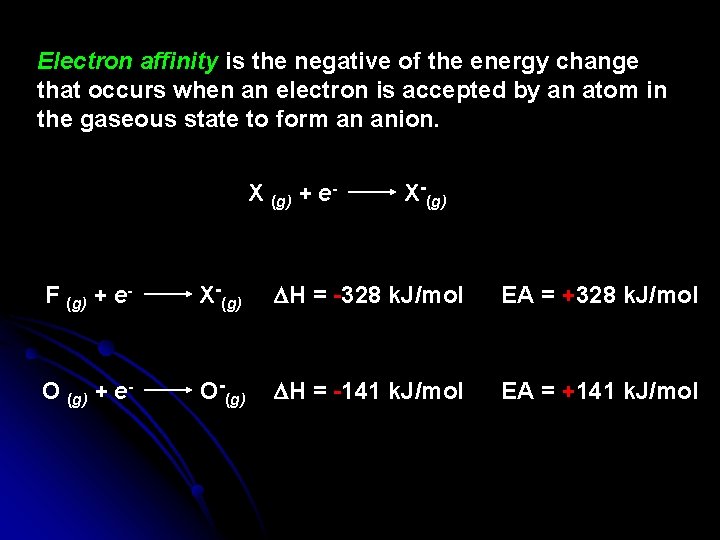
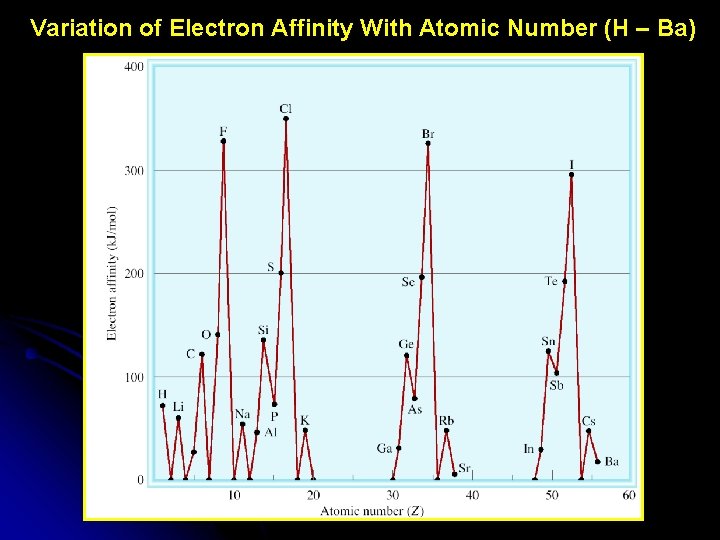
Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion. X (g) + e- X-(g) F (g) + e- X-(g) DH = -328 k. J/mol EA = +328 k. J/mol O (g) + e- O-(g) DH = -141 k. J/mol EA = +141 k. J/mol


Variation of Electron Affinity With Atomic Number (H – Ba)

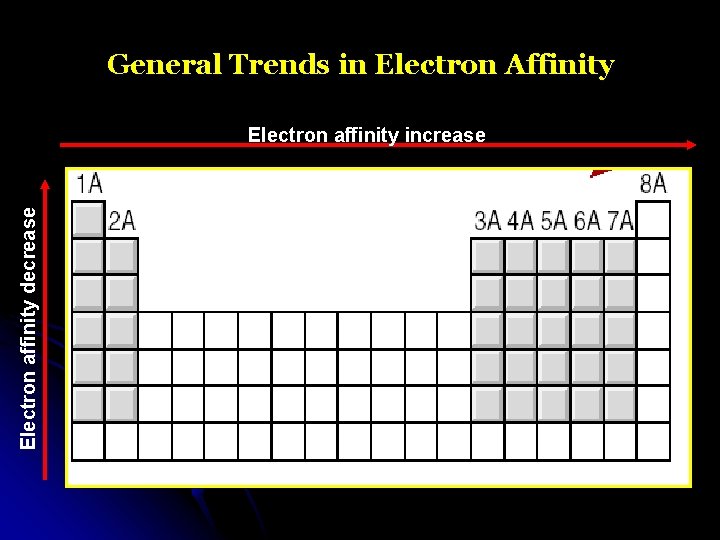
General Trends in Electron Affinity Electron affinity decrease Electron affinity increase

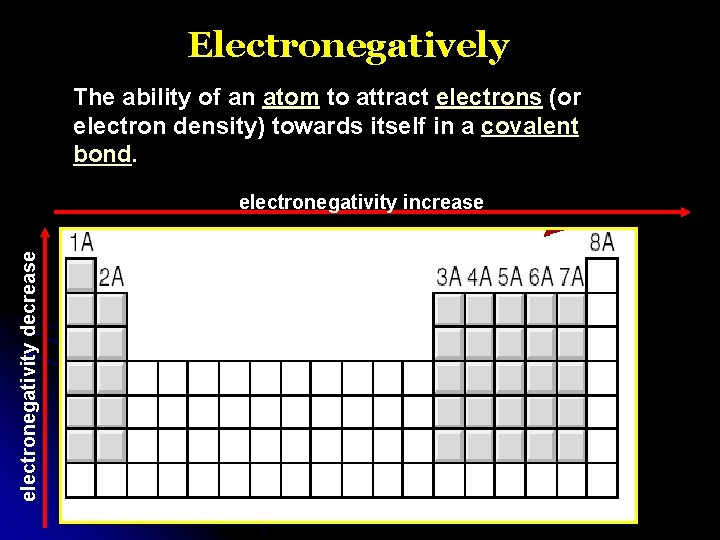
Electronegatively The ability of an atom to attract electrons (or electron density) towards itself in a covalent bond. electronegativity decrease electronegativity increase

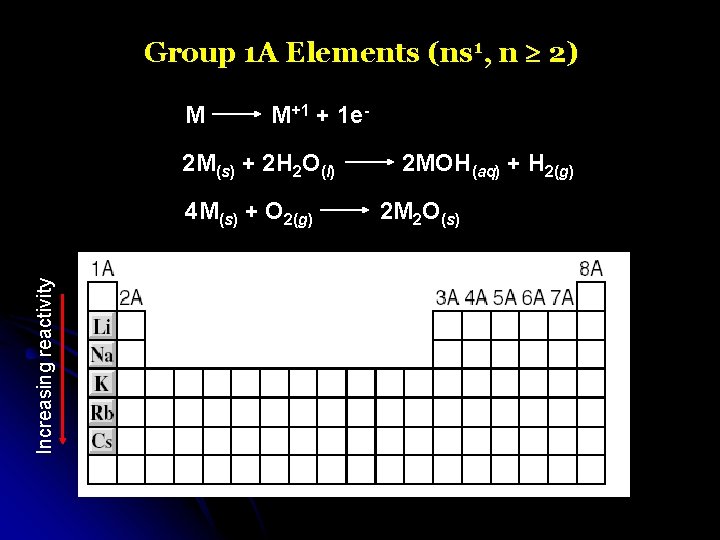
Group 1 A Elements (ns 1, n 2) M M+1 + 1 e- 2 M(s) + 2 H 2 O(l) Increasing reactivity 4 M(s) + O 2(g) 2 MOH(aq) + H 2(g) 2 M 2 O(s)

Group 1 A Elements (ns 1, n 2)

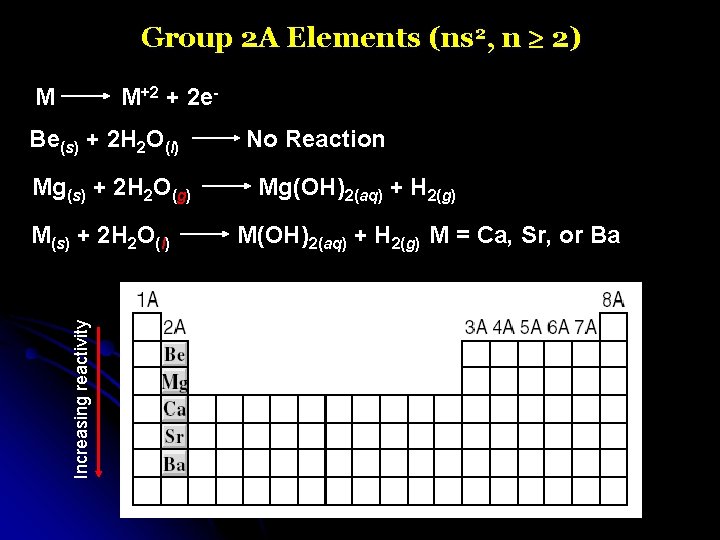
Group 2 A Elements (ns 2, n 2) M M+2 + 2 e- Be(s) + 2 H 2 O(l) Mg(s) + 2 H 2 O(g) Increasing reactivity M(s) + 2 H 2 O(l) No Reaction Mg(OH)2(aq) + H 2(g) M = Ca, Sr, or Ba

Group 2 A Elements (ns 2, n 2)

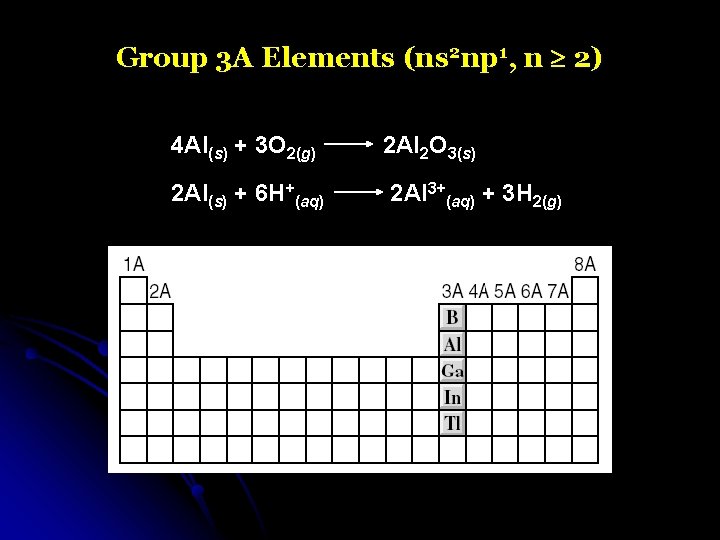
Group 3 A Elements (ns 2 np 1, n 2) 4 Al(s) + 3 O 2(g) 2 Al(s) + 6 H+(aq) 2 Al 2 O 3(s) 2 Al 3+(aq) + 3 H 2(g)

Group 3 A Elements (ns 2 np 1, n 2)

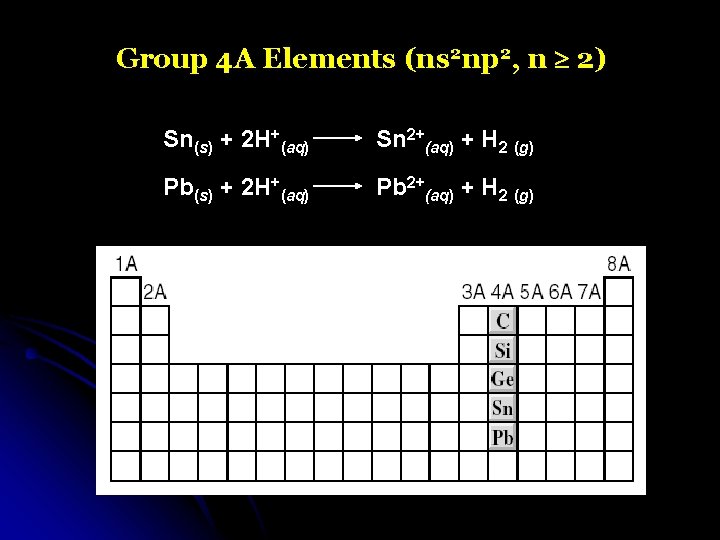
Group 4 A Elements (ns 2 np 2, n 2) Sn(s) + 2 H+(aq) Sn 2+(aq) + H 2 (g) Pb(s) + 2 H+(aq) Pb 2+(aq) + H 2 (g)

Group 4 A Elements (ns 2 np 2, n 2)

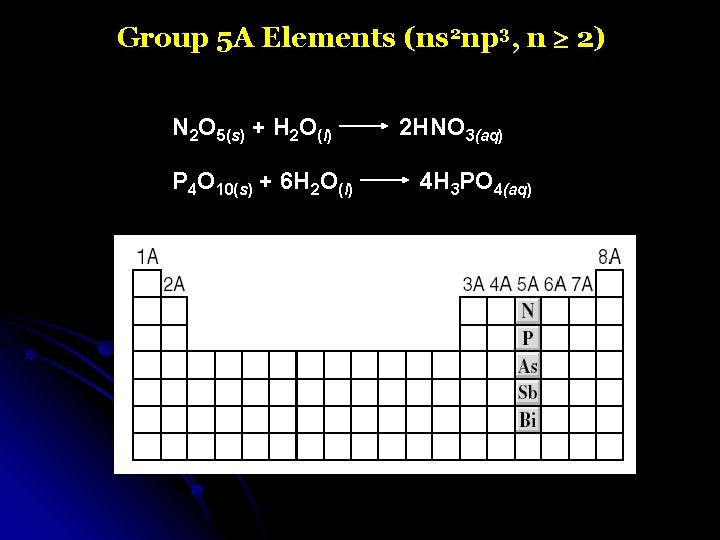
Group 5 A Elements (ns 2 np 3, n 2) N 2 O 5(s) + H 2 O(l) P 4 O 10(s) + 6 H 2 O(l) 2 HNO 3(aq) 4 H 3 PO 4(aq)

Group 5 A Elements (ns 2 np 3, n 2)

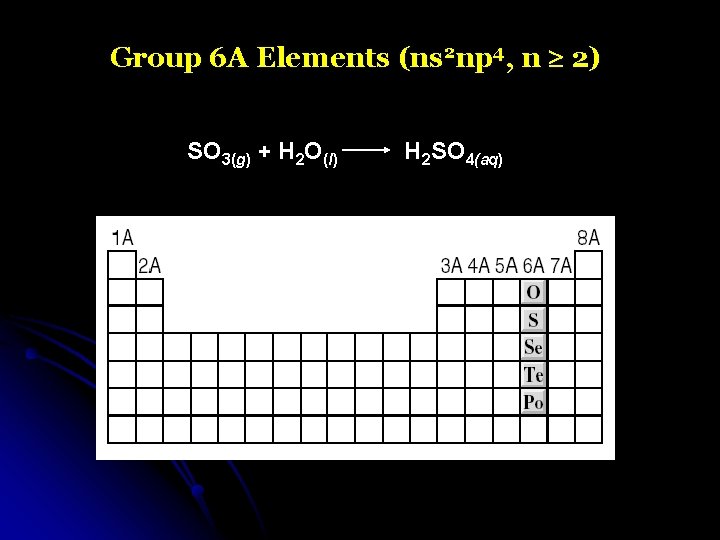
Group 6 A Elements (ns 2 np 4, n 2) SO 3(g) + H 2 O(l) H 2 SO 4(aq)

Group 6 A Elements (ns 2 np 4, n 2)

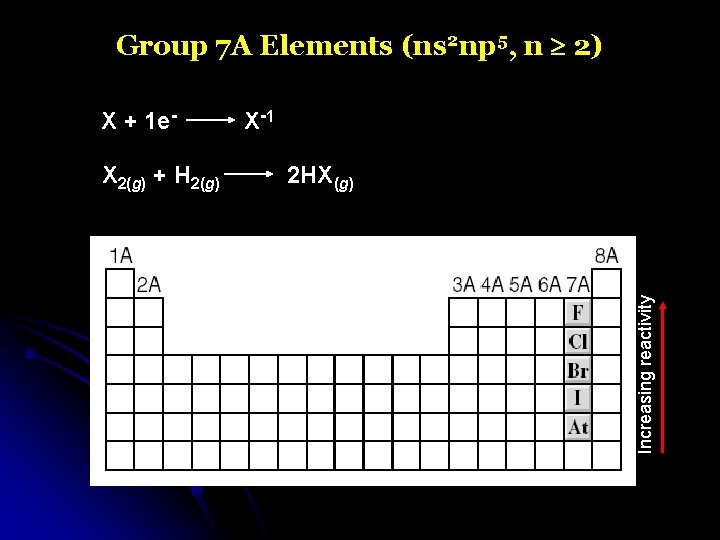
Group 7 A Elements (ns 2 np 5, n 2) X 2(g) + H 2(g) X -1 2 HX(g) Increasing reactivity X + 1 e-

Group 7 A Elements (ns 2 np 5, n 2)

Group 8 A Elements (ns 2 np 6, n 2) Completely filled ns and np subshells. Highest ionization energy of all elements. No tendency to accept extra electrons.

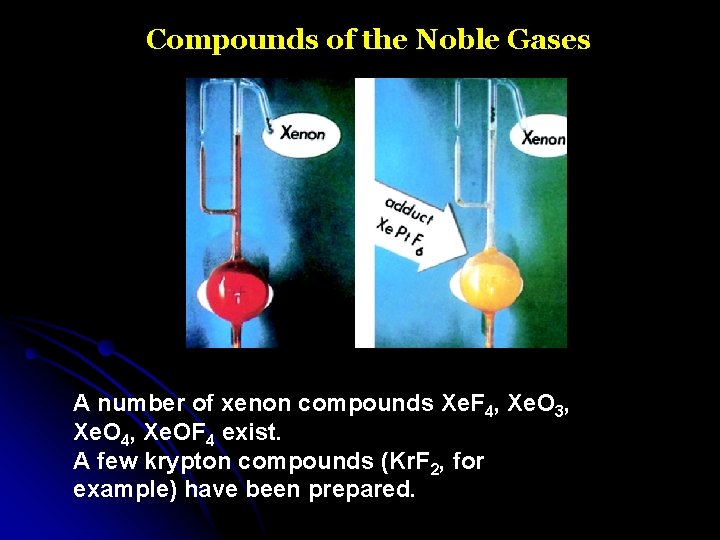
Compounds of the Noble Gases A number of xenon compounds Xe. F 4, Xe. O 3, Xe. O 4, Xe. OF 4 exist. A few krypton compounds (Kr. F 2, for example) have been prepared.

Comparison of Group 1 A and 1 B The metals in these two groups have similar outer electron configurations, with one electron in the outermost s orbital. Chemical properties are quite different due to difference in the ionization energy. Lower I 1, more reactive