Topic 4 Classifying Elements Elements All elements were























- Slides: 40

Topic 4 – Classifying Elements

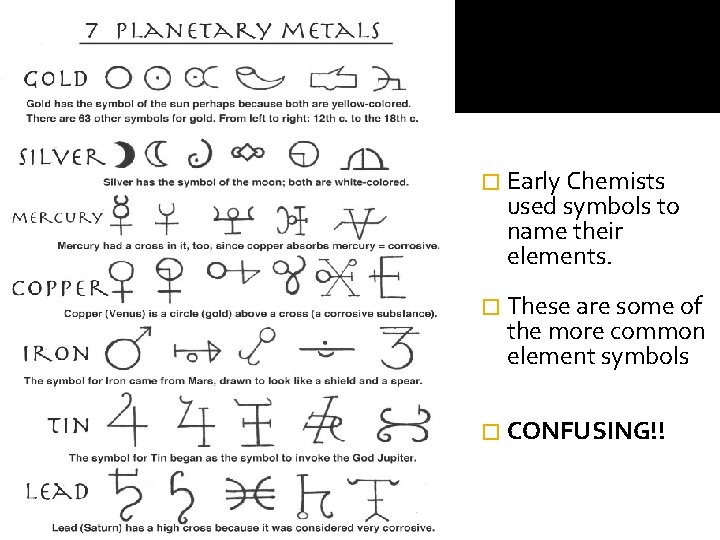
Elements �All elements were named as they were discovered. The person who discovered the elements got to choose how it was named. �Just think… Watsonium – has a nice ring to it

Naming Elements � Most people named their discovered elements after: Latin/Greek based on their properties Themselves Place of discovery, country or planets From the mineral in which it was found Named to honor a place or person

� Early Chemists used symbols to name their elements. � These are some of the more common element symbols � CONFUSING!!

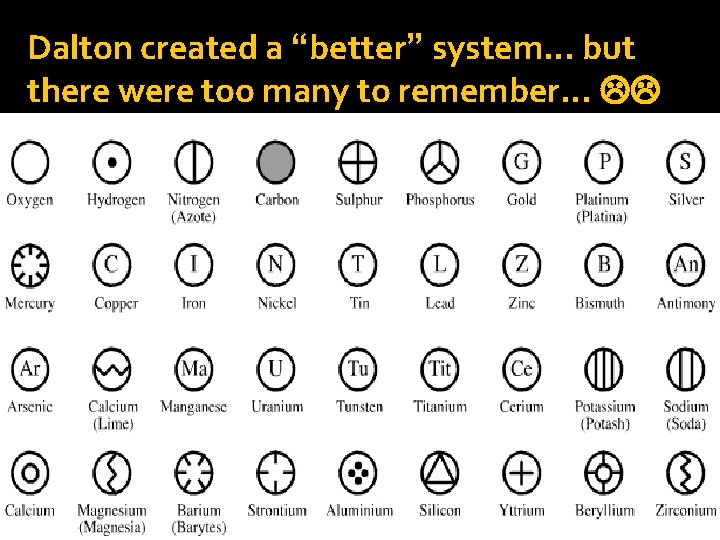
Dalton created a “better” system… but there were too many to remember…

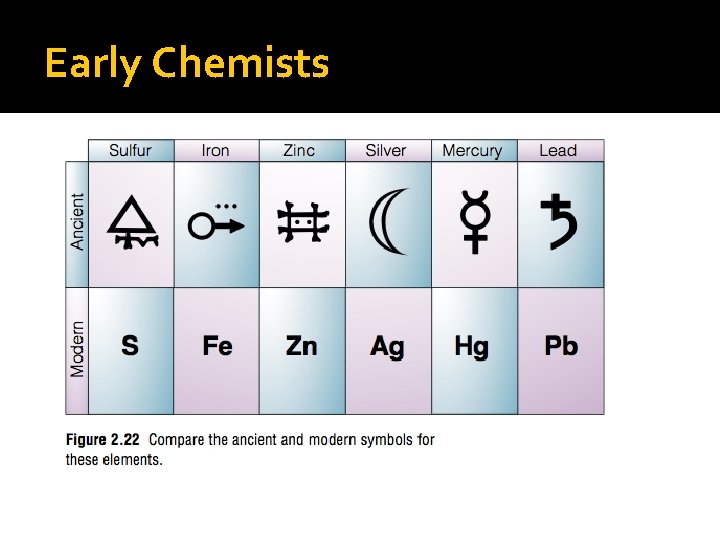
Early Chemists

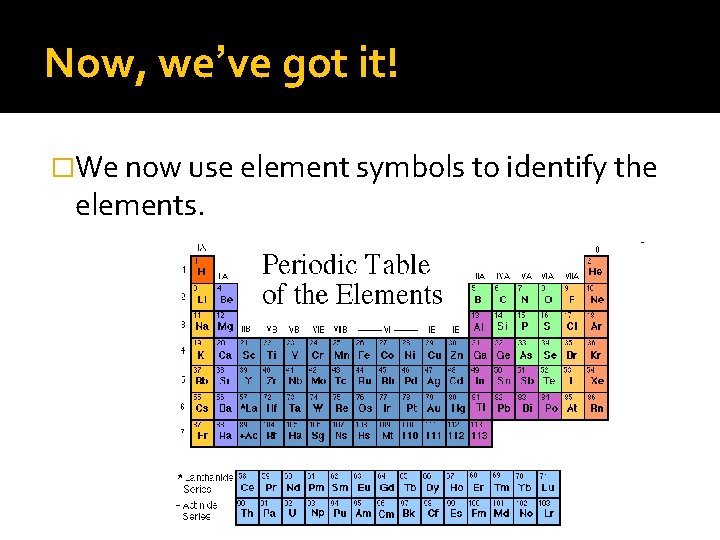
Now, we’ve got it! �We now use element symbols to identify the elements.

New Elements!

�Nihonium - symbol Nh, for the element 113, �Moscovium - symbol Mc, for the element 115, �Tennessine - symbol Ts, for the element 117, and. �Oganesson - symbol Og, for the element 118. http: //www. nydailynews. com/news/national/na mes-origin-stories-new-elements-article 1. 2667280

The current names came from…. � The FIRST initial of the common name H for Hydrogen O for Oxygen C for Carbon � The FIRST TWO letters (or 1 st and 3 rd) of the common name Mg for Magnesium Al for Aluminum � Initials of the Latin name Cu for Copper (Cuprum) Au for Gold (Aurum)

Elements 104 to 109 �were created by laboratories in the USA, Germany and Russia over the last two decades (all discovered 1977 -1997) �Only a few atoms of these elements ever existed and none survived after its creation for more than a few seconds before decaying radioactively into atomic debris.

Recognize any of these? � � � 104 -Rutherfordium (Rd) 105 -Dubnium (Db) 106 -Seaborgium (Sg) 107 -Bohroum (Bh) 108 -Hassium (Hs)

HUGE MESS �Because so many people made these elements there were HUGE arguments about who would name them. �IUPAC (International Union of Pure and Applied Chemistry) eventually came up with the names in 1997. �There is as of this week debate over what to name elements the 4 newest elements.

Synthentic Elements �A synthetic element is an element that does not occur in nature. are produced by converting a lighter element into a heavier one

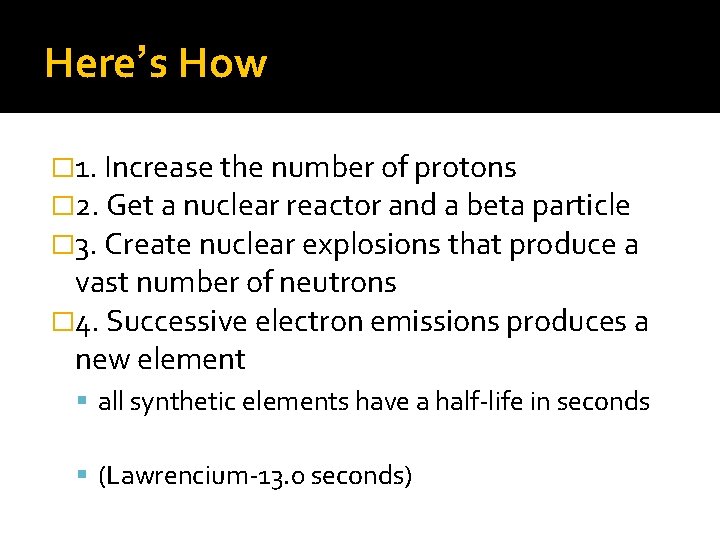
Here’s How � 1. Increase the number of protons � 2. Get a nuclear reactor and a beta particle � 3. Create nuclear explosions that produce a vast number of neutrons � 4. Successive electron emissions produces a new element all synthetic elements have a half-life in seconds (Lawrencium-13. 0 seconds)

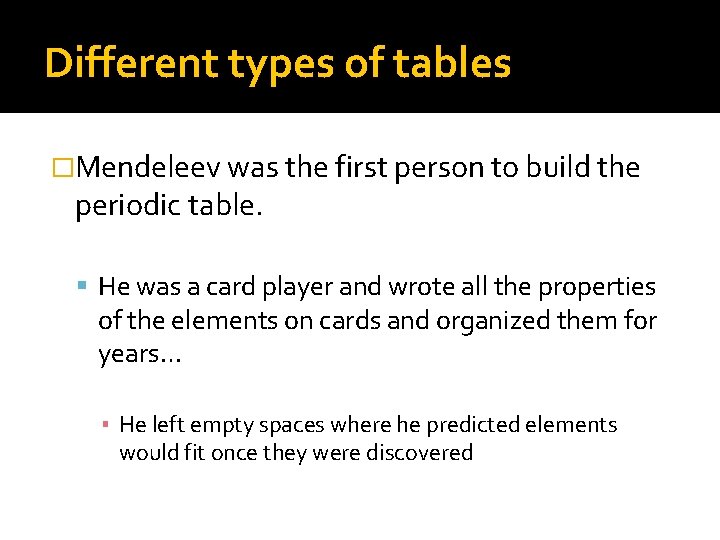
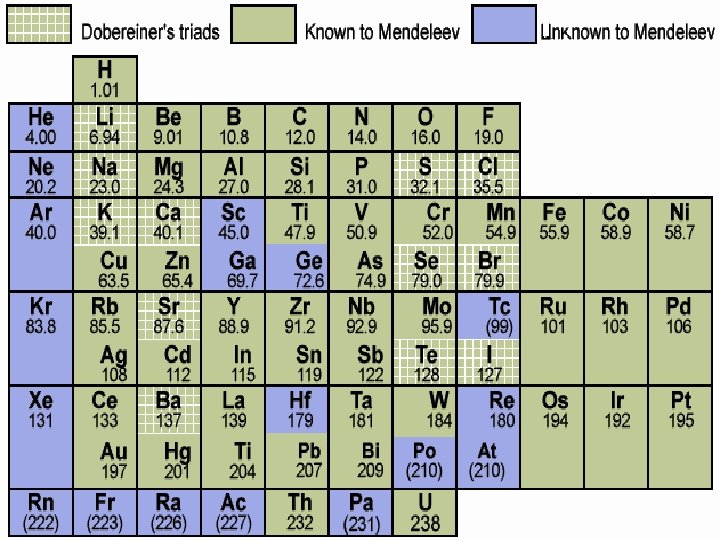
Different types of tables �Mendeleev was the first person to build the periodic table. He was a card player and wrote all the properties of the elements on cards and organized them for years… ▪ He left empty spaces where he predicted elements would fit once they were discovered


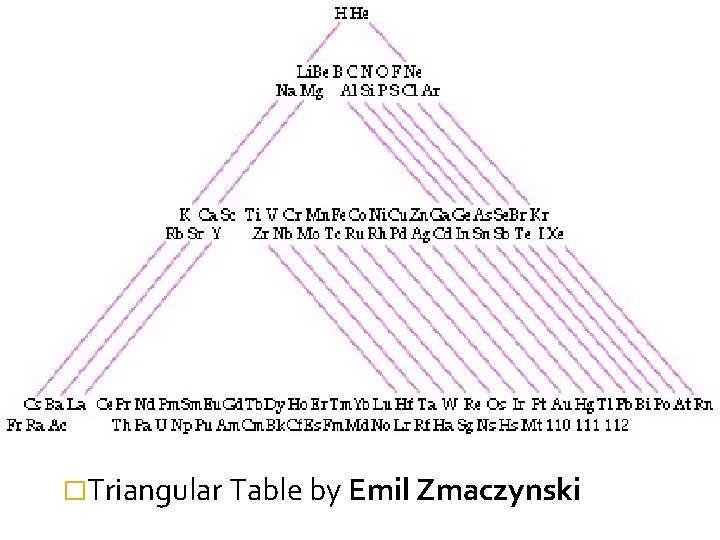
�Triangular Table by Emil Zmaczynski

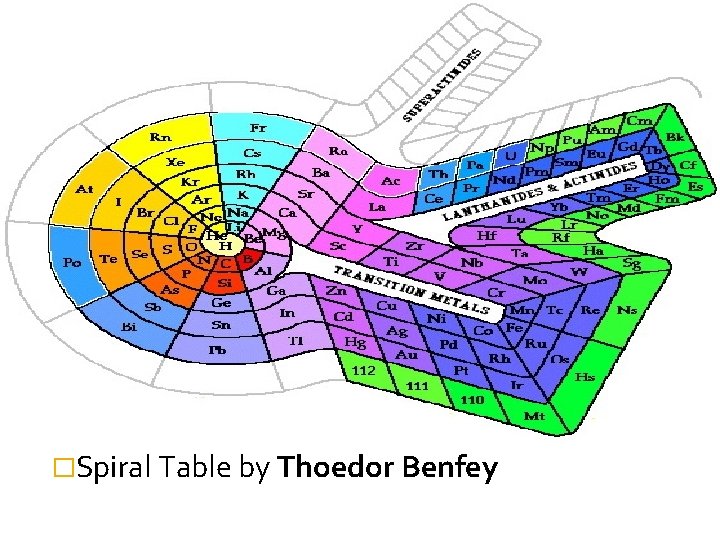
�Spiral Table by Thoedor Benfey

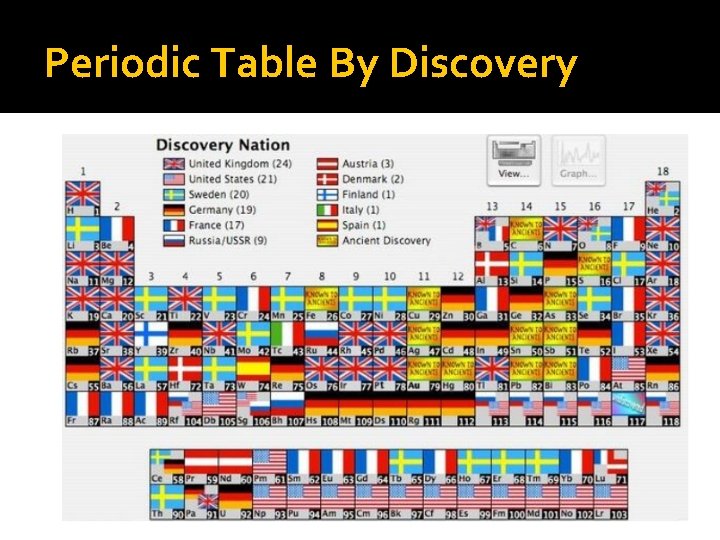
Periodic Table By Discovery

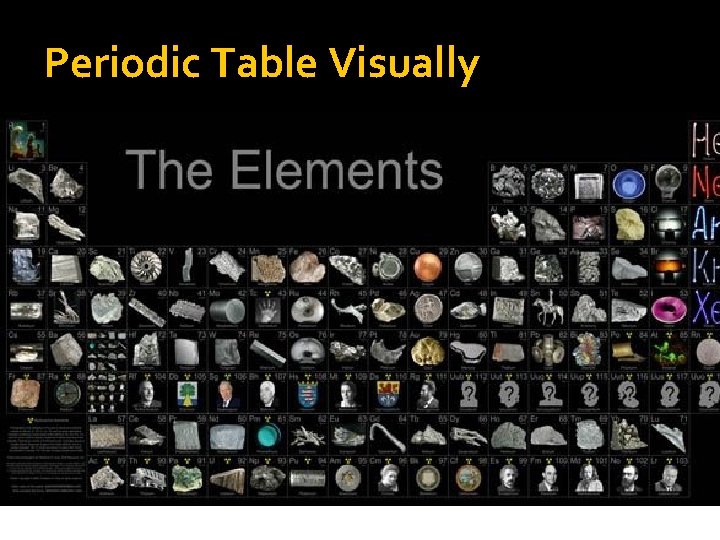
Periodic Table Visually

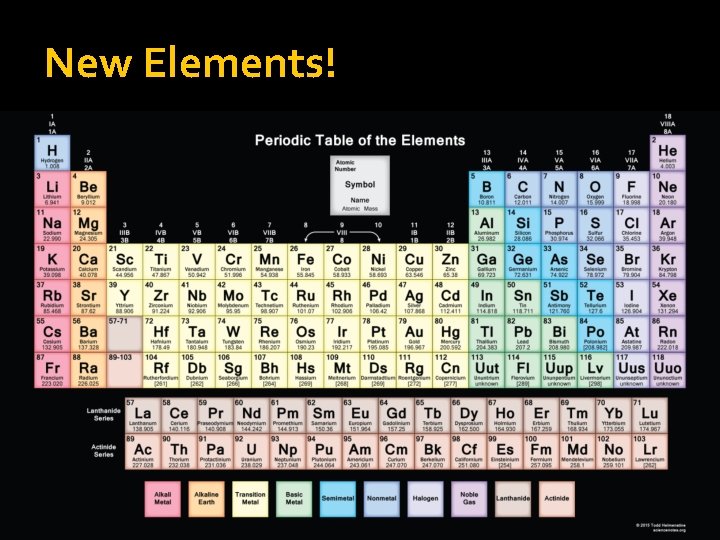
Atomic Number �The Atomic number indicates the number of protons in the nucleus of an atom. The biggest, and most obvious number on the table (and they go in order, 1, 2, 3, 4, …)

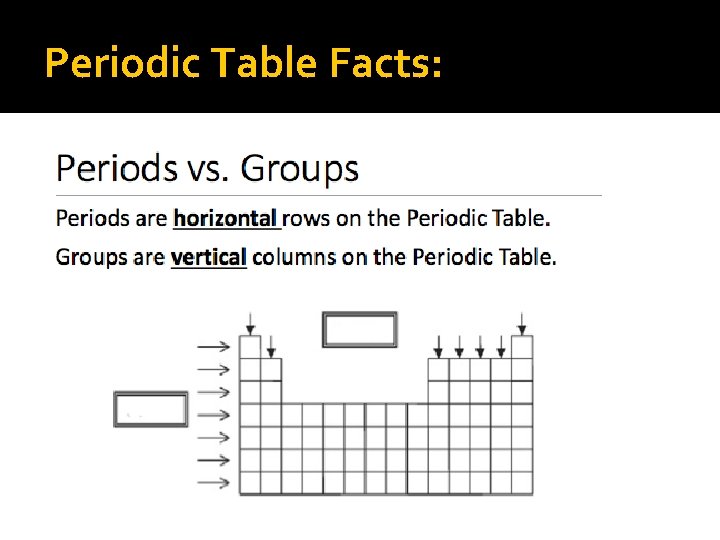
Periodic Table Facts: As you go across the horizontal rows or PERIODS the atomic number increases by one � The vertical columns are called groups and are also known as FAMILIES � (each family has the same number of electrons in their outer shell)

Periodic Table Facts:

Metals

Non-Metals

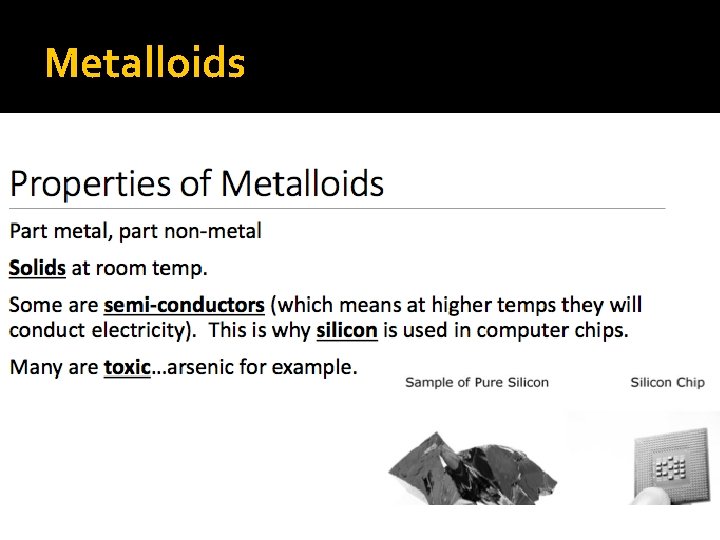
Metalloids

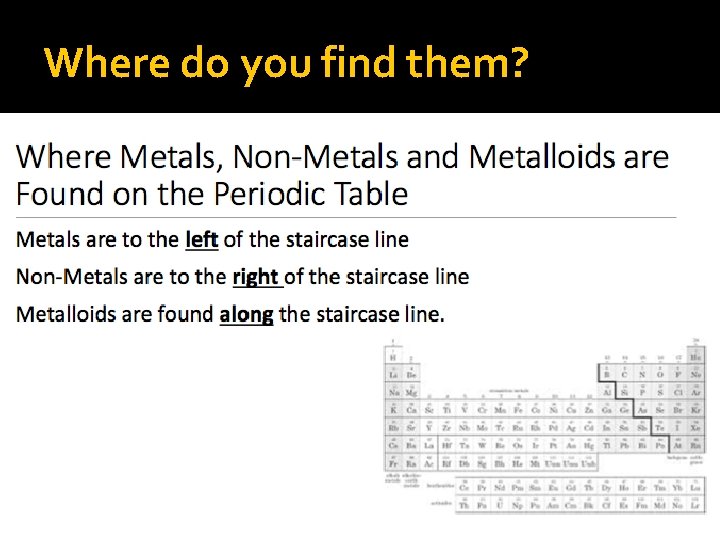
Where do you find them?

Where do you find them?

Families of the Periodic Table �You will be able to locate major families of the periodic table.

Alkali Metals Family �Found in Group/Family 1 Li, Na, K, Rb, Cs, Fr �Soft, silvery, white metals �Very malleable �Good conductors of heat �REACT HIGHLY WITH WATER

Alkali Metals Family �CANNOT BE FOUND IN NATURE IN A PURE STATE �Alkali Metals Video �Have one electron in their outer shell (group 1 = 1 electron)

Alkaline Earth Metals Family

Alkaline Earth Metals Family �Found in group 2 �Silvery, white metals �Soft but not as soft as the Alkali metals �Not as reactive as Alkali Metals so can be found in nature in a pure state. �Has 2 electrons in the outer shell (group 2 = 2 electrons)

Alkaline Earth Metals Family �Alkali and Alkaline Earth metals burn very bright colors. Potassium (K) Barium (Ba) Sodium (Na) Calcium (Ca) Magnesium (Mg)

The Noble Gases

The Noble Gases �INCLUDE: He, Ne, Ar, Kr, Xe, Rn �Un-reactive �All are Gases �Odorless, tasteless and colorless �Outer Shell is full of electrons �Noble Gases and Balloons

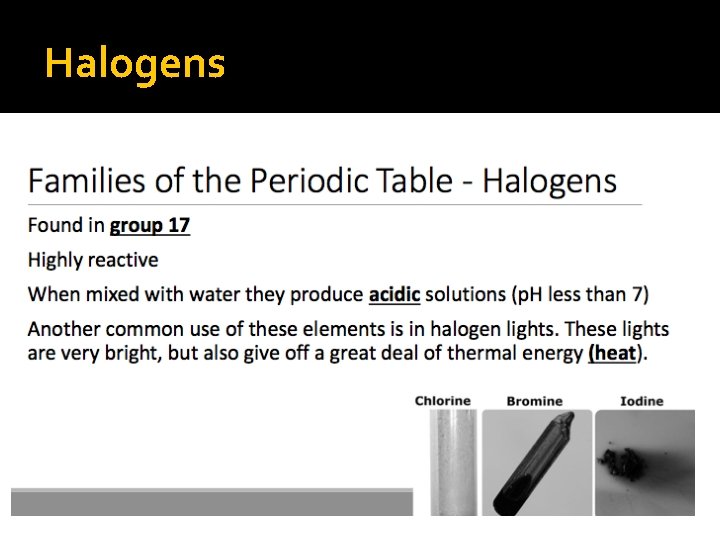
Halogens

Halogens � INCLUDE: F, Cl, Br, I, At � Gas, Liquids and Solids � Extremely corrosive and harmful � Poisonous � Naturally found in the form of compounds because they react with almost every other element � Compounds formed are useful (salt, toothpaste) � Have 7 unpaired electrons in the outer shell � physical properties of Halogens

Transition Metals �There are 38 elements in groups 3 -12 called the Transition Metals � 3 elements that are important in this group are nickel, cobalt and iron These are the ONLY elements known to produce a magnetic field