Chapter 22 The Organic Chemistry of Amino Acids























- Slides: 75

Chapter 22 The Organic Chemistry of Amino Acids, Peptides, and Proteins Paula Yurkanis Bruice University of California, Santa Barbara © 2014 Pearson Education, Inc.

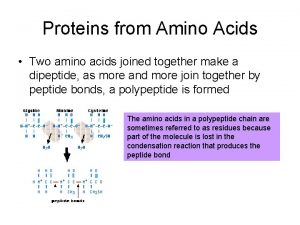
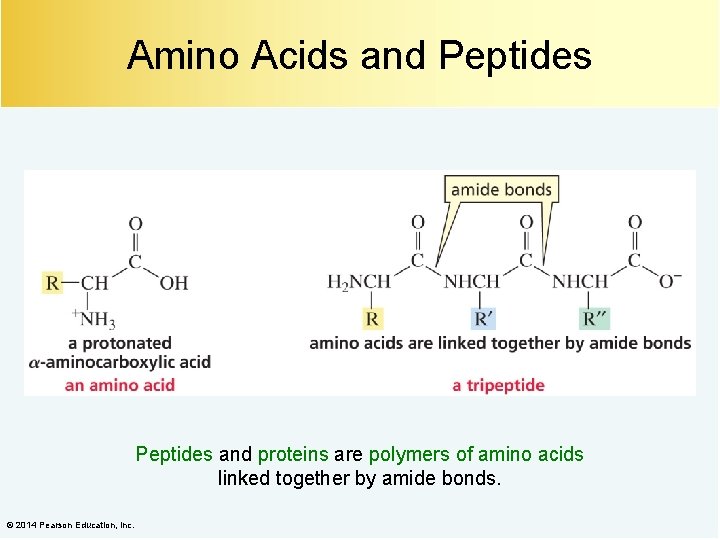
Amino Acids and Peptides and proteins are polymers of amino acids linked together by amide bonds. © 2014 Pearson Education, Inc.

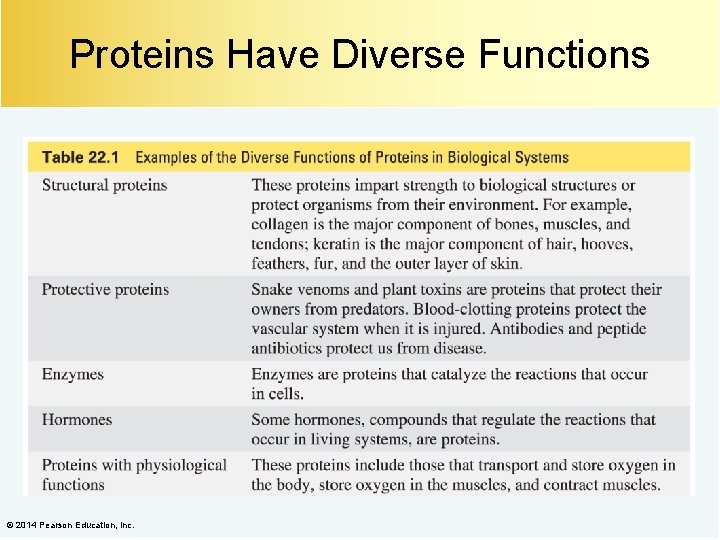
Proteins Have Diverse Functions © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

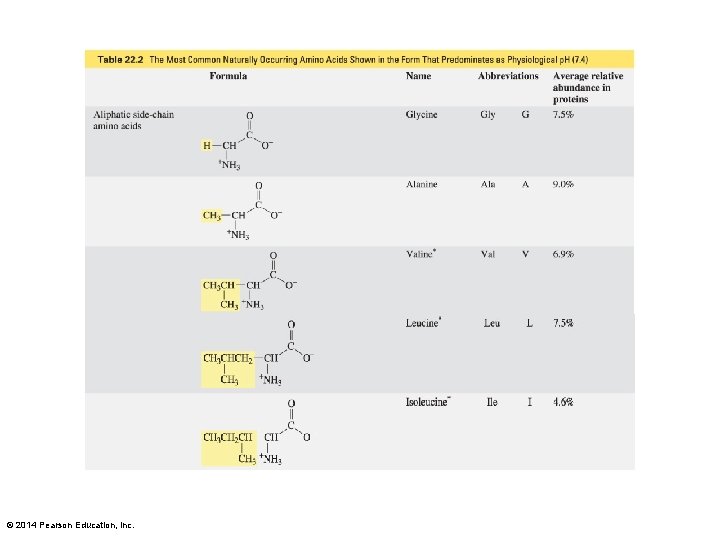
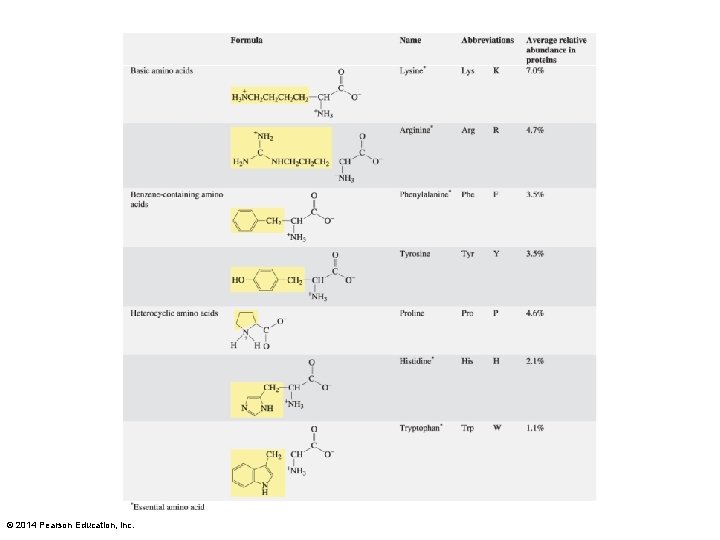
Lysine and Arginine The amino group is on the epsilon carbon. The guanidino group is on the delta carbon. © 2014 Pearson Education, Inc.

Histidine and Tryptophan Histidine is an imidazole-substituted alanine. Tryptophan is an indole-substituted alanine. © 2014 Pearson Education, Inc.

D-Sugars © 2014 Pearson Education, Inc. and L-Amino Acids

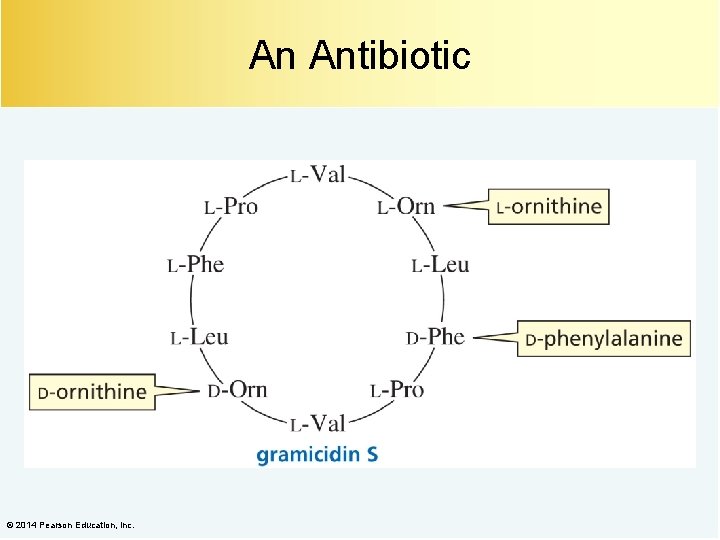
An Antibiotic © 2014 Pearson Education, Inc.

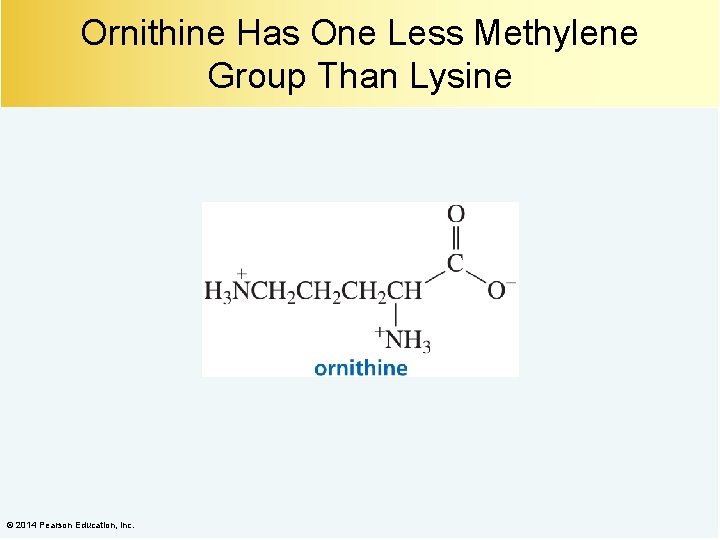
Ornithine Has One Less Methylene Group Than Lysine © 2014 Pearson Education, Inc.

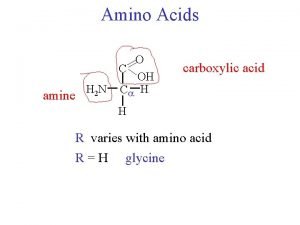
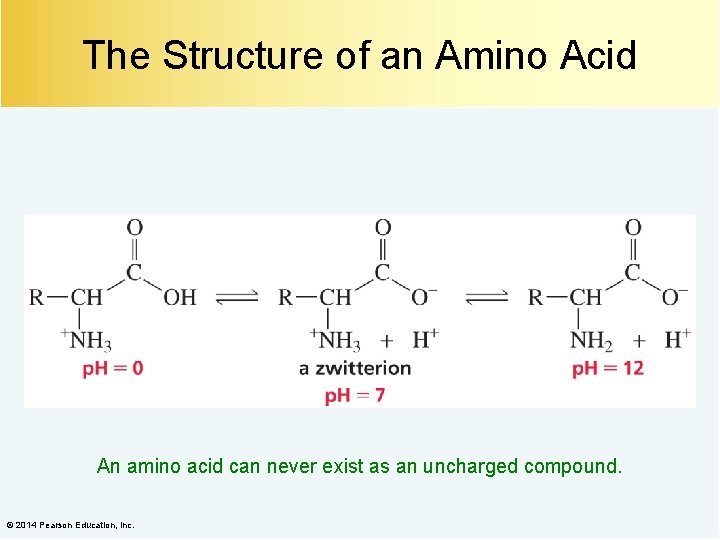
The Structure of an Amino Acid An amino acid can never exist as an uncharged compound. © 2014 Pearson Education, Inc.

© 2014 Pearson Education, Inc.

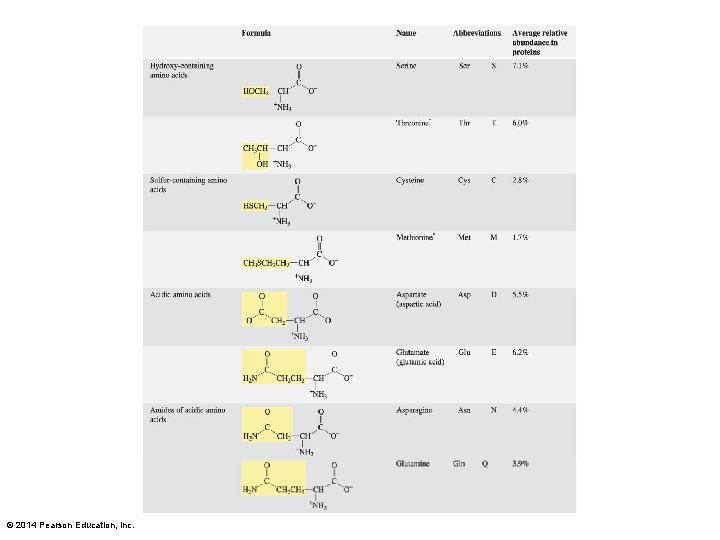
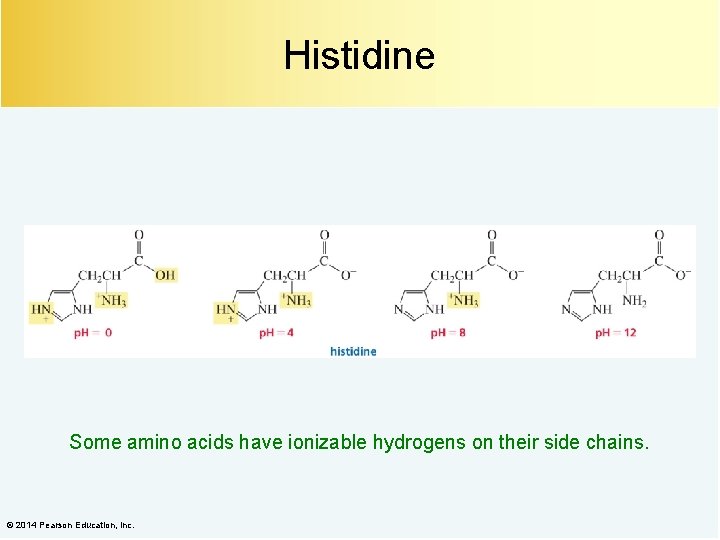
Histidine Some amino acids have ionizable hydrogens on their side chains. © 2014 Pearson Education, Inc.

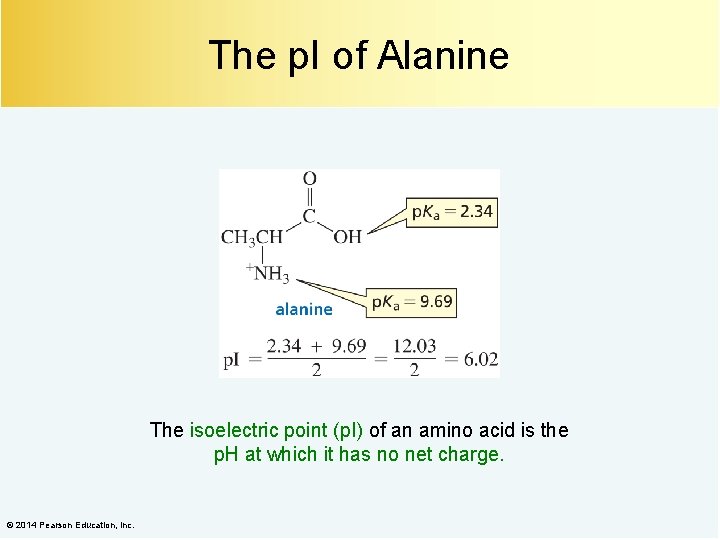
The p. I of Alanine The isoelectric point (p. I) of an amino acid is the p. H at which it has no net charge. © 2014 Pearson Education, Inc.

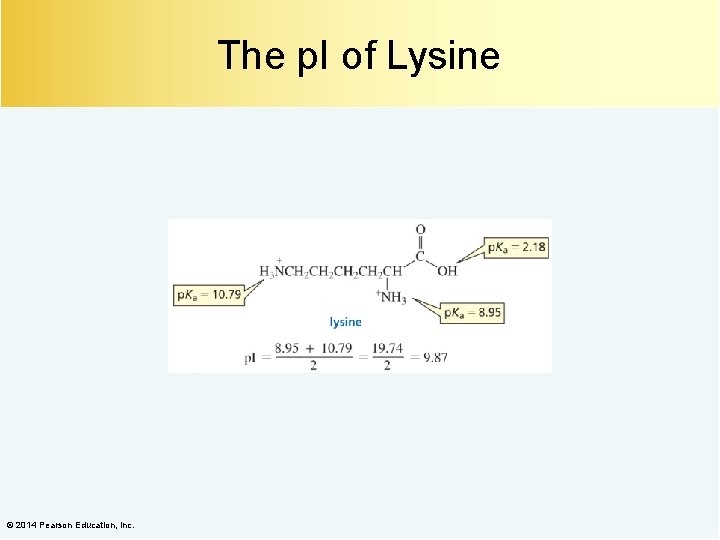
The p. I of Lysine © 2014 Pearson Education, Inc.

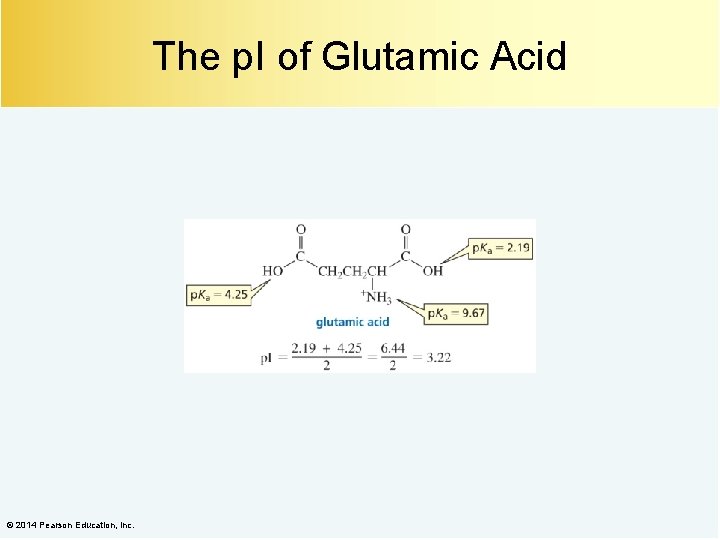
The p. I of Glutamic Acid © 2014 Pearson Education, Inc.

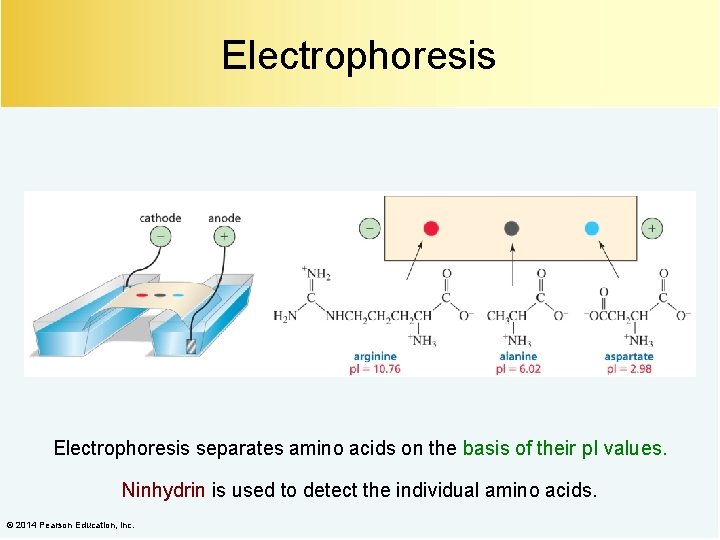
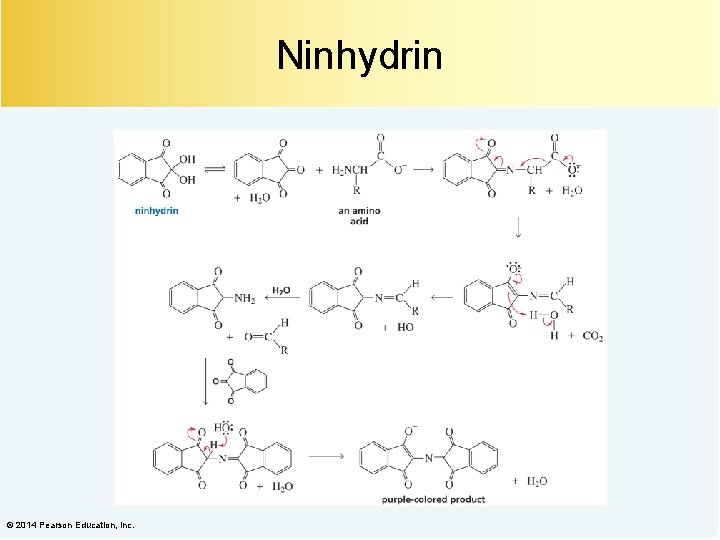
Electrophoresis separates amino acids on the basis of their p. I values. Ninhydrin is used to detect the individual amino acids. © 2014 Pearson Education, Inc.

Ninhydrin © 2014 Pearson Education, Inc.

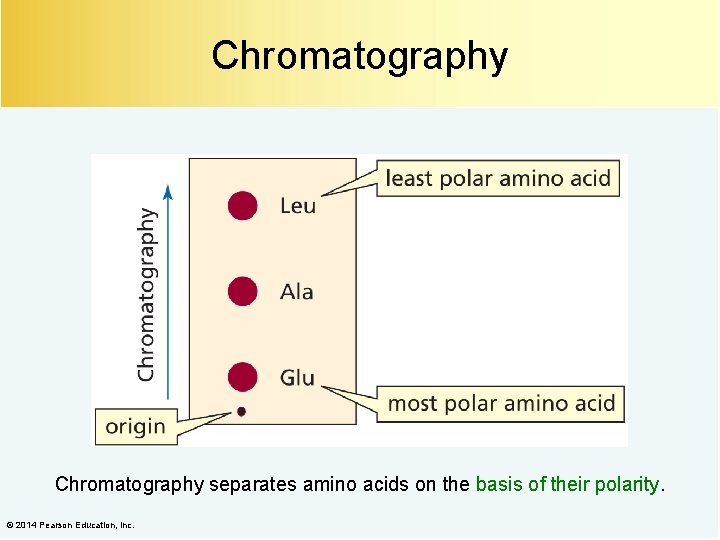
Chromatography separates amino acids on the basis of their polarity. © 2014 Pearson Education, Inc.

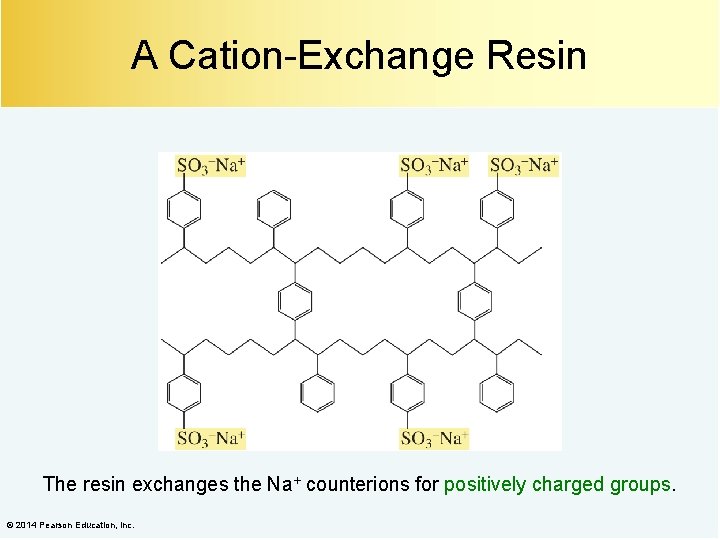
A Cation-Exchange Resin The resin exchanges the Na+ counterions for positively charged groups. © 2014 Pearson Education, Inc.

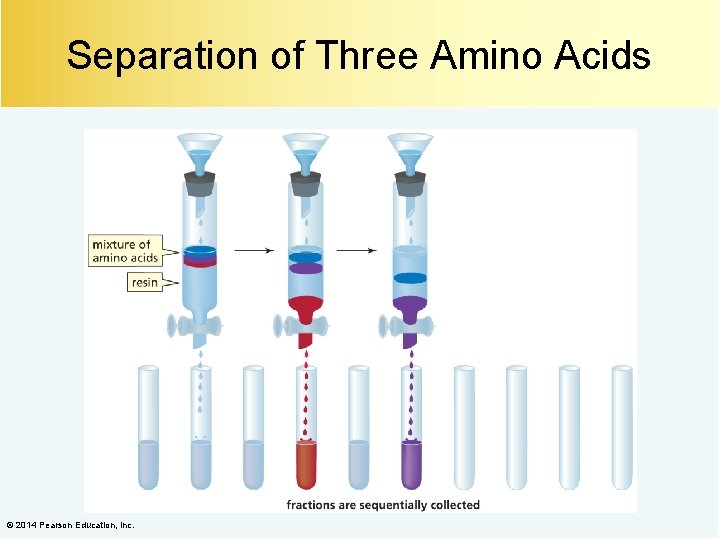
Separation of Three Amino Acids © 2014 Pearson Education, Inc.

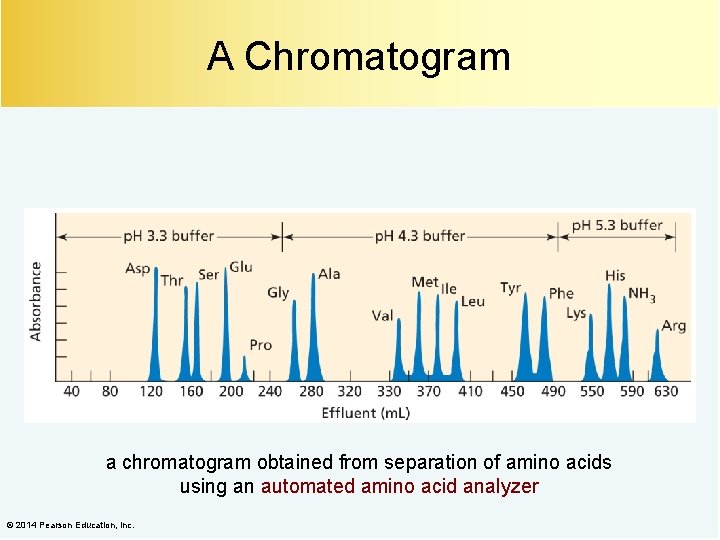
A Chromatogram a chromatogram obtained from separation of amino acids using an automated amino acid analyzer © 2014 Pearson Education, Inc.

Synthesizing an Amino Acid Using an HVZ Reaction © 2014 Pearson Education, Inc.

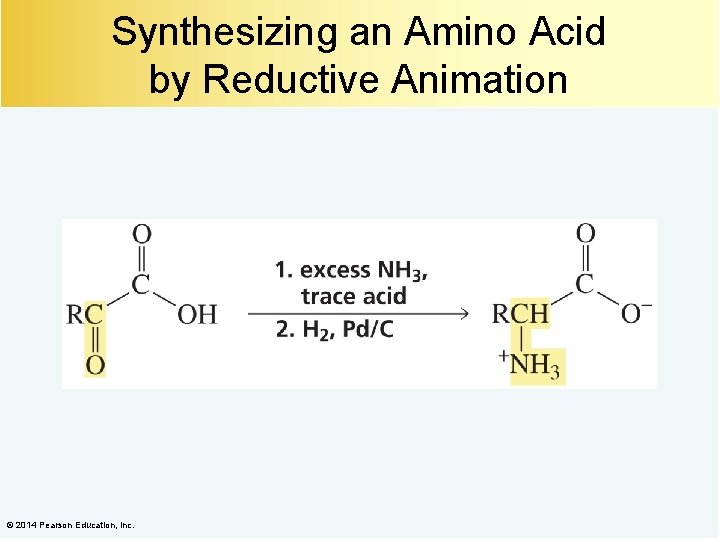
Synthesizing an Amino Acid by Reductive Animation © 2014 Pearson Education, Inc.

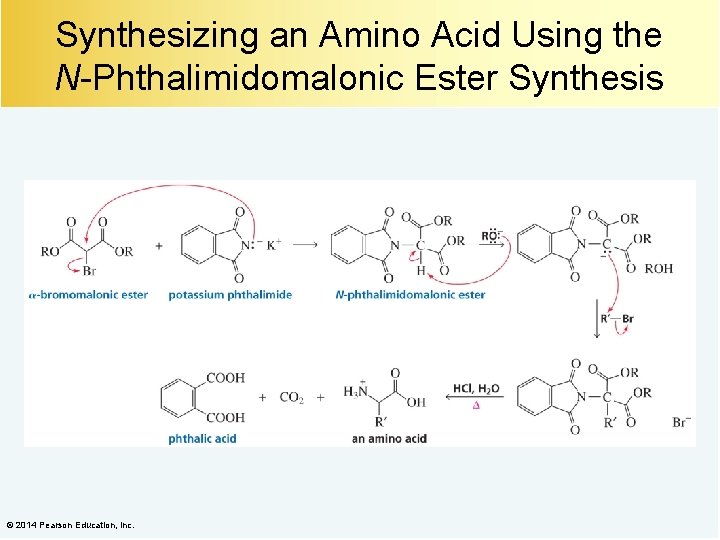
Synthesizing an Amino Acid Using the N-Phthalimidomalonic Ester Synthesis © 2014 Pearson Education, Inc.

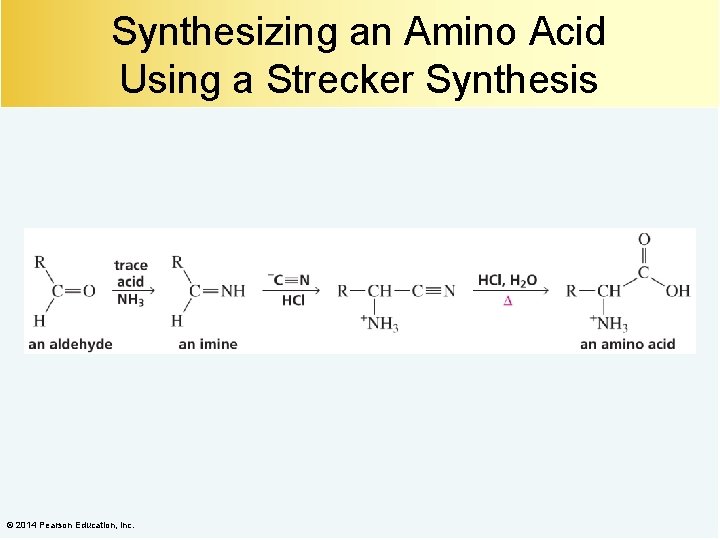
Synthesizing an Amino Acid Using a Strecker Synthesis © 2014 Pearson Education, Inc.

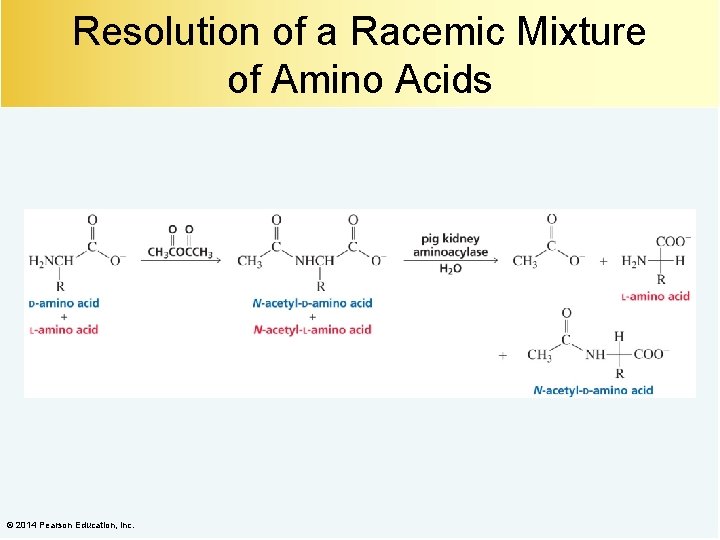
Resolution of a Racemic Mixture of Amino Acids © 2014 Pearson Education, Inc.

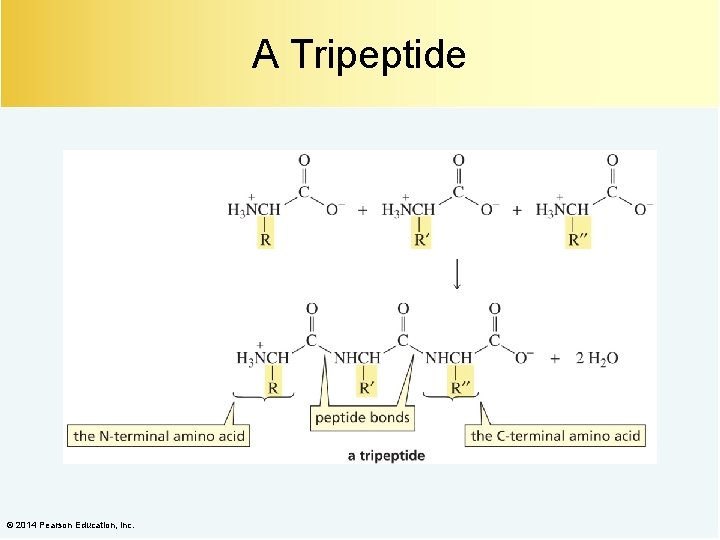
A Tripeptide © 2014 Pearson Education, Inc.

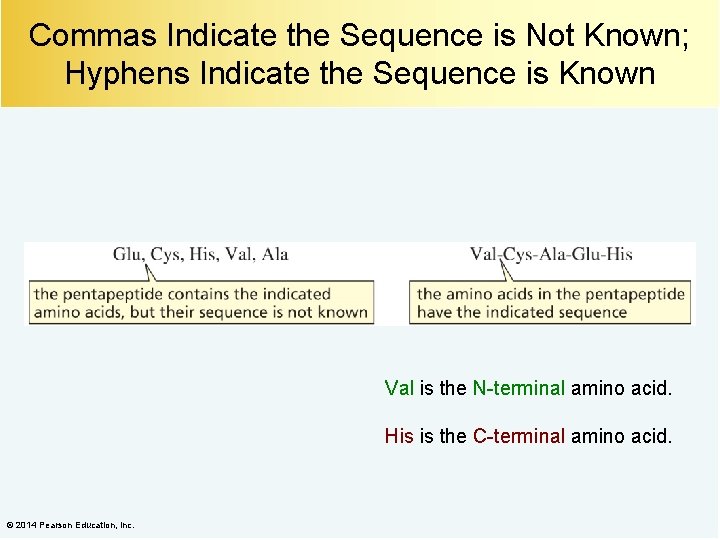
Commas Indicate the Sequence is Not Known; Hyphens Indicate the Sequence is Known Val is the N-terminal amino acid. His is the C-terminal amino acid. © 2014 Pearson Education, Inc.

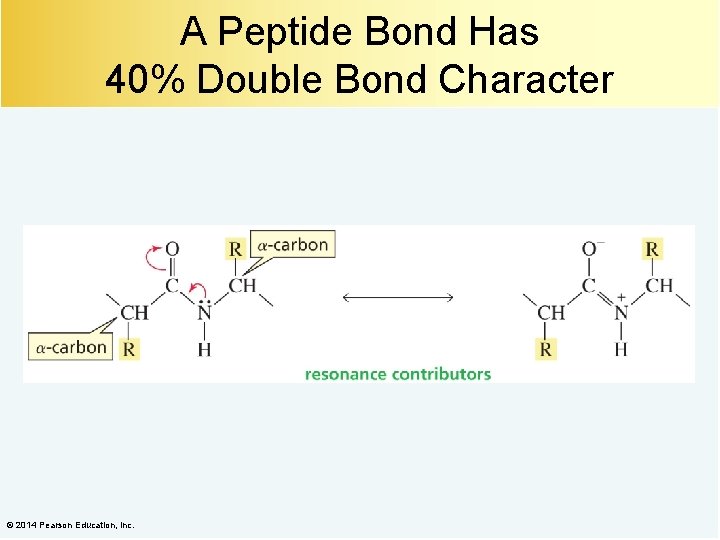
A Peptide Bond Has 40% Double Bond Character © 2014 Pearson Education, Inc.

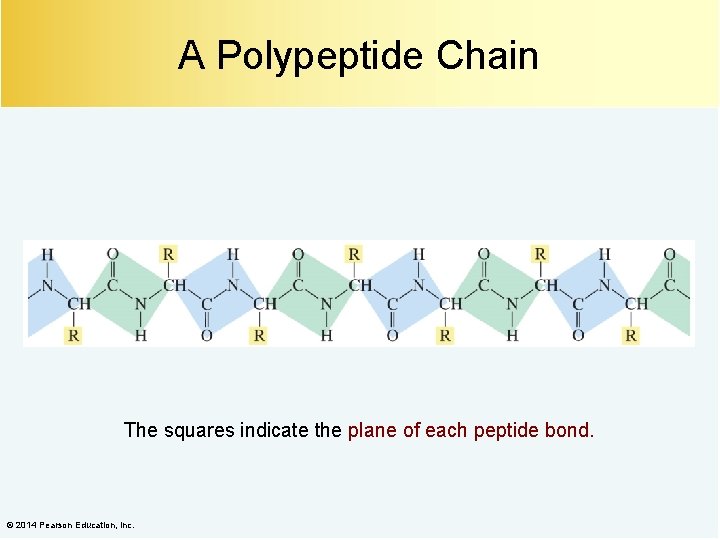
A Polypeptide Chain The squares indicate the plane of each peptide bond. © 2014 Pearson Education, Inc.

Mild Oxidation of a Thiol © 2014 Pearson Education, Inc.

The Mechanism © 2014 Pearson Education, Inc.

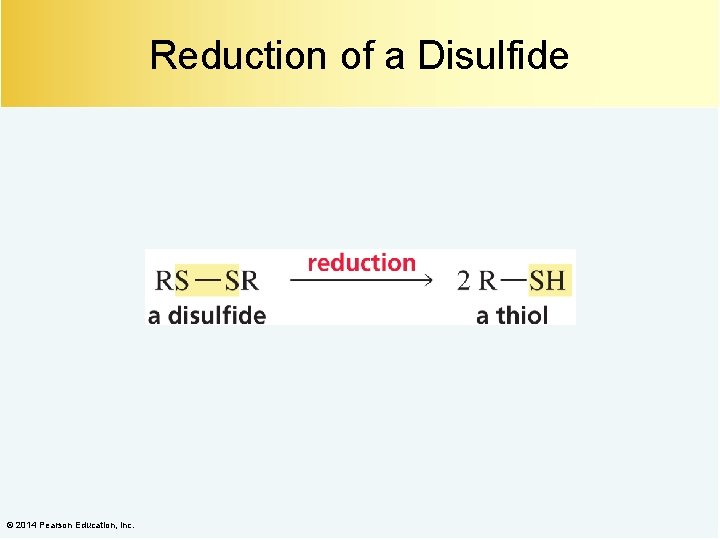
Reduction of a Disulfide © 2014 Pearson Education, Inc.

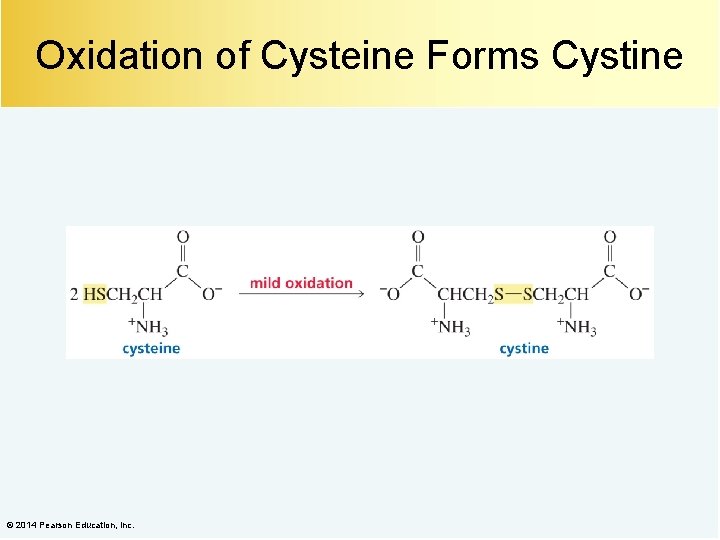
Oxidation of Cysteine Forms Cystine © 2014 Pearson Education, Inc.

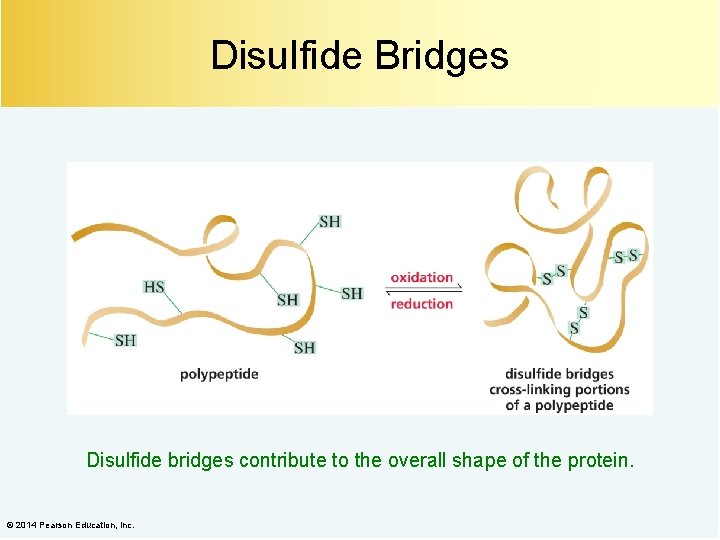
Disulfide Bridges Disulfide bridges contribute to the overall shape of the protein. © 2014 Pearson Education, Inc.

Straight and Curly Hair © 2014 Pearson Education, Inc.

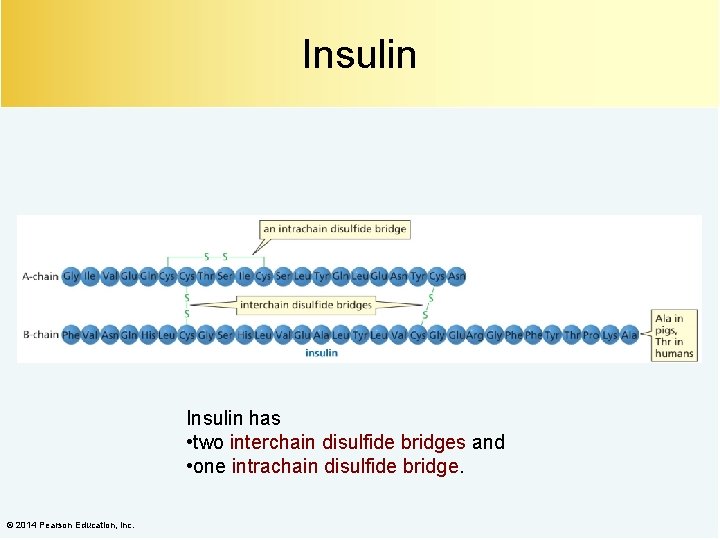
Insulin has • two interchain disulfide bridges and • one intrachain disulfide bridge. © 2014 Pearson Education, Inc.

Enkephalins peptides synthesized by the body to control pain © 2014 Pearson Education, Inc.

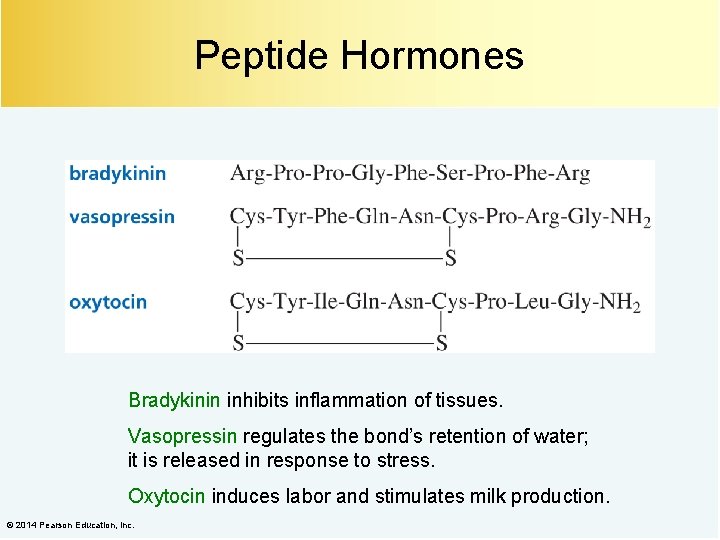
Peptide Hormones Bradykinin inhibits inflammation of tissues. Vasopressin regulates the bond’s retention of water; it is released in response to stress. Oxytocin induces labor and stimulates milk production. © 2014 Pearson Education, Inc.

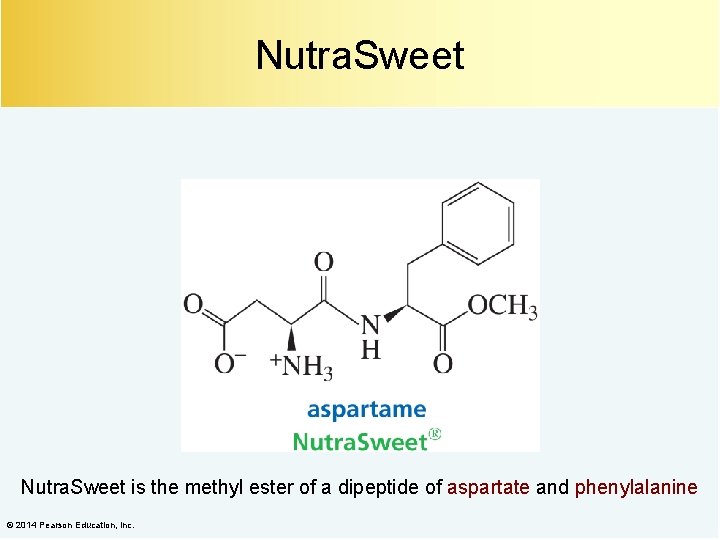
Nutra. Sweet is the methyl ester of a dipeptide of aspartate and phenylalanine © 2014 Pearson Education, Inc.

Synthesizing Gly-Ala Because amino acids have two functional groups, mixing Gly and Ala (and heating) would lead to four different dipeptides. © 2014 Pearson Education, Inc.

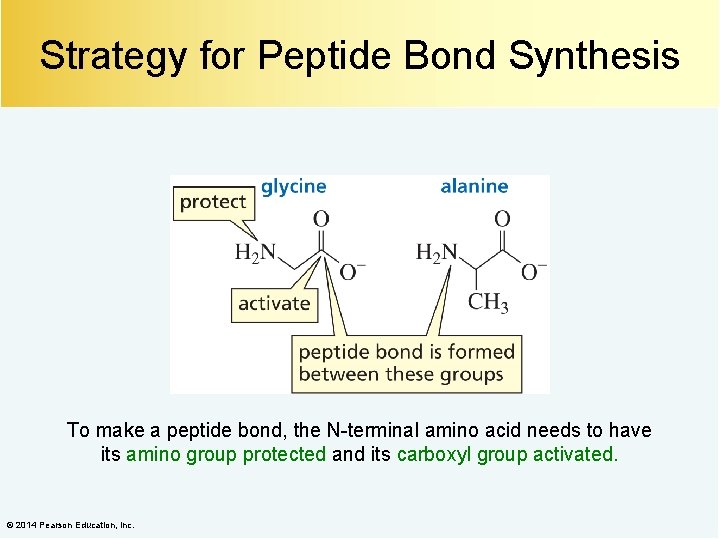
Strategy for Peptide Bond Synthesis To make a peptide bond, the N-terminal amino acid needs to have its amino group protected and its carboxyl group activated. © 2014 Pearson Education, Inc.

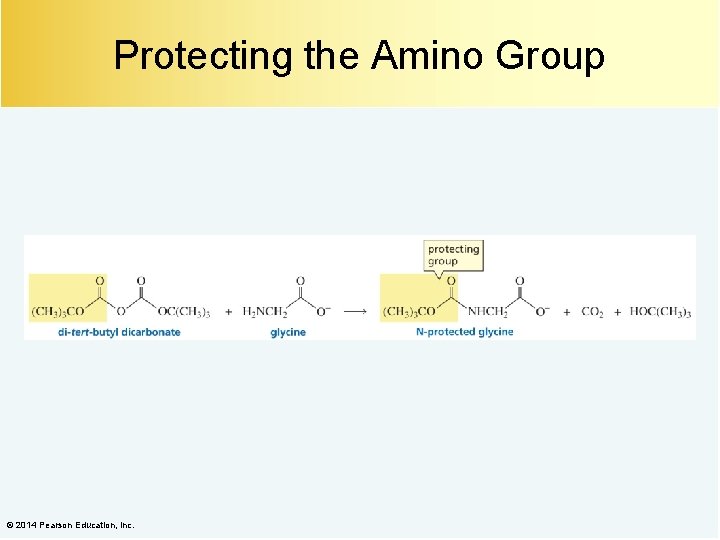
Protecting the Amino Group © 2014 Pearson Education, Inc.

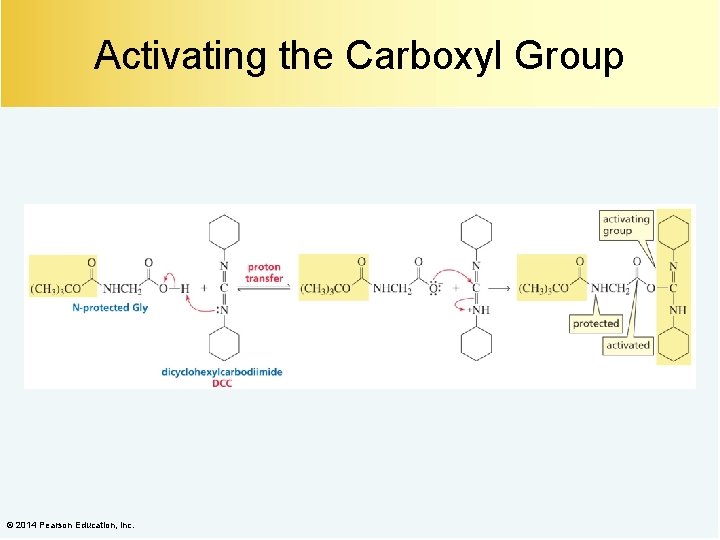
Activating the Carboxyl Group © 2014 Pearson Education, Inc.

Forming a New Peptide Bond © 2014 Pearson Education, Inc.

Adding a Third Amino Acid © 2014 Pearson Education, Inc.

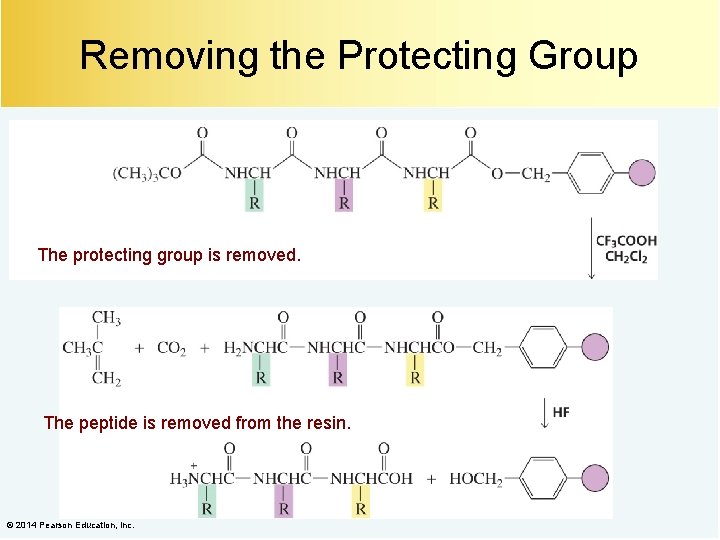
Removing the Protecting Group When the desired number of amino acids has been added to the chain, the protecting group can be removed. © 2014 Pearson Education, Inc.

Assuming an 80% Yield for Formation of Each Peptide Bond © 2014 Pearson Education, Inc.

Automated Solid-Phase Peptide Synthesis The N-protected C-terminal amino acid is added to the resin. © 2014 Pearson Education, Inc.

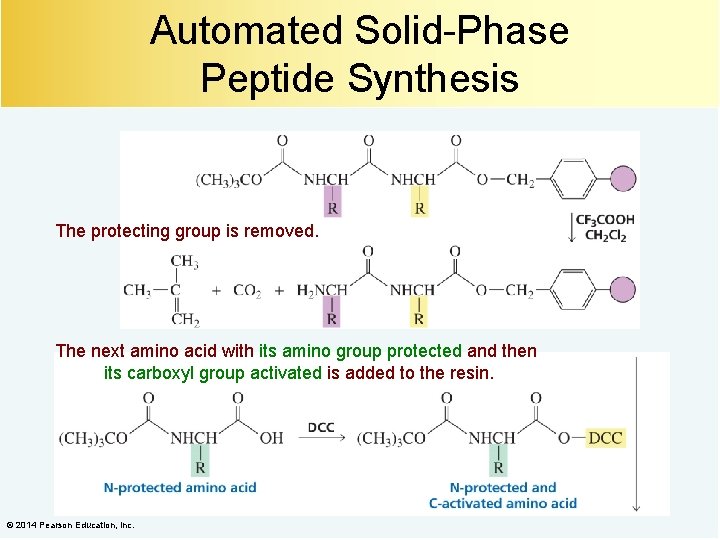
Automated Solid-Phase Peptide Synthesis The protecting group is removed. The next amino acid with its amino group protected and then its carboxyl group activated is added to the resin. © 2014 Pearson Education, Inc.

Automated Solid-Phase Peptide Synthesis The protecting group is removed. The next amino acid with its amino group protected and then its carboxyl group activated is added to the resin. © 2014 Pearson Education, Inc.

Removing the Protecting Group The protecting group is removed. The peptide is removed from the resin. © 2014 Pearson Education, Inc.

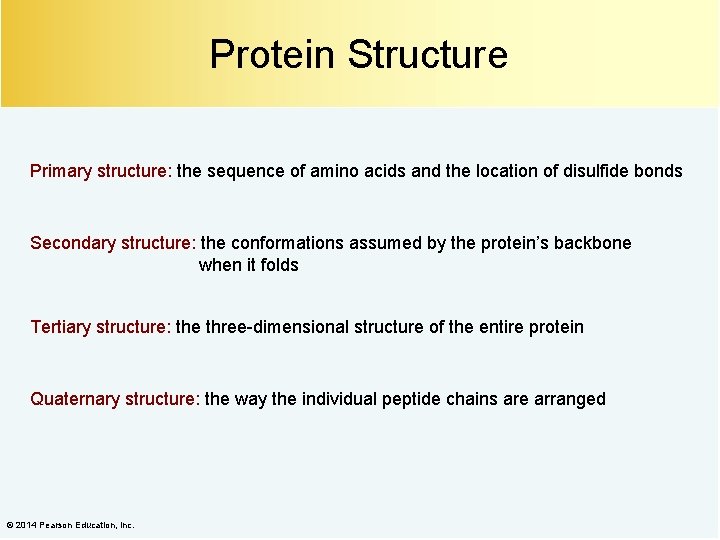
Protein Structure Primary structure: the sequence of amino acids and the location of disulfide bonds Secondary structure: the conformations assumed by the protein’s backbone when it folds Tertiary structure: the three-dimensional structure of the entire protein Quaternary structure: the way the individual peptide chains are arranged © 2014 Pearson Education, Inc.

Reducing the Disulfide Bridges in Proteins The first step in determining the sequence of amino acids is cleaving the disulfide bridges. © 2014 Pearson Education, Inc.

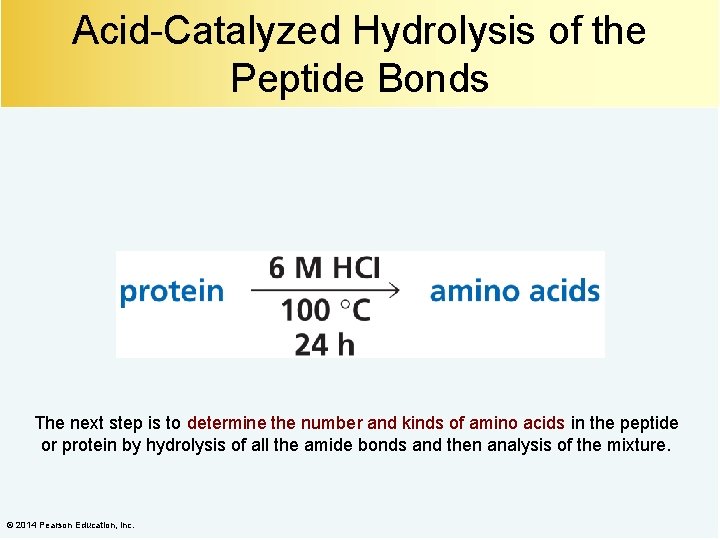
Acid-Catalyzed Hydrolysis of the Peptide Bonds The next step is to determine the number and kinds of amino acids in the peptide or protein by hydrolysis of all the amide bonds and then analysis of the mixture. © 2014 Pearson Education, Inc.

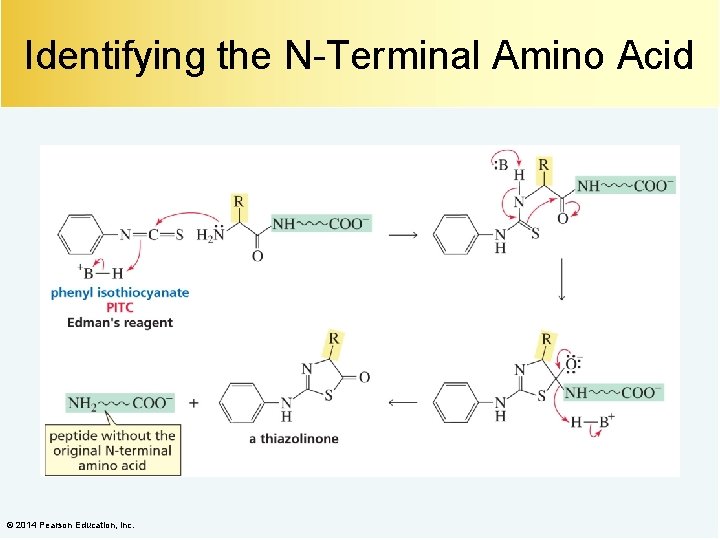
Identifying the N-Terminal Amino Acid © 2014 Pearson Education, Inc.

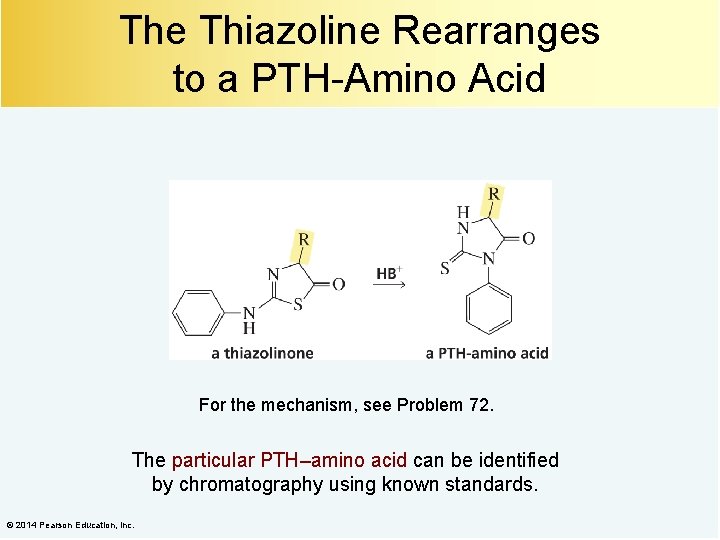
The Thiazoline Rearranges to a PTH-Amino Acid For the mechanism, see Problem 72. The particular PTH–amino acid can be identified by chromatography using known standards. © 2014 Pearson Education, Inc.

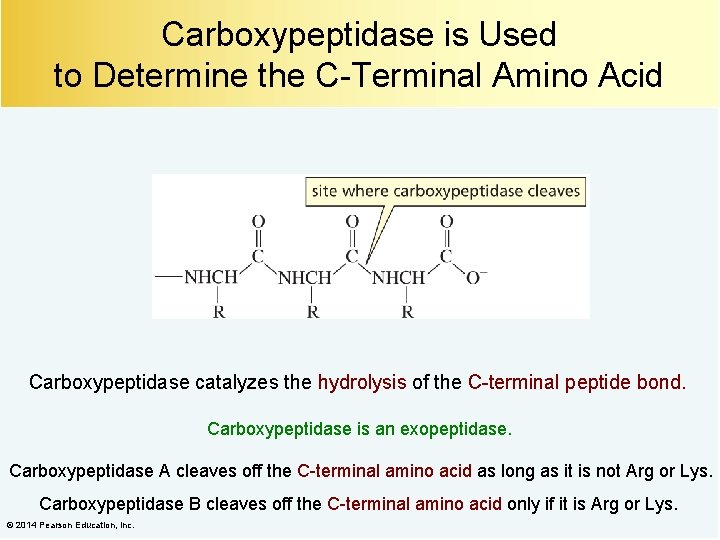
Carboxypeptidase is Used to Determine the C-Terminal Amino Acid Carboxypeptidase catalyzes the hydrolysis of the C-terminal peptide bond. Carboxypeptidase is an exopeptidase. Carboxypeptidase A cleaves off the C-terminal amino acid as long as it is not Arg or Lys. Carboxypeptidase B cleaves off the C-terminal amino acid only if it is Arg or Lys. © 2014 Pearson Education, Inc.

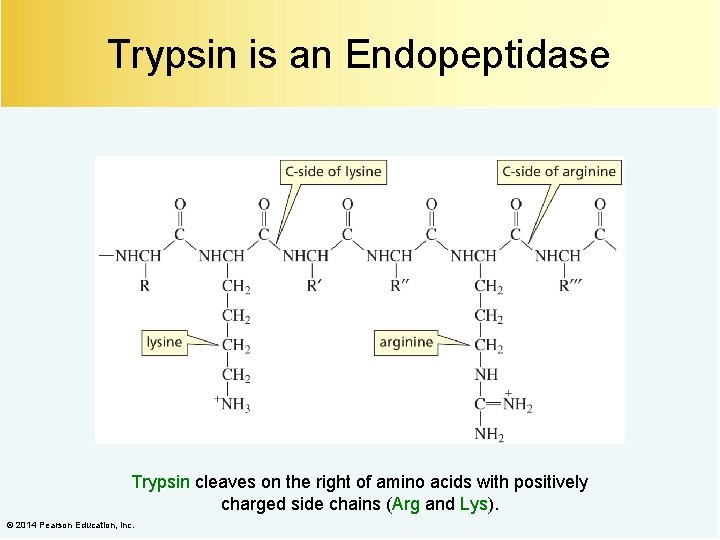
Trypsin is an Endopeptidase Trypsin cleaves on the right of amino acids with positively charged side chains (Arg and Lys). © 2014 Pearson Education, Inc.

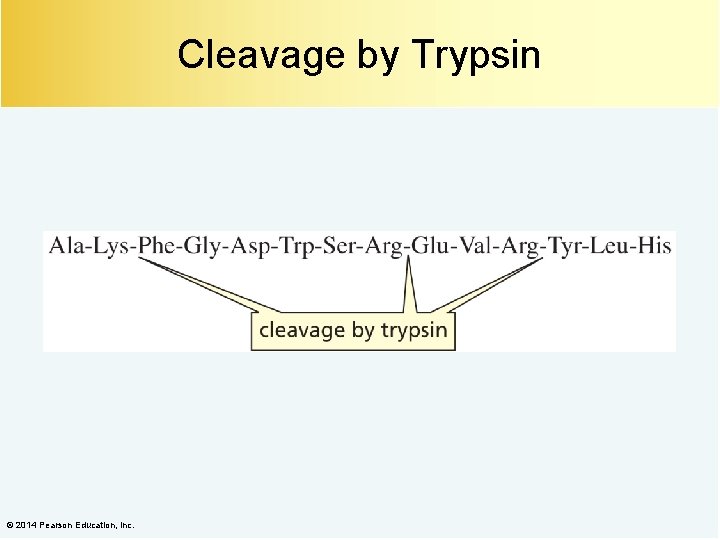
Cleavage by Trypsin © 2014 Pearson Education, Inc.

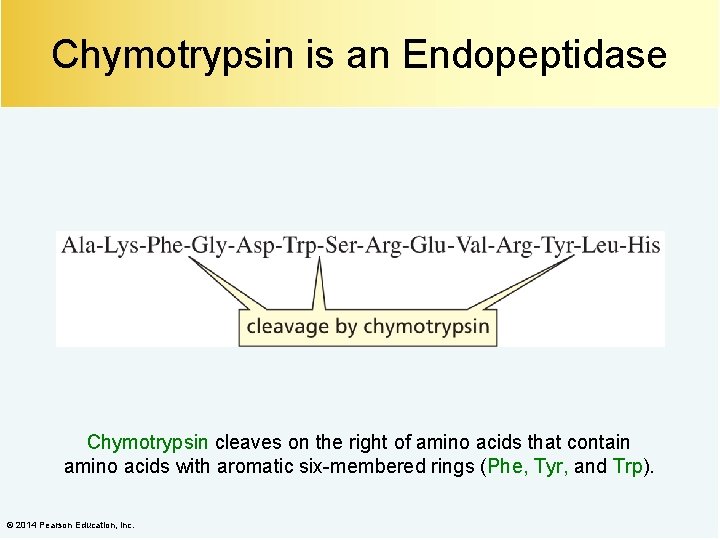
Chymotrypsin is an Endopeptidase Chymotrypsin cleaves on the right of amino acids that contain amino acids with aromatic six-membered rings (Phe, Tyr, and Trp). © 2014 Pearson Education, Inc.

Elastase is an Endopeptidase Elastase cleaves on the right of small amino acids (Gly and Ala). © 2014 Pearson Education, Inc.

An Enzyme Will Not Hydrolyze a Peptide Bond if Proline is at the Cleavage Site © 2014 Pearson Education, Inc.

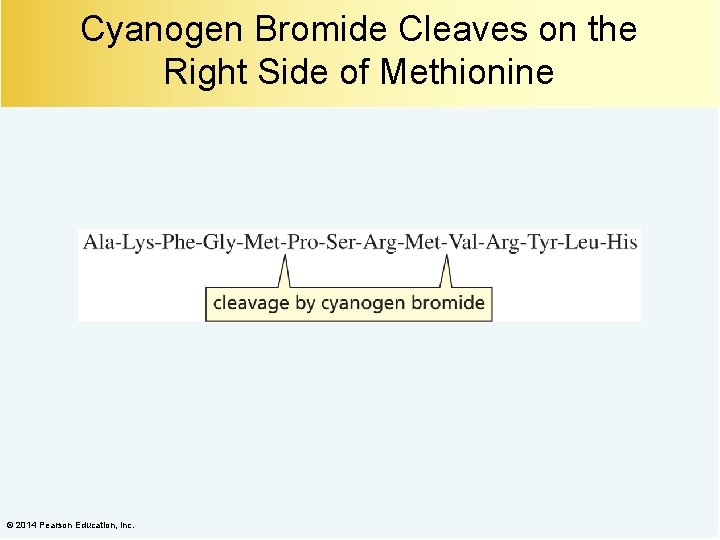
Cyanogen Bromide Cleaves on the Right Side of Methionine © 2014 Pearson Education, Inc.

The Mechanism © 2014 Pearson Education, Inc.

Secondary Structure Three factors determine the secondary structure: • The regional planarity about each peptide bond limits the conformations of the peptide chain. • The number of peptide groups that engage in hydrogen bonding is maximized. • The need for adequate separation between nearby R groups © 2014 Pearson Education, Inc.

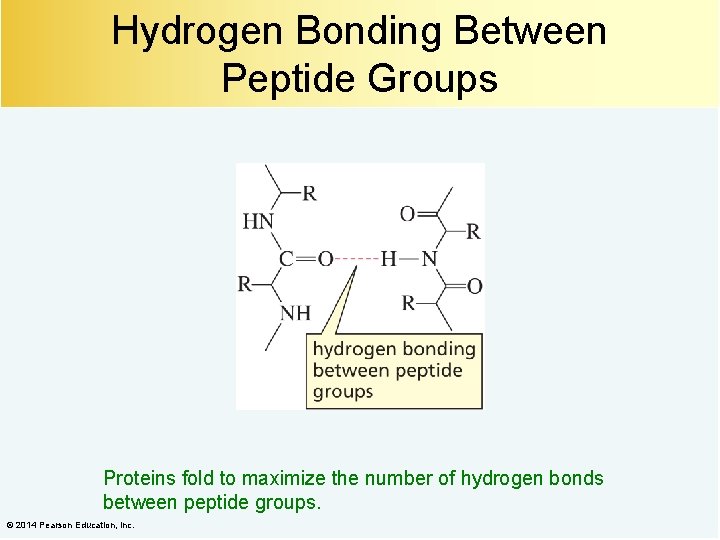
Hydrogen Bonding Between Peptide Groups Proteins fold to maximize the number of hydrogen bonds between peptide groups. © 2014 Pearson Education, Inc.

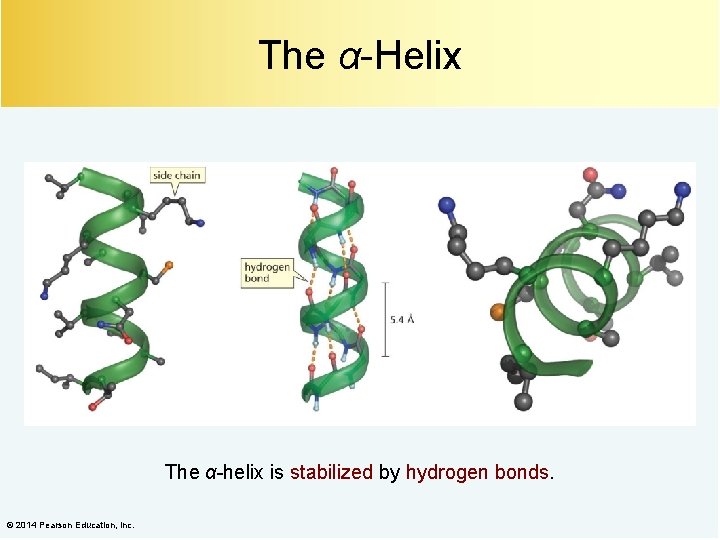
The α-Helix The α-helix is stabilized by hydrogen bonds. © 2014 Pearson Education, Inc.

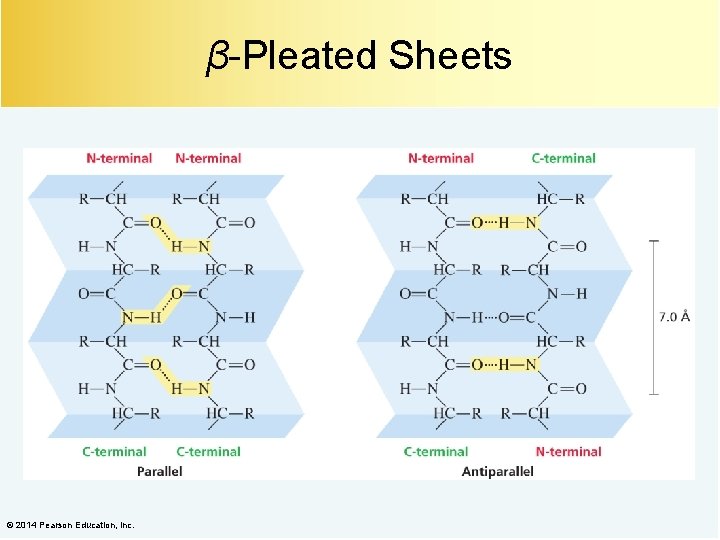
β-Pleated Sheets © 2014 Pearson Education, Inc.

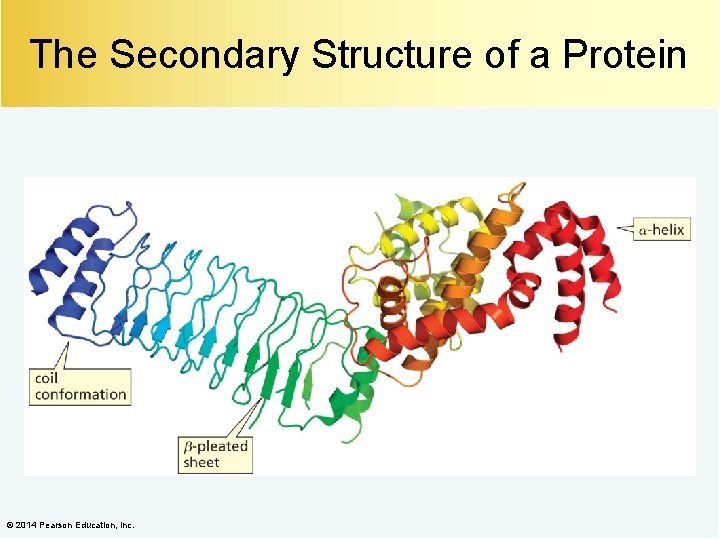
The Secondary Structure of a Protein © 2014 Pearson Education, Inc.

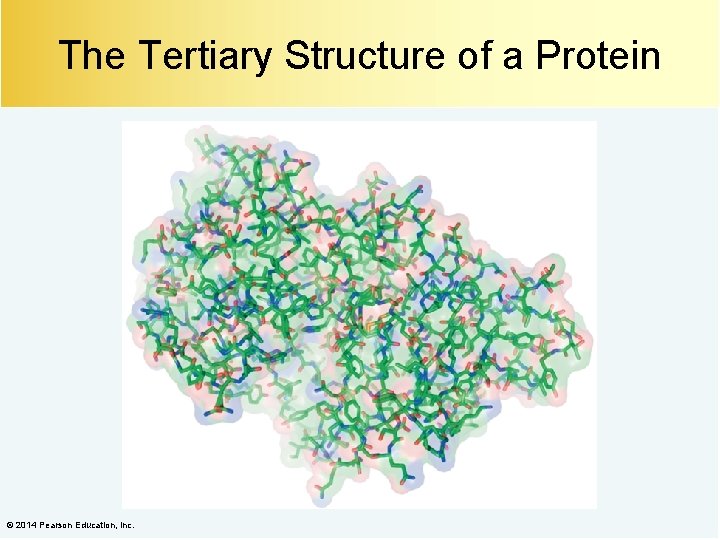
The Tertiary Structure of a Protein © 2014 Pearson Education, Inc.

Stabilizing Interactions © 2014 Pearson Education, Inc.

The Quaternary Structure of Hemoglobin The individual chains are called subunits. © 2014 Pearson Education, Inc.
 Acid base chemistry of amino acids
Acid base chemistry of amino acids Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Titration curves for amino acids
Titration curves for amino acids Titration curve of amino acids
Titration curve of amino acids Deamination of amino acids
Deamination of amino acids Properties of amino acids slideshare
Properties of amino acids slideshare Deamination of glutamine
Deamination of glutamine 20 amino acid structure
20 amino acid structure Early man
Early man Carbohydrates gives
Carbohydrates gives Amino acid titration curves
Amino acid titration curves Gluconeogenesis slideshare
Gluconeogenesis slideshare Purely ketogenic amino acids
Purely ketogenic amino acids Plp mechanism transamination
Plp mechanism transamination Amino acids groups
Amino acids groups Solubility of amino acids in water
Solubility of amino acids in water Www.chemsheets.co.uk
Www.chemsheets.co.uk Optical properties of amino acids
Optical properties of amino acids Chymotrypsin cleaves which amino acids
Chymotrypsin cleaves which amino acids Serylglycyltyrosylalanylleucine
Serylglycyltyrosylalanylleucine Diphthamide
Diphthamide Non essential amino acids mnemonics
Non essential amino acids mnemonics Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Aromatic amino acids
Aromatic amino acids Phenol containing amino acids
Phenol containing amino acids Amino acid with phenol group
Amino acid with phenol group Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Properties of amino acids
Properties of amino acids Biomedical importance of amino acids
Biomedical importance of amino acids Are amino acids negatively charged
Are amino acids negatively charged Conditionally essential amino acids
Conditionally essential amino acids Non essential amino acids mnemonics
Non essential amino acids mnemonics Essential amino acids arginine
Essential amino acids arginine Glucogenic amino acids
Glucogenic amino acids Amino acids characteristics
Amino acids characteristics Deamination of amino acids
Deamination of amino acids Oxidative deamination of amino acids
Oxidative deamination of amino acids Difference between hydrophobic and hydrophilic amino acids
Difference between hydrophobic and hydrophilic amino acids Non essential amino acids mnemonics
Non essential amino acids mnemonics What is proteins
What is proteins Quantitative qualitative estimation
Quantitative qualitative estimation 64 amino acids
64 amino acids Biliverdin color
Biliverdin color Transdeamination of amino acids
Transdeamination of amino acids 17/35
17/35 Classify amino acids
Classify amino acids Salt bridges in proteins
Salt bridges in proteins Pvt tim hall
Pvt tim hall Upon hydrolysis of fibron which amino acids are produced?
Upon hydrolysis of fibron which amino acids are produced? R groups amino acids
R groups amino acids Acid base properties of amino acids
Acid base properties of amino acids Transdeamination of amino acids
Transdeamination of amino acids Ketogenic amino acids
Ketogenic amino acids Lysine titration curve
Lysine titration curve What is made of amino acids
What is made of amino acids Wikipedia amino acids
Wikipedia amino acids Importance of sulphur containing amino acids
Importance of sulphur containing amino acids What are enzymes made of
What are enzymes made of Importance of sulphur containing amino acids
Importance of sulphur containing amino acids Charged amino acids
Charged amino acids Peptide bond definition milady
Peptide bond definition milady Protein amino acids
Protein amino acids B-pleated sheet
B-pleated sheet Net charges of amino acids
Net charges of amino acids 191 amino acid
191 amino acid Amino acids classification
Amino acids classification Nitrogen removal from amino acids
Nitrogen removal from amino acids Urea cycle definition in biochemistry
Urea cycle definition in biochemistry Gluconeogenesis meaning
Gluconeogenesis meaning Aug amino acid
Aug amino acid Quaternary structure of protein
Quaternary structure of protein Structure of amino acid
Structure of amino acid Mixed amino acids
Mixed amino acids Properties of amino acids slideshare
Properties of amino acids slideshare