CHAPTER 11 Proteins Structure and Function OUTLINE 11




























- Slides: 80

CHAPTER 11: Proteins: Structure and Function OUTLINE • 11. 1 Amino Acids • 11. 2 Chirality and Amino Acids • 11. 3 Peptides • 11. 4 Protein Architecture • 11. 5 Enzymes

CHAPTER 11: Proteins: Structure and Function BIOMOLECULES • Biomolecules are large, complex organic molecules. • They are present in all living things. • They include: • Proteins • Carbohydrates • Lipids • Nucleic acids (DNA and RNA) • The first of these is presented below, the remainder in subsequent sets of slides. • All obey general principles of chemical reactions discussed previously.

CHAPTER 11: Proteins: Structure and Function PROTEINS • Proteins have some of the most diverse functions of all biological molecules, ranging from the hemoglobin that transports oxygen to tissues, to collagen and elastin that provide structure to ligaments, tendons, and blood vessels, to the enzymes that catalyze all biochemical reactions. • They are composed of amino acids linked together in chains, folded into complex structures with specific biological functions.

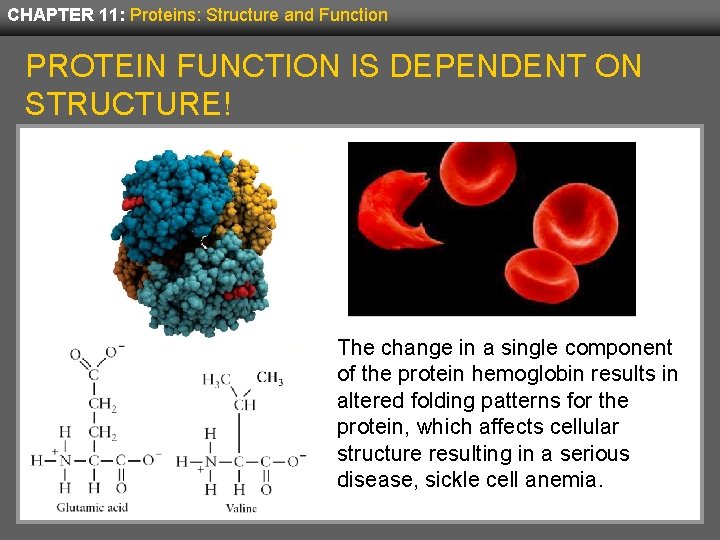
CHAPTER 11: Proteins: Structure and Function PROTEIN FUNCTION IS DEPENDENT ON STRUCTURE! The change in a single component of the protein hemoglobin results in altered folding patterns for the protein, which affects cellular structure resulting in a serious disease, sickle cell anemia.

CHAPTER 11: Proteins: Structure and Function DID YOU KNOW? 1. What is the chemical basis for sickle cell anemia? 2. How does molecular polarity play a role in hemoglobin function?

CHAPTER 11: Proteins: Structure and Function DID YOU ANSWER? Hemoglobin, like all globular proteins, has surface polar amino acids and internal nonpolar amino acids. In sickle cell anemia, one of its surface amino acids, glutamic acid, is genetically exchanged for the nonpolar amino acid, valine. This simple shift results in the incorrect conformation, or folding, of the protein, causing the disease.

CHAPTER 11: Proteins: Structure and Function 11. 1 AMINO ACIDS

CHAPTER 11: Proteins: Structure and Function RECALL • Chemical properties of organic functional groups • Effect of p. H on acidic and basic groups

CHAPTER 11: Proteins: Structure and Function PARTS OF AMINO ACIDS • An amino acids consists of • an amine • a carboxylic acid • a hydrogen atom • one of about 20 side chains (R groups or residues) • All bonded to a central atom, the -carbon • The identity of an amino acid is determined by its side chain.

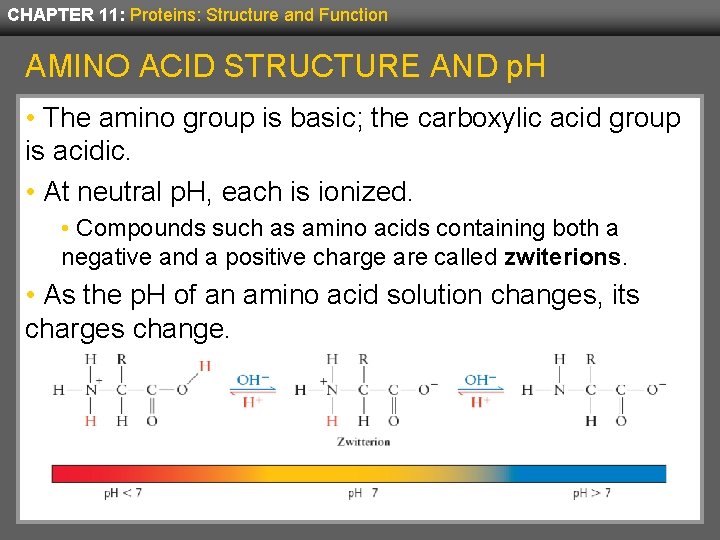
CHAPTER 11: Proteins: Structure and Function AMINO ACID STRUCTURE AND p. H • The amino group is basic; the carboxylic acid group is acidic. • At neutral p. H, each is ionized. • Compounds such as amino acids containing both a negative and a positive charge are called zwiterions. • As the p. H of an amino acid solution changes, its charges change.

CHAPTER 11: Proteins: Structure and Function AMINO ACID SIDE CHAINS • The 20 amino acids are differentiated by their side chains. • The side chains may be either polar or nonpolar. • Nonpolar side chains are generally alkanes or have aromatic ring structures. • Polar side chains may be divided into acidic, basic, or neutral side chains. • At physiological p. H, • the 3 basic side chains have positive charge • the 2 acidic side chains have negative charge

CHAPTER 11: Proteins: Structure and Function EXAMPLES OF AMINO ACIDS • Aspartic acid has an acidic side chain, lysine has a basic side chain, and cysteine has a neutral side chain. • Each amino acid may be designated by a 3 -letter abbreviation, as shown.

CHAPTER 11: Proteins: Structure and Function NONPOLAR AMINO ACIDS *Essential Amino Acid

CHAPTER 11: Proteins: Structure and Function POLAR AMINO ACIDS

CHAPTER 11: Proteins: Structure and Function ESSENTIAL AMINO ACIDS • Essential amino acids are amino acids required in our diets. • About half may be synthesized by our bodies, the rest must be consumed in the foods we eat. • Essential amino acids include the following:

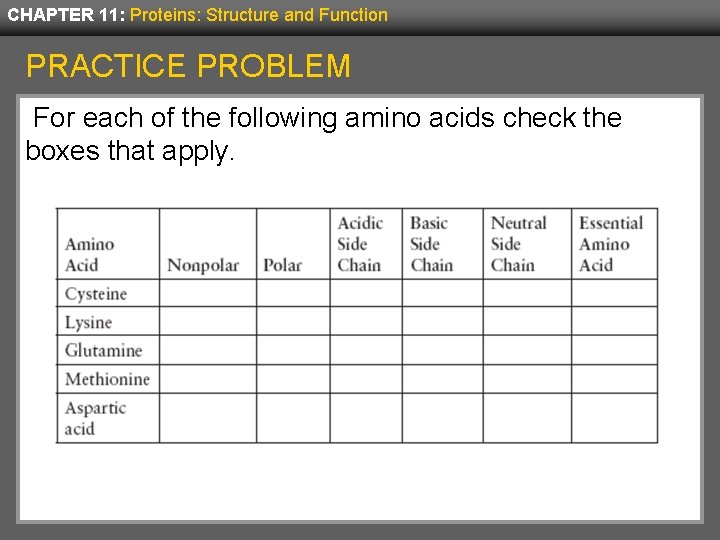
CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEM For each of the following amino acids check the boxes that apply.

CHAPTER 11: Proteins: Structure and Function 11. 2 CHIRALITY AND AMINO ACIDS

CHAPTER 11: Proteins: Structure and Function AMINO ACID CHIRALITY • Chirality is a property associated with many molecules, particularly those with biomedical applications such as most amino acids. • Objects, such as a glove or certain molecules, are chiral if their mirror images are non-superposable. • Your right and left hands have chirality because they are mirror images, and yet are not the same as each other. • The term “chiral” means “handed. ” • Objects, such as a sock or mitten, are achiral because their mirror images are superposable. • Chiral molecules have at least one tetrahedral atom with four different atoms or groups attached.

CHAPTER 11: Proteins: Structure and Function ENANTIOMERS • Enantiomers are pairs of chiral objects, one being the mirror image of the other. • Amino acids may consist of enantiomers. • But only one of the pair is found in nature. • It is customary to refer to each member of the enantiomeric pair with the prefixes D- or L-. • Only the L-form of amino acids is common. Other common classes of materials are in the D-form. • Other naming rules may use R- and S, (+) and (-), or d-, and l-.

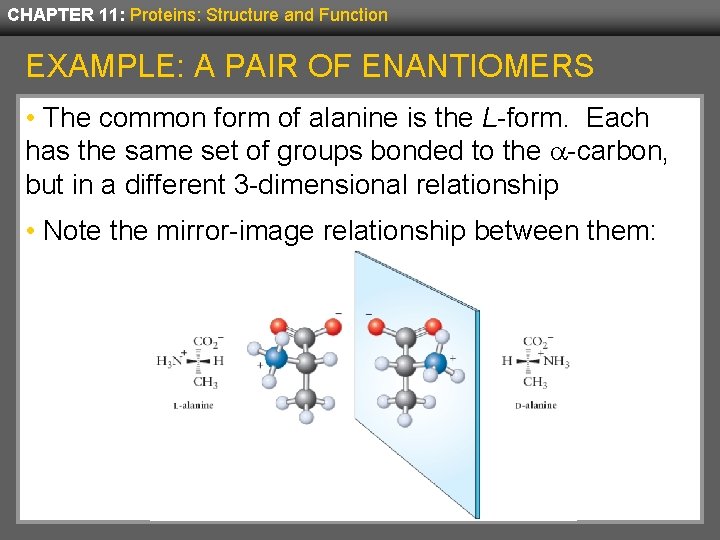
CHAPTER 11: Proteins: Structure and Function EXAMPLE: A PAIR OF ENANTIOMERS • The common form of alanine is the L-form. Each has the same set of groups bonded to the -carbon, but in a different 3 -dimensional relationship • Note the mirror-image relationship between them:

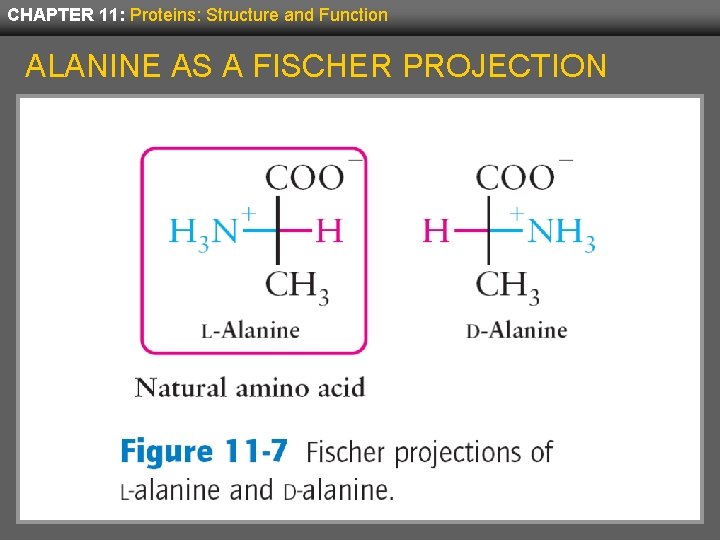
CHAPTER 11: Proteins: Structure and Function FISCHER PROJECTIONS • A simplified way to indicate the structure of enantiomers is to use Fischer projections. • These indicate chiral atoms as crosshairs (+) with the main carbon chain written vertically. • For L-amino acids, the alpha carbon is shown at the crosshair with the carboxylate to the top, the amino group to the left and the side chain down. • D-amino acids would be the mirror image of this.

CHAPTER 11: Proteins: Structure and Function ALANINE AS A FISCHER PROJECTION

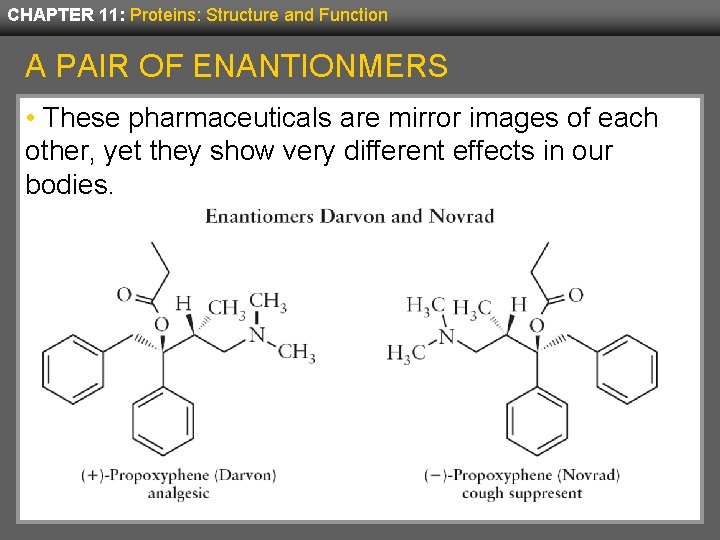
CHAPTER 11: Proteins: Structure and Function PROPERTIES OF ENANTIONMERS • When enantionmers are in an achiral environment, their physical and chemical properties are identical. • In a chiral environment, such as within a cell, their properties may be quite different. • Because proteins are composed of L-amino acids, they are chiral. • For example two common drugs, Darvon and Novrad are enantiomers, but one is an analgesic and the other is a cough suppressant.

CHAPTER 11: Proteins: Structure and Function A PAIR OF ENANTIONMERS • These pharmaceuticals are mirror images of each other, yet they show very different effects in our bodies.

CHAPTER 11: Proteins: Structure and Function RACEMIC MIXTURES • A racemic mixture is a mixture containing both enantiomers, the L- form and the D-form. • Since one of the pair usually does not have biological activity, the racemic mixture is likely to have reduced potency. • These are usually designated by indicating both forms, such as D/L. • In many cases, the enantiomer without biological activity displays adverse effects if consumed. • Most pharmaceuticals contain only one of the two enantiomers.

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS 1. Indicate whether the following statements are TRUE or FALSE. A chiral molecule: a. is superposable on its mirror image b. has an enantiomer c. may exhibit different chemical properties from its enantiomer in the body d. typically exhibits different chemical properties from its enantiomer in an achiral environment 2. Ibuprofen, the active ingredient in Motrin and other OTC analgesics, is a chiral drug sold as a racemic mixture. What does this mean?

CHAPTER 11: Proteins: Structure and Function 11. 3 PEPTIDES

CHAPTER 11: Proteins: Structure and Function PEPTIDE BONDS • Peptides are molecules formed by joining two or more amino acids. • Peptides may be • small oligopeptides (2 amino acids to about a dozen) • larger polypeptides (up to about 50 amino acids) • very large molecules called proteins (>50 amino acids) • To form these, amino acids are joined by peptide bonds, essentially an amide group. • Peptide bonds form between the carboxylate group of one amino acid and the ammonium on another.

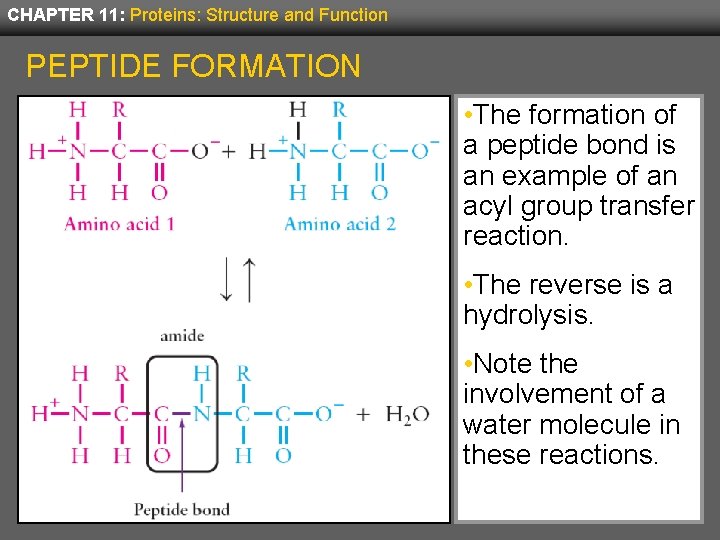
CHAPTER 11: Proteins: Structure and Function PEPTIDE FORMATION • The formation of a peptide bond is an example of an acyl group transfer reaction. • The reverse is a hydrolysis. • Note the involvement of a water molecule in these reactions.

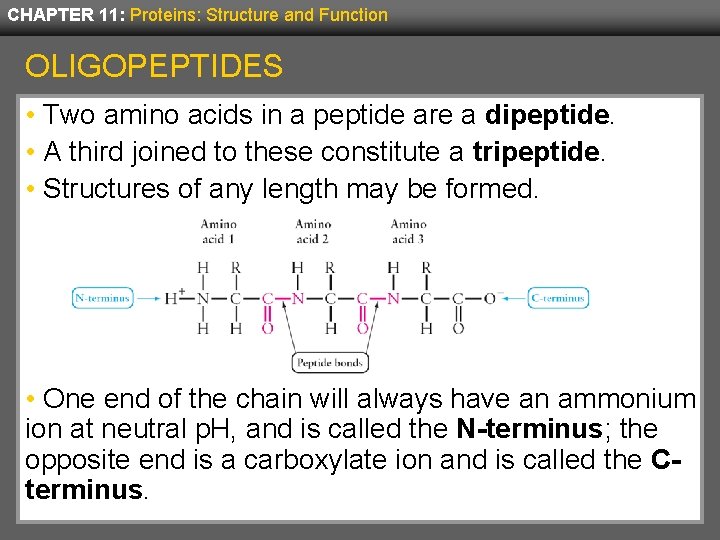
CHAPTER 11: Proteins: Structure and Function OLIGOPEPTIDES • Two amino acids in a peptide are a dipeptide. • A third joined to these constitute a tripeptide. • Structures of any length may be formed. • One end of the chain will always have an ammonium ion at neutral p. H, and is called the N-terminus; the opposite end is a carboxylate ion and is called the Cterminus.

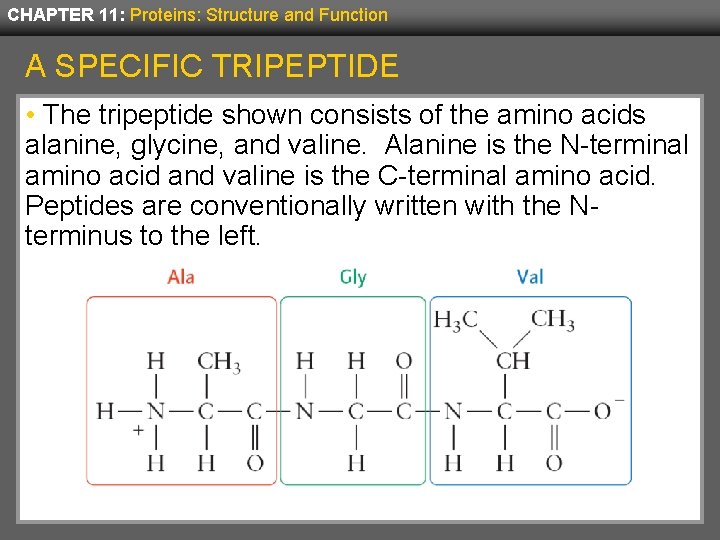
CHAPTER 11: Proteins: Structure and Function A SPECIFIC TRIPEPTIDE • The tripeptide shown consists of the amino acids alanine, glycine, and valine. Alanine is the N-terminal amino acid and valine is the C-terminal amino acid. Peptides are conventionally written with the Nterminus to the left.

CHAPTER 11: Proteins: Structure and Function COMMON OLIGONUCLEOTIDES • Aspartame, commercially the artificial sweetener Nutrasweet™, is the dipeptide asp-phe. • Oxytocin, a hormone involved in contractions during labor and in lactation, consists of the 9 amino acids: cys - tyr - ile - gln - asn - cys - pro - leu – gly • The endorphin, met-enkephalin, mentioned in chapter 7, has 5 amino acids. This helps reduce pain following injury.

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEM Are the dipeptides gly-phe and phe-gly different compounds? Are they structural isomers or stereoisomers?

CHAPTER 11: Proteins: Structure and Function 11. 4 PROTEIN ARCHITECTURE

CHAPTER 11: Proteins: Structure and Function PROTEIN STRUCTURE • Proteins are composed of the 20 L-amino acids. • A protein may have from about 50 to many thousands of amino acids, joined linearly by way of peptide bonds. • The information for determining the sequence of amino acids resides in the DNA of most cells. • A gene is the region of DNA responsible for the coding of a protein. • There are thousands of different proteins in the body, each has a specific function dependent on its structure.

CHAPTER 11: Proteins: Structure and Function THREE-DIMENSIONAL SHAPE OF PROTEINS • Proteins are not strictly linear structures. • The chain of amino acids folds into a threedimensional structure termed its native conformation. • Electrostatic interactions between atoms within the protein and between protein atoms and external atoms, such as solvent, determine folding patterns. • Amino acid side chains contribute interacting groups to this folding.

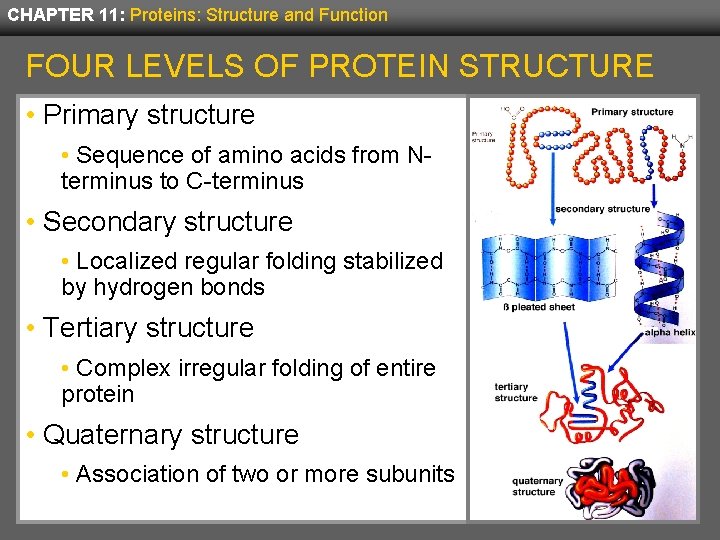
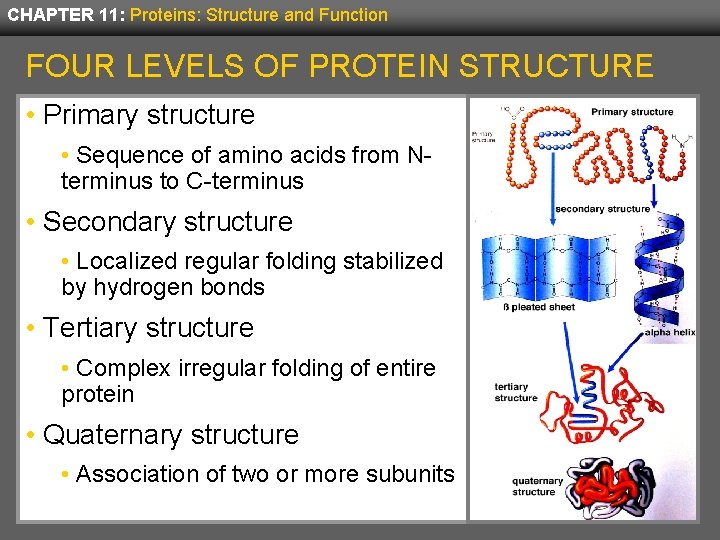
CHAPTER 11: Proteins: Structure and Function FOUR LEVELS OF PROTEIN STRUCTURE • Primary structure • Sequence of amino acids from Nterminus to C-terminus • Secondary structure • Localized regular folding stabilized by hydrogen bonds • Tertiary structure • Complex irregular folding of entire protein • Quaternary structure • Association of two or more subunits

CHAPTER 11: Proteins: Structure and Function PRIMARY STRUCTURE OF A PROTEIN • The sequence of amino acids determines all other aspects of a protein’s structure and function. • If this sequence is altered, the protein may not function properly. • Genetic disease is usually a disruption of the primary structure, often by replacing a single amino acid. • In sickle cell anemia, a single amino acid change from glutamic acid to valine results in improper function of hemoglobin. • An amino acid is located in sequence by the number of its position from the N-terminus.

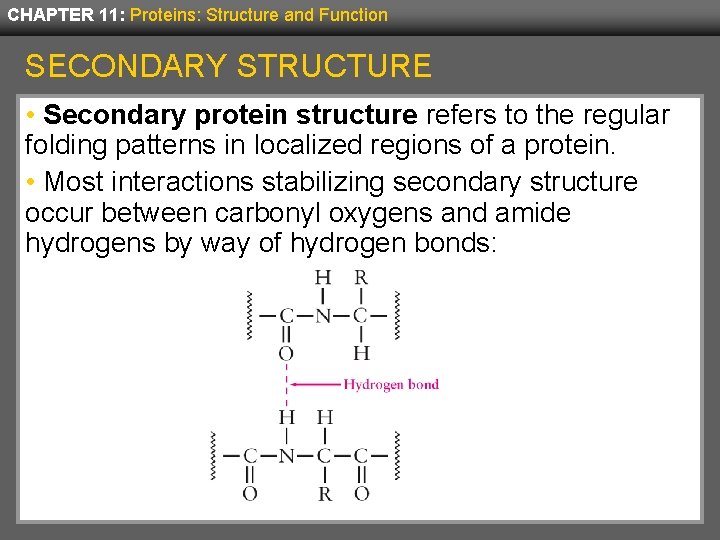
CHAPTER 11: Proteins: Structure and Function SECONDARY STRUCTURE • Secondary protein structure refers to the regular folding patterns in localized regions of a protein. • Most interactions stabilizing secondary structure occur between carbonyl oxygens and amide hydrogens by way of hydrogen bonds:

CHAPTER 11: Proteins: Structure and Function PATTERNS IN SECONDARY STRUCTURE • There are many examples of extended patterns of secondary structure. • The two most common types of secondary structure are • -helix • b-pleated sheet • Typical proteins may contain some of each of these or be mostly one or the other; some proteins contain neither.

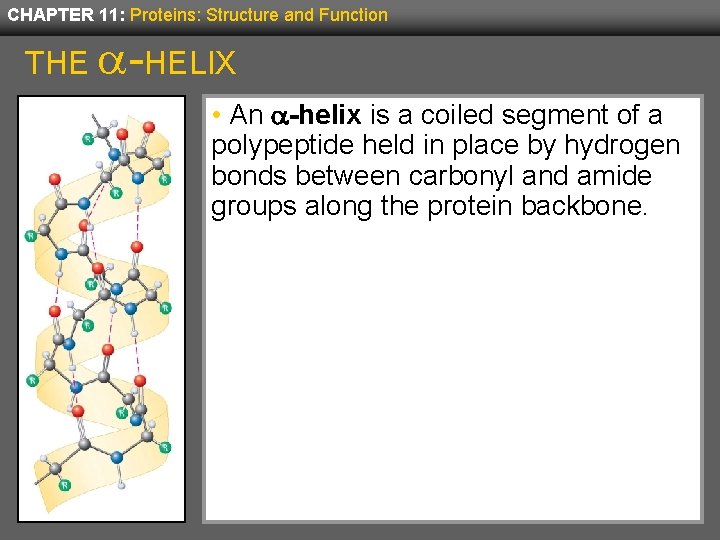
CHAPTER 11: Proteins: Structure and Function THE -HELIX • An a-helix is a coiled segment of a polypeptide held in place by hydrogen bonds between carbonyl and amide groups along the protein backbone.

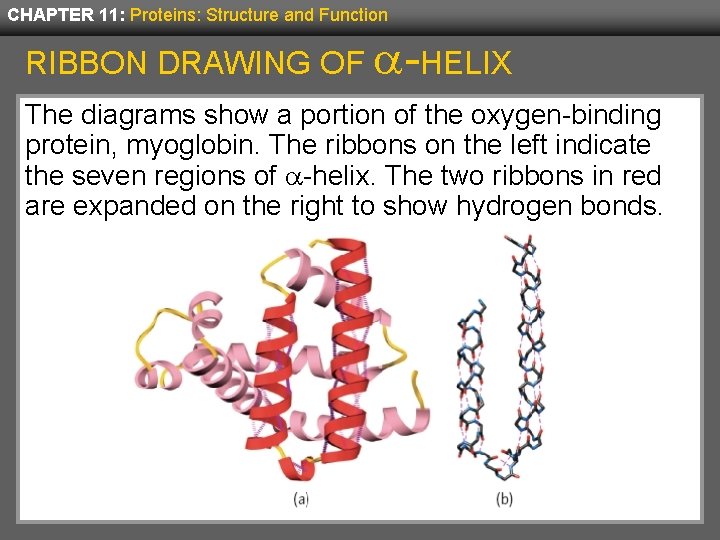
CHAPTER 11: Proteins: Structure and Function RIBBON DRAWING OF -HELIX The diagrams show a portion of the oxygen-binding protein, myoglobin. The ribbons on the left indicate the seven regions of -helix. The two ribbons in red are expanded on the right to show hydrogen bonds.

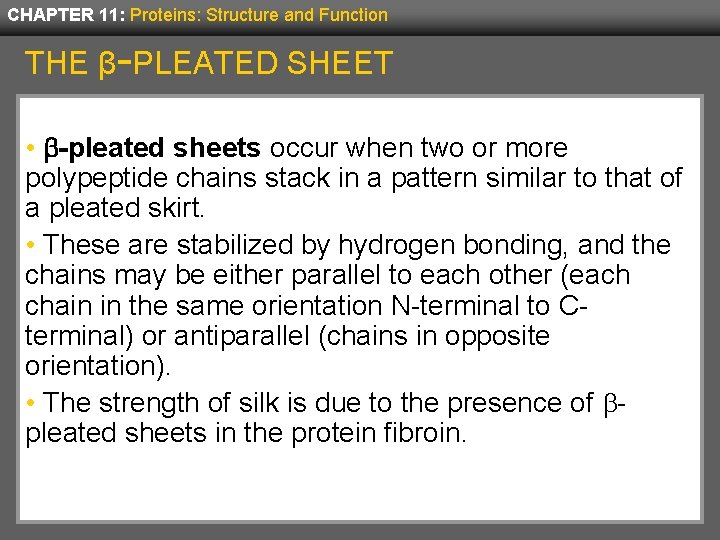
CHAPTER 11: Proteins: Structure and Function THE β-PLEATED SHEET • b-pleated sheets occur when two or more polypeptide chains stack in a pattern similar to that of a pleated skirt. • These are stabilized by hydrogen bonding, and the chains may be either parallel to each other (each chain in the same orientation N-terminal to Cterminal) or antiparallel (chains in opposite orientation). • The strength of silk is due to the presence of bpleated sheets in the protein fibroin.

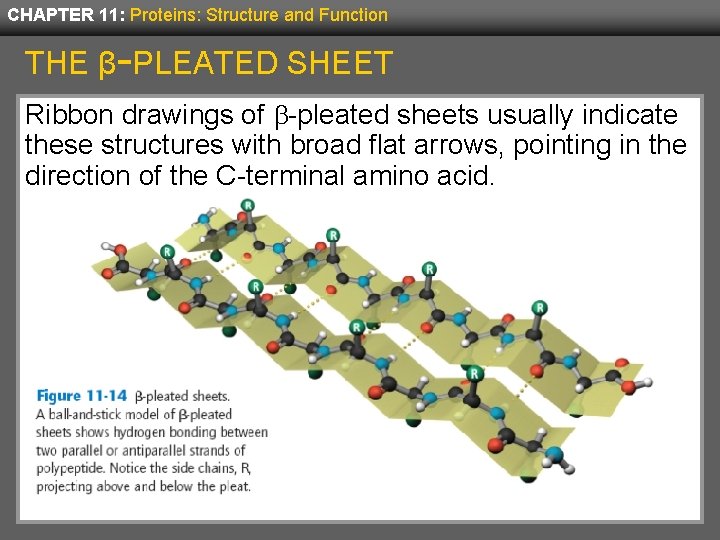
CHAPTER 11: Proteins: Structure and Function THE β-PLEATED SHEET Ribbon drawings of b-pleated sheets usually indicate these structures with broad flat arrows, pointing in the direction of the C-terminal amino acid.

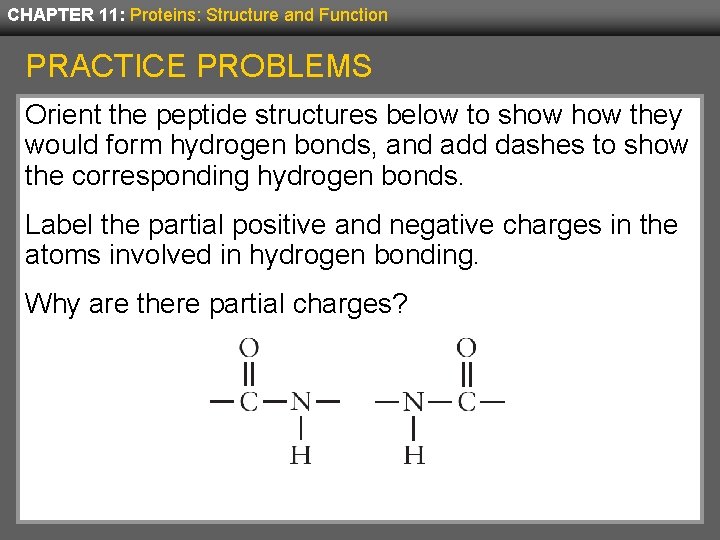
CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS Orient the peptide structures below to show they would form hydrogen bonds, and add dashes to show the corresponding hydrogen bonds. Label the partial positive and negative charges in the atoms involved in hydrogen bonding. Why are there partial charges?

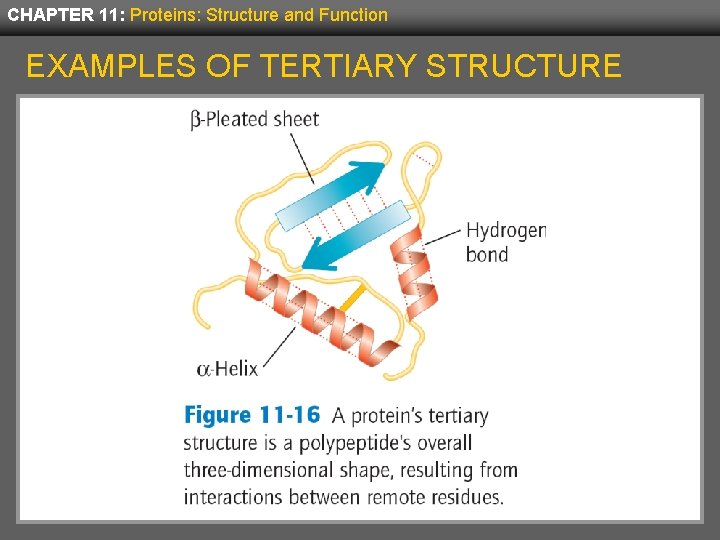
CHAPTER 11: Proteins: Structure and Function TERTIARY STRUCTURE • The tertiary structure of proteins describes the entire folding pattern of the protein and consists of complex and irregular folding, not patterned as found in secondary structure. • Tertiary structure is determined by interactions that include distant amino acid residues as well as the surrounding environment. • For some proteins, tertiary structure also includes prosthetic groups—non-peptide organic molecules or metal ions that are strongly bound to the protein.

CHAPTER 11: Proteins: Structure and Function EXAMPLES OF TERTIARY STRUCTURE

CHAPTER 11: Proteins: Structure and Function FOUR LEVELS OF PROTEIN STRUCTURE • Primary structure • Sequence of amino acids from Nterminus to C-terminus • Secondary structure • Localized regular folding stabilized by hydrogen bonds • Tertiary structure • Complex irregular folding of entire protein • Quaternary structure • Association of two or more subunits

CHAPTER 11: Proteins: Structure and Function WHAT CAUSES A PROTEIN TO FOLD? • The fundamental driving force behind protein folding is energy. • A folded protein and its environment are lower in potential energy than the unfolded protein. • Proteins fold spontaneously into their native conformation under physiological conditions. • Protein folding is not random, but is defined by the sum of all the electrostatic interactions present in the protein and its surroundings.

CHAPTER 11: Proteins: Structure and Function TYPES OF ELECTROSTATIC INTERACTIONS IN PROTEINS • There are 4 main types of electrostatic interactions resulting in the tertiary structure of a protein: • Disulfide bridges (covalent bond, hydrophobic) • Salt bridges (ionic bond, hydrophilic) • Hydrogen bonding (hydrophilic) • Dispersion forces (hydrophobic)

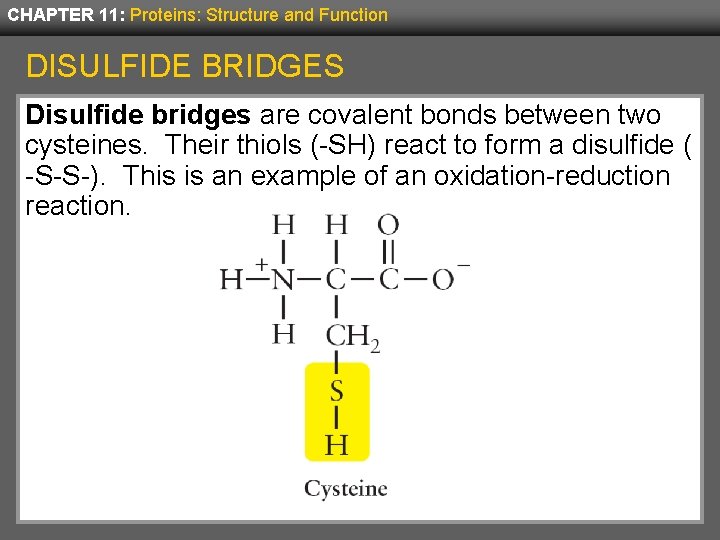
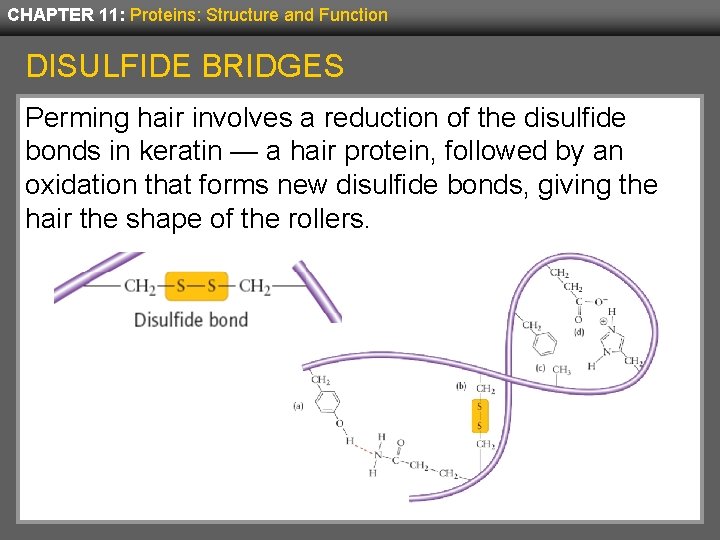
CHAPTER 11: Proteins: Structure and Function DISULFIDE BRIDGES Disulfide bridges are covalent bonds between two cysteines. Their thiols (-SH) react to form a disulfide ( -S-S-). This is an example of an oxidation-reduction reaction.

CHAPTER 11: Proteins: Structure and Function DISULFIDE BRIDGES Perming hair involves a reduction of the disulfide bonds in keratin — a hair protein, followed by an oxidation that forms new disulfide bonds, giving the hair the shape of the rollers.

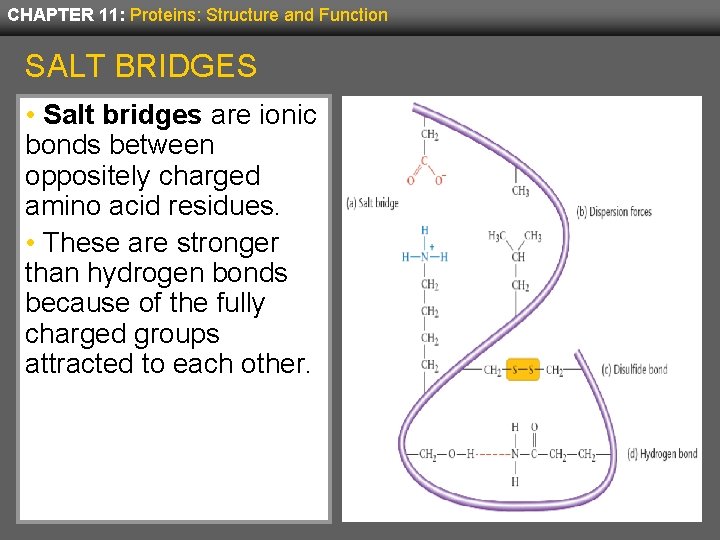
CHAPTER 11: Proteins: Structure and Function SALT BRIDGES • Salt bridges are ionic bonds between oppositely charged amino acid residues. • These are stronger than hydrogen bonds because of the fully charged groups attracted to each other.

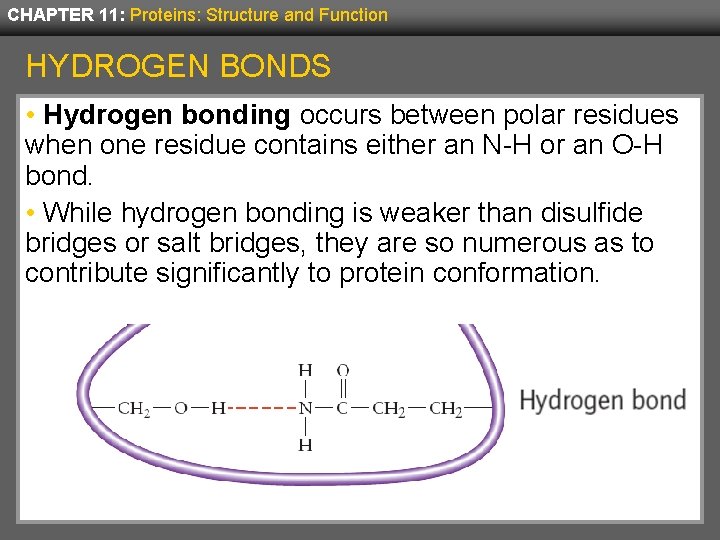
CHAPTER 11: Proteins: Structure and Function HYDROGEN BONDS • Hydrogen bonding occurs between polar residues when one residue contains either an N-H or an O-H bond. • While hydrogen bonding is weaker than disulfide bridges or salt bridges, they are so numerous as to contribute significantly to protein conformation.

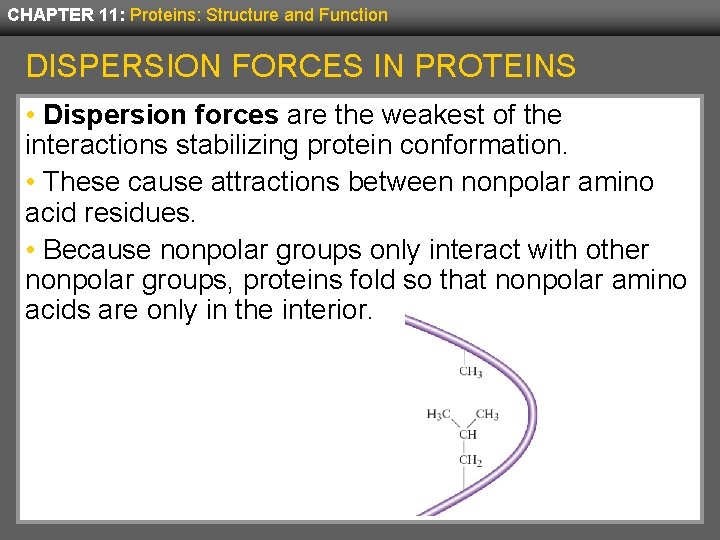
CHAPTER 11: Proteins: Structure and Function DISPERSION FORCES IN PROTEINS • Dispersion forces are the weakest of the interactions stabilizing protein conformation. • These cause attractions between nonpolar amino acid residues. • Because nonpolar groups only interact with other nonpolar groups, proteins fold so that nonpolar amino acids are only in the interior.

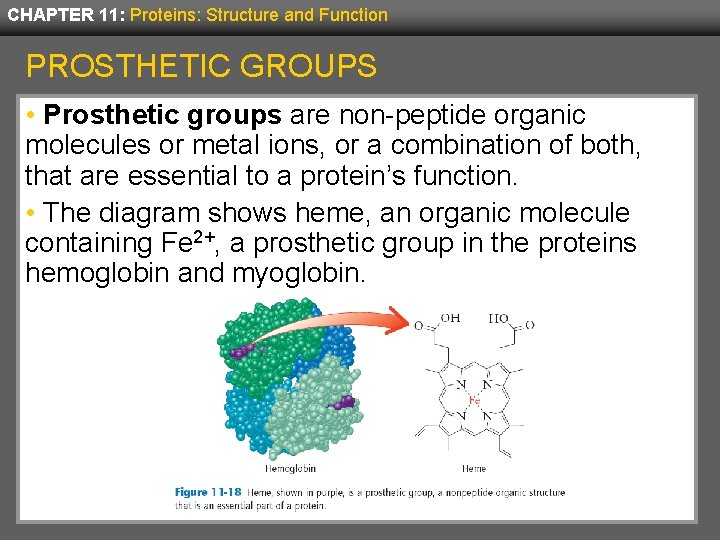
CHAPTER 11: Proteins: Structure and Function PROSTHETIC GROUPS • Prosthetic groups are non-peptide organic molecules or metal ions, or a combination of both, that are essential to a protein’s function. • The diagram shows heme, an organic molecule containing Fe 2+, a prosthetic group in the proteins hemoglobin and myoglobin.

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS Cystic Fibrosis (CF) is a disease caused by a missing phenylalanine-508 in a protein containing 1480 amino acids. The protein is necessary for chloride ions (Cl-) to pass through the cell membrane. The abnormal CF protein does not fold correctly as a result of the missing amino acid. As a result, mucus accumulates in the lungs causing respiratory infections and difficulty breathing. Many individuals with CF die by age 30, and require a lifetime of specialized care. a. Write the structure of the missing amino acid. b. Where in the primary structure is a phe missing? c. What is a gene? Would you expect cystic fibrosis to be a hereditary disease or an infectious disease? Explain. d. What causes the protein that causes CF not to fold correctly?

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS 1. Write the products formed in the following reduction. 2. To remove the odor from a dog sprayed by a skunk, the dog can be bathed in water containing a small amount of bleach (Na. OCl) or hydrogen peroxide (H 2 O 2). Both bleach and hydrogen peroxide are strong oxidizing agents. The chemical structure of one of the compounds that give skunks their offensive odor is shown to the right. Write the oxidation reaction that occurs when the thiol functional group in this compound reacts with an oxidizing agent.

CHAPTER 11: Proteins: Structure and Function QUATERNARY STRUCTURE • Some proteins consist of more than one polypeptide chain; each is termed a subunit. • The quaternary structure of a protein is the relative arrangement and position of the subunits within the protein. • The subunits are stabilized by the same interactions responsible for tertiary structure: disulfide bridges, salt bridges, hydrogen bonds, and dispersion forces.

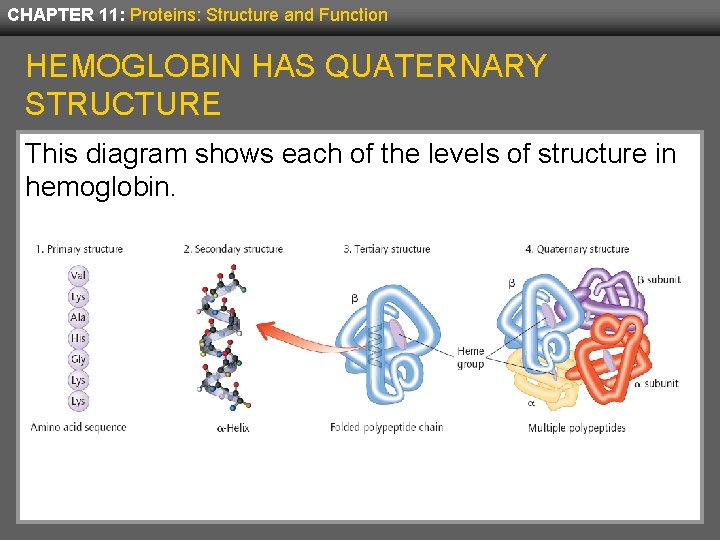
CHAPTER 11: Proteins: Structure and Function HEMOGLOBIN HAS QUATERNARY STRUCTURE This diagram shows each of the levels of structure in hemoglobin.

CHAPTER 11: Proteins: Structure and Function PROTEIN DENATURATION • The denaturation of a protein is any process disrupting its folding. • Denaturation does not alter the primary structure. • Denaturation is only rarely reversible. • Denaturants are agents capable of denaturing proteins and include the following: • Heat • p. H changes • Detergents • Some metal ions such as Pb+2, Hg+2 • Cooking an egg is an example of irreversible protein denaturation.

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS For the following pairs of amino acids, indicate how they might interact to contribute to the tertiary or quaternary structure of a protein. Choose from the following: disulfide bond, salt bridge, hydrogen bonding, or dispersion forces. aspartic acid and histidine serine and lysine leucine and valine two cysteines tryptophan and isoleucine

CHAPTER 11: Proteins: Structure and Function TYPES OF PROTEINS • There are several ways to categorize proteins. One way is by solubility properties: • Fibrous proteins • Insoluble, consists of long fibers or sheets • Examples include collagen, keratin, elastins • Globular proteins • Compact, soluble • Examples include enzymes, antibodies, and hormones • Membrane proteins • Contained within cell membrane; nonpolar exterior • Examples include receptors, channels

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS 1. List three ways to denature a protein. 2. State two differences between globular and fibrous proteins.

CHAPTER 11: Proteins: Structure and Function 11. 5 ENZYMES

CHAPTER 11: Proteins: Structure and Function HOW DO ENZYMES WORK? • Enzymes are globular proteins able to catalyze many chemical reactions in living things. • Enzymes work by lowering the activation energy, EA, for a reaction. • Enzymes do not alter the overall change in energy of a reaction, ∆E. • The reactant in an enzyme-catalyzed reaction is called the substrate.

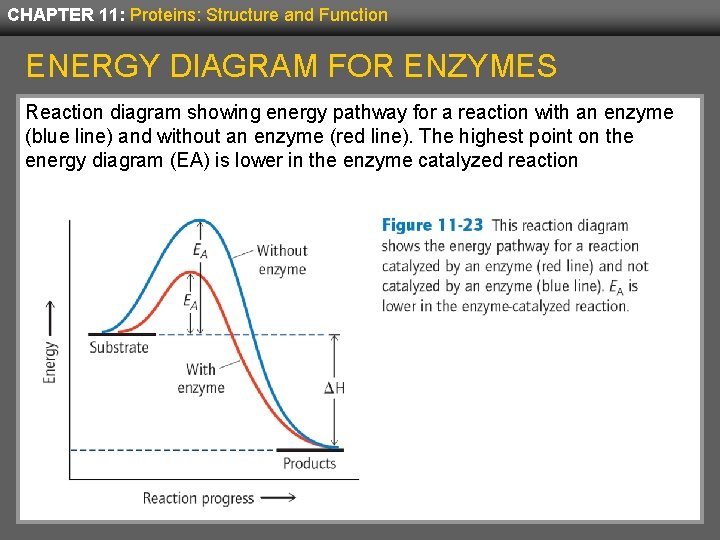
CHAPTER 11: Proteins: Structure and Function ENERGY DIAGRAM FOR ENZYMES Reaction diagram showing energy pathway for a reaction with an enzyme (blue line) and without an enzyme (red line). The highest point on the energy diagram (EA) is lower in the enzyme catalyzed reaction

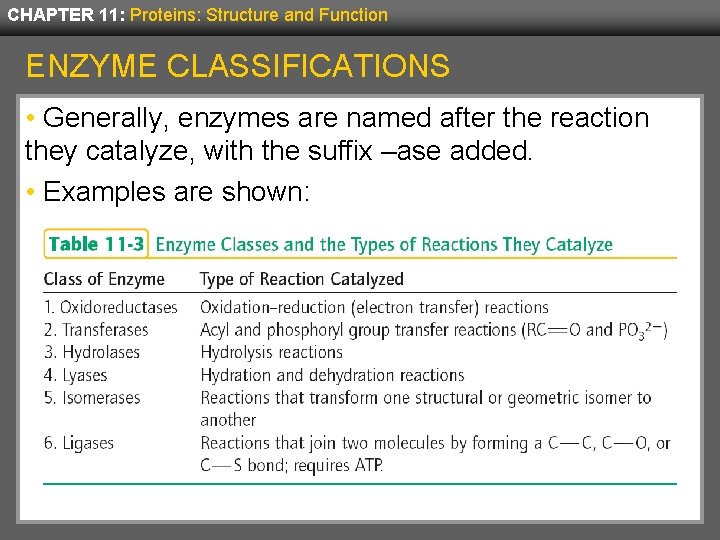
CHAPTER 11: Proteins: Structure and Function ENZYME CLASSIFICATIONS • Generally, enzymes are named after the reaction they catalyze, with the suffix –ase added. • Examples are shown:

CHAPTER 11: Proteins: Structure and Function THE ENZYME-SUBSTRATE COMPLEX • An enzyme is able to act on its substrate by binding to it at the enzyme’s active site. • The shape of the substrate is complementary to the shape of the active site. • Binding occurs through weak intermolecular forces such as hydrogen bonds, dipole-dipole interactions, and dispersion forces. • The relationship between the active site and the bound substrate is termed the enzyme-substrate complex. • This relationship is described through the lock-andkey model: they fit the same way a key fits in a lock.

CHAPTER 11: Proteins: Structure and Function ENZYME RATES • An enzyme speeds up a reaction by a considerable factor. • Reactions mediated by enzymes may proceed as slowly as two substrate molecules acted on per second to as fast as 10 million molecules per second! • These reactions tend to be reversible.

CHAPTER 11: Proteins: Structure and Function COFACTORS AND COENZYMES • Cofactors are generally metal ions required by the enzyme for its catalytic function. • Examples include Mg 2+, Zn 2+, and Fe 2+. • Coenzymes are organic molecules with a similar function. • Examples include NAD+/NADH, FAD/FADH 2, and coenzyme A, all derived from B vitamins. • While enzymes are not changed through the reaction, coenzymes are changed and must be recycled to be used again.

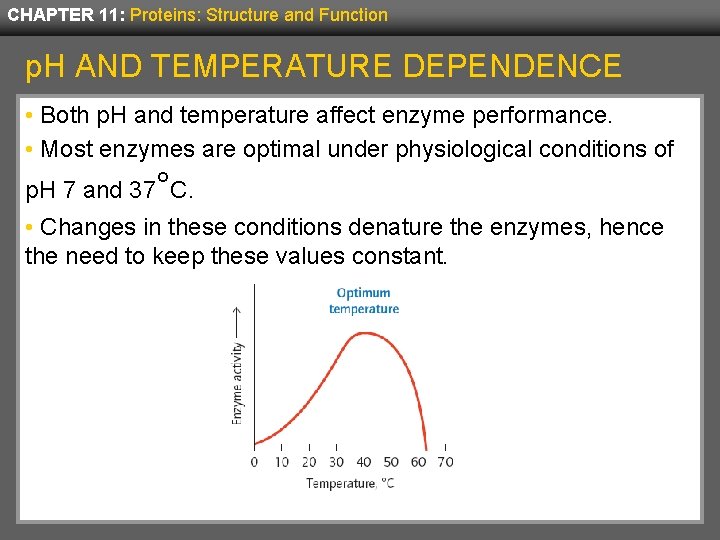
CHAPTER 11: Proteins: Structure and Function p. H AND TEMPERATURE DEPENDENCE • Both p. H and temperature affect enzyme performance. • Most enzymes are optimal under physiological conditions of p. H 7 and 37°C. • Changes in these conditions denature the enzymes, hence the need to keep these values constant.

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEMS 1. What is the active site of an enzyme? 2. Which of the following changes when a catalyst is added to a reaction: • EA or ∆E? • The heat released from the reaction or the rate of the reaction? 3. How is the rate of an enzyme catalyzed reaction affected by the following changes? a. an increase in p. H b. a decrease in p. H c. a decrease in temperature d. an increase in temperature

CHAPTER 11: Proteins: Structure and Function ENZYME INHIBITORS • Enzyme inhibitors prevent an enzyme from performing its function. • Non-specific inhibitors act on all enzymes. • Examples include p. H and temperature. • Specific inhibitors act on a selected few enzymes. Two categories of these: • Competitive inhibitors • Noncompetitive inhibitors

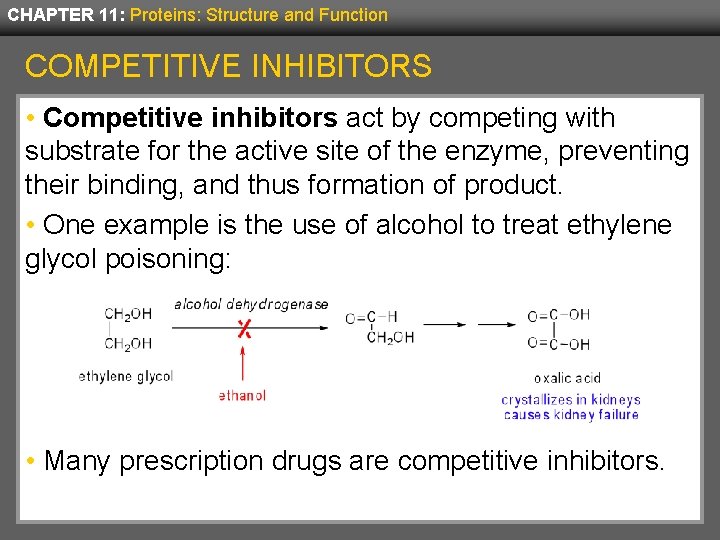
CHAPTER 11: Proteins: Structure and Function COMPETITIVE INHIBITORS • Competitive inhibitors act by competing with substrate for the active site of the enzyme, preventing their binding, and thus formation of product. • One example is the use of alcohol to treat ethylene glycol poisoning: • Many prescription drugs are competitive inhibitors.

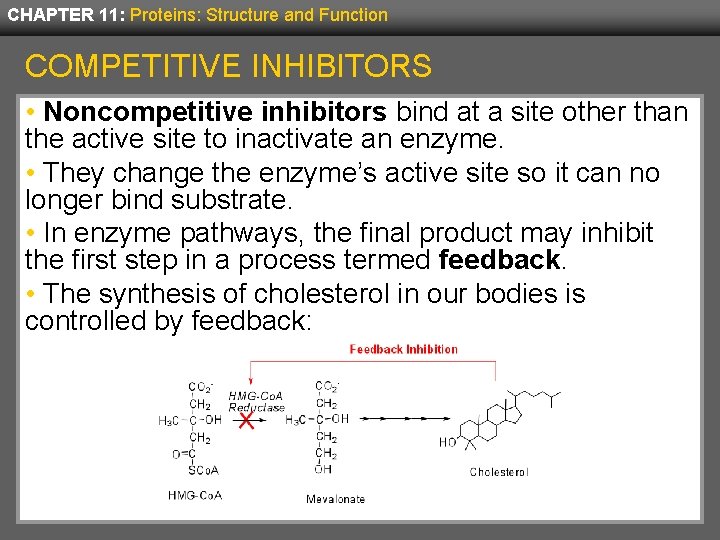
CHAPTER 11: Proteins: Structure and Function COMPETITIVE INHIBITORS • Noncompetitive inhibitors bind at a site other than the active site to inactivate an enzyme. • They change the enzyme’s active site so it can no longer bind substrate. • In enzyme pathways, the final product may inhibit the first step in a process termed feedback. • The synthesis of cholesterol in our bodies is controlled by feedback:

CHAPTER 11: Proteins: Structure and Function PRACTICE PROBLEM Which type of enzyme inhibitor binds to the active site of an enzyme: a competitive inhibitor or a noncompetitive inhibitor?

CHAPTER 11: Proteins: Structure and Function SUMMARY OF MAIN CHAPTER POINTS, P 1 • Amino Acids • Proteins are constructed from a set of 20 -22 amino acids • An amino acid contains an amine and a carboxylic acid covalently bonded to a carbon atom—the -carbon. The -carbon also contains a side chain, R, which determines the identity of the amino acid. • The side chains of the 20 natural amino acids can be classified as either nonpolar or polar. • The polar side chains can be classified as acidic, basic, and neutral. • Chirality and Amino Acids • A chiral object has a non-superposable mirror image. • Nineteen of the amino acids are chiral. • Peptides are molecules composed of two or more amino acids held together by peptide bonds. • A peptide bond is an amide formed between the carboxylic acid of one amino acid and the amine of another amino acid.

CHAPTER 11: Proteins: Structure and Function SUMMARY OF MAIN CHAPTER POINTS, P 2 • Protein Architecture • Proteins are composed of anywhere from 50 to thousands of amino acids. • The four levels of protein architecture are defined as primary structure, secondary structure, tertiary structure, and quaternary structure. • The primary structure of a protein is its amino acid sequence. • Secondary protein structure refers to regular folding patterns in local regions of the polypeptide backbone. • The tertiary structure of a protein is the complex and irregular folding of the entire polypeptide. • The quaternary structure of a protein is the relative arrangement and position of each of the subunits within the overall structure of the protein. • Denaturation of a protein is the disruption of the secondary, tertiary, or quaternary structure of a protein so that it can no longer perform its function.

CHAPTER 11: Proteins: Structure and Function SUMMARY OF MAIN CHAPTER POINTS, P 3 • Enzymes act as catalysts to speed up chemical reactions. • Enzymes work by lowering the energy of activation, EA, for a reaction. • Cofactors and coenzymes sometimes help an enzyme achieve its catalytic effect. • Enzyme inhibitors are compounds that prevent an enzyme from performing its function. • A competitive inhibitor competes with the substrate for the active site of the enzyme. • Noncompetitive inhibitors bind at a location on the enzyme other than the active site and prevent the substrate from binding to the substrate.