Titration curves Of amino acids and weak acidsacetic










- Slides: 21

Titration curves Of amino acids and weak acids(acetic acid)

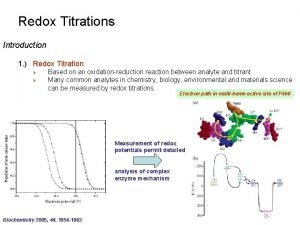
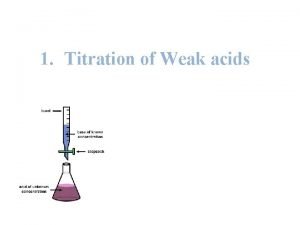
Titration • Titration curves are produced by monitoring the p. H of given volume of a sample solution after successive addition of acid or alkali • The curves are usually plots of p. H against the volume of titrant added or more correctly against the number of equivalents added per mole of the sample

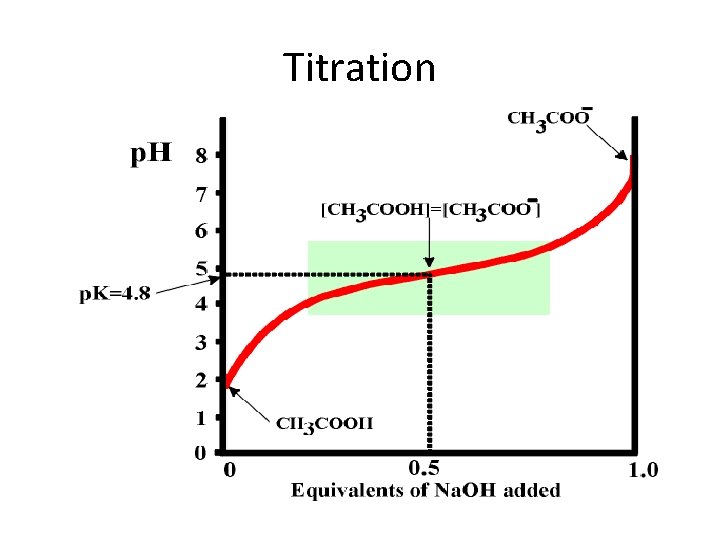
Titration of acetic acid • At the starting point the acid form predominates (CH 3 COOH). • As strong base is added (e. g. Na. OH), the acid is converted to its conjugate base. • At the mid point of the titration, where p. H=p. K, the concentrations of the acid and the conjugate base are equal. • At the end point(equivalence point), the conjugate base predominates, and the total amount of OH added is equivalent to the amount of acid that was present in the starting point.

Titration

Titration Determination of p. Ka values: p. Ka values can be obtained from the titration data by the following methods: 1. The p. H at the point of inflection is the p. Ka value and this may be read directly 2. By definition the p. Ka value is equal to the p. H at which the acid is half titrated. The p. Ka can therefore be obtained from the knowledge of the end point of the titration.

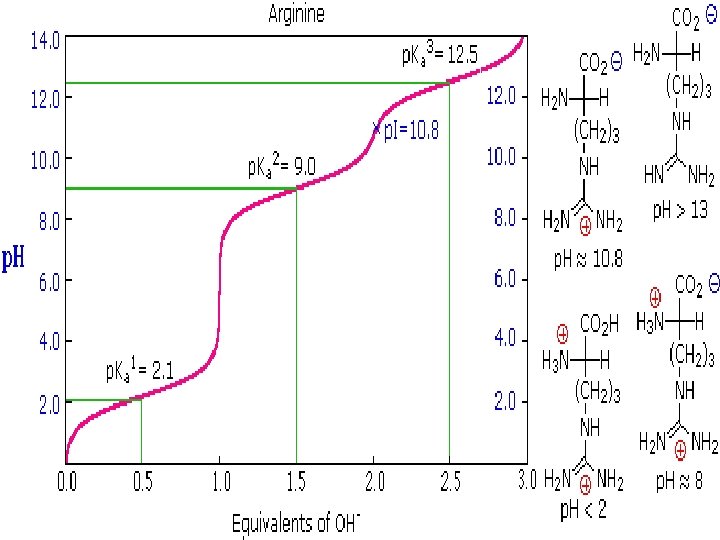
Titration of amino acids • Titration of glycine • Titration of arginine

Titration • When an amino acid is dissolved in water it exists predominantly in the isoelectric form. • Upon titration with acid, it acts as a base, and upon titration with base, it acts as an acid( a compound that can act as either an acid or a base is known as an amphoteric compound).

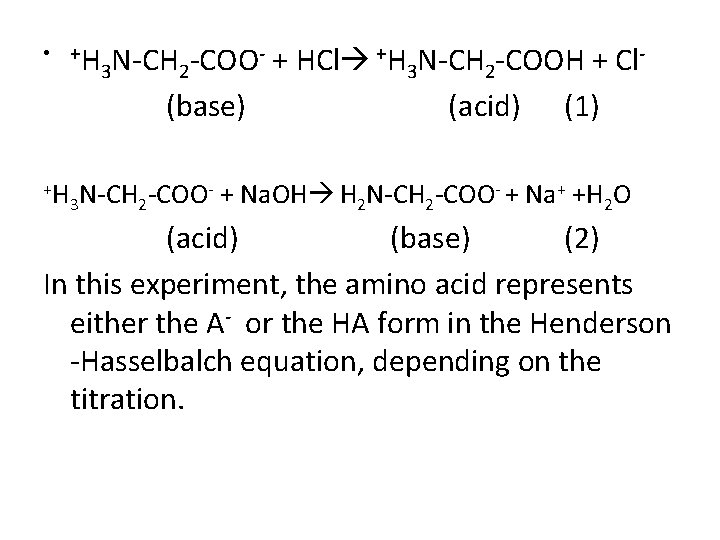
• +H +H - + HCl +H N-CH -COOH + Cl. N-CH -COO 3 2 (base) (acid) (1) - + Na. OH H N-CH -COO- + Na+ +H O N-CH -COO 3 2 2 (acid) (base) (2) In this experiment, the amino acid represents either the A- or the HA form in the Henderson -Hasselbalch equation, depending on the titration.

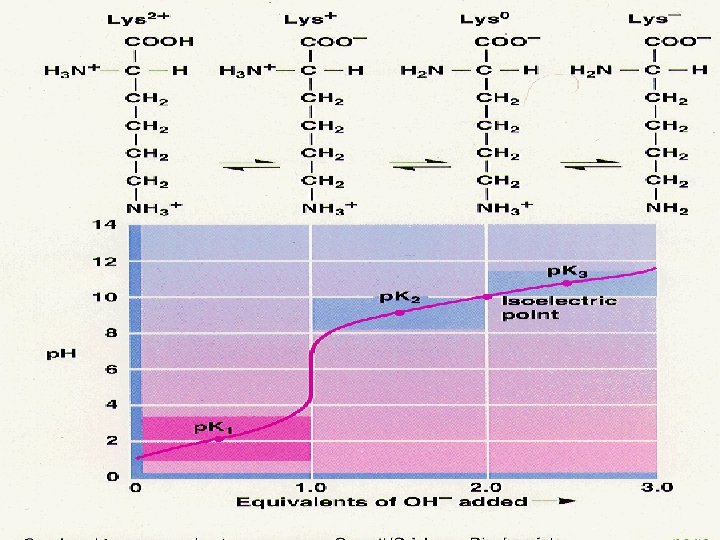
Acid–base properties • All of the amino acids have an acidic group (COOH) and a basic group (NH 2) attached to the α carbon. • Two of the amino acids have acidic side chains: aspartate and glutamate. • Three of the amino acids have basic side chains: arginine, histidine, and lysine.

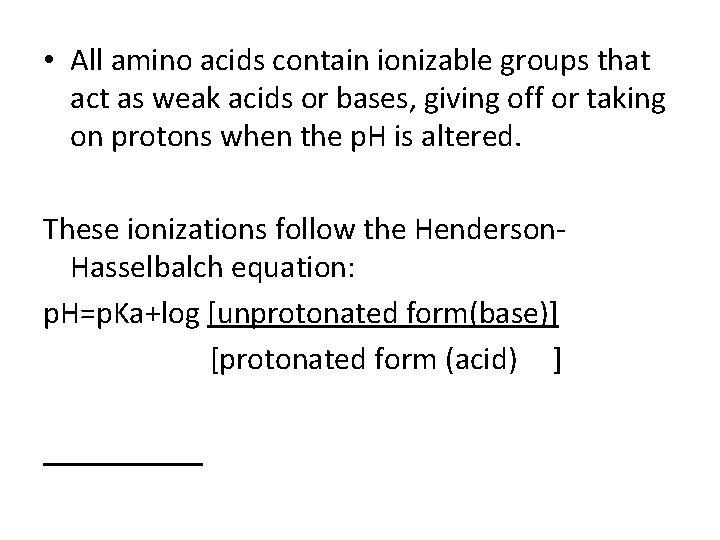
• All amino acids contain ionizable groups that act as weak acids or bases, giving off or taking on protons when the p. H is altered. These ionizations follow the Henderson. Hasselbalch equation: p. H=p. Ka+log [unprotonated form(base)] [protonated form (acid) ]

• When the conc of the unprotonated form equals that of the unprotonated form, the ratio of their concentrations equals 1, and log 1=0. • Hence, p. Ka can be defined as the p. H at which the concentrations of the protonated and unprotonated forms of a particular ionizable species are equal. • The p. Ka also equals the p. H at which the ionizable group is at its best buffering capacity; that is the p. H at which the solution resists changes in p. H most effectively.

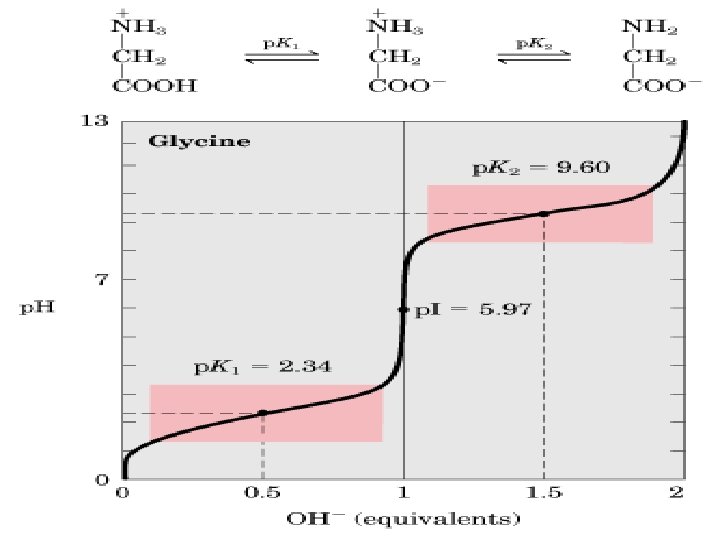
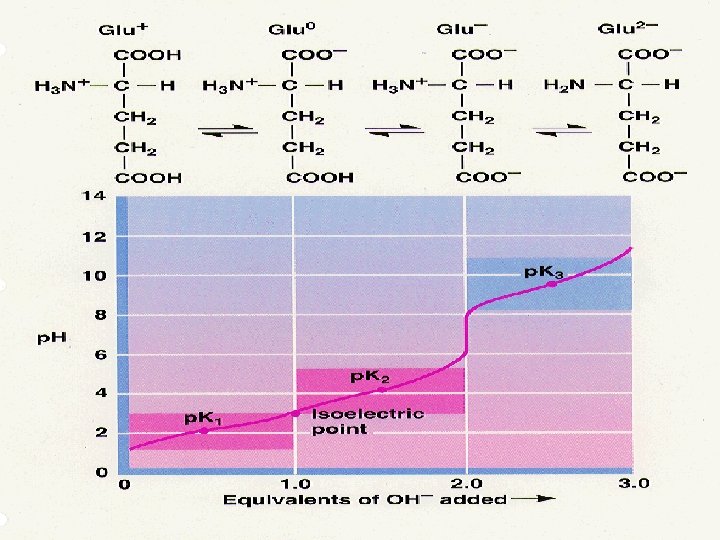
• Consider applying the Henderson-Hasselbalch equation to the titration of glycine with acid and base. • Glycine has two ionizable groups: a corboxyl group and an amino group, with p. Ka values of 2. 4 and 9. 6 respectively. • In water at p. H 6, glycine exists as a dipolar ion, or zwitterion, in which the carboxyl group is unprotonated(-COO- ) and the amino group is protonated to give the substituted ammonium ion(-NH 3+).

• Addition of acid to the solution lowers the p. H rapidly at first and then more slowly as the buffering action of the carboxyl is exerted. • At p. H 2. 4 the p. Ka is reached, one-half the acid has been consumed, and the carboxyl group is half ionized and is most effective as a buffer. • Titration of the amino group with base follows a similar curve into the alkaline region. • The intersection between the titration of the carboxyl group and the titration of the amino group describes in this case the point at which glycine has no net charge, and is called the isoelectric point (p. I).

The isoelectric point (p. I) • the isoelectric point, p. I, is the p. H of an aqueous solution of an amino acid at which the molecules have no net charge. In other words, the positively charged groups are exactly balanced by the negatively charged groups. • For simple amino acids such as alanine, the p. I is an average of the p. Ka's of the carboxyl (2. 34) and ammonium (9. 69) groups. Thus, the p. I for alanine is calculated to be: (2. 34 + 9. 69)/2 = 6. 02. • If additional acidic or basic groups are present as side-chain functions, the p. I is the average of the p. Ka's of the two most similar acids.

Cont. . (p. I) • In the case of aspartic acid, the similar acids are the alpha-carboxyl function (p. Ka = 2. 1) and the side-chain carboxyl function (p. Ka = 3. 9), so p. I = (2. 1 + 3. 9)/2 = 3. 0. • For arginine, the similar acids are the guanidinium species on the side-chain (p. Ka = 12. 5) and the alpha-ammonium function (p. Ka = 9. 0), so the calculated p. I = (12. 5 + 9. 0)/2 = 10. 75.


• Most amino acids contain carboxyl and amino groups having p. Ka values similar to those of glycine. • In addition to these groups, many amino acids contain other ionizable groups, which introduce other “steps” or p. Ka values into their titration curves.

Titration curves • The p. K is the p. H at the midpoint of the buffering region (where the p. H changes only slightly upon addition of either acid or base). • The p. K is the p. H corresponding to the inflection point in the titration curve. • The end point of a titration curve represents the observed end of the titration. • The isoelectric point (isoelectric p. H; p. I) is the p. H at which the amino acid has a net zero charge. For a simple diprotic amino acid, the p. I falls halfway between the two p. K values. For acidic amino acids, the p. I is given by ½(p. K 1 + p. K 2) and for basic amino acids it’s given by ½(p. K 2 + p. K 3)



 Titration curve for amino acids
Titration curve for amino acids Glycine titration curve
Glycine titration curve Lysine titration
Lysine titration Titration curve of amino acids
Titration curve of amino acids Strong acid-strong base titration indicator
Strong acid-strong base titration indicator Redoxtitration
Redoxtitration Strong acid-strong base titration indicator
Strong acid-strong base titration indicator Principle of conductometric titration
Principle of conductometric titration Why do titration curves flatten out
Why do titration curves flatten out Why do titration curves flatten out
Why do titration curves flatten out Acid base titration equivalence point
Acid base titration equivalence point Ketogenic vs glucogenic amino acids
Ketogenic vs glucogenic amino acids Why is lysine basic
Why is lysine basic Classification of amino acids
Classification of amino acids Difference between hydrophobic and hydrophilic amino acids
Difference between hydrophobic and hydrophilic amino acids Titration vs back titration
Titration vs back titration Titration vs back titration
Titration vs back titration Define redox titration
Define redox titration Difference between a strong and weak acid
Difference between a strong and weak acid Weak acid and weak base reaction
Weak acid and weak base reaction Strong acid examples
Strong acid examples Genetic code wheel
Genetic code wheel