Amino acids Proteins Amino Acids Amino acids Are











- Slides: 36

Amino acids & Proteins

Amino Acids Amino acids § Are the building blocks of proteins. § Contain a carboxylic acid group and an amino group on the alpha ( ) carbon. § Are ionized in solution. § Each contain a different side group (R). R │ H 2 N—C —COOH │ H R + │ H 3 N—C —COO− │ H Ionized form

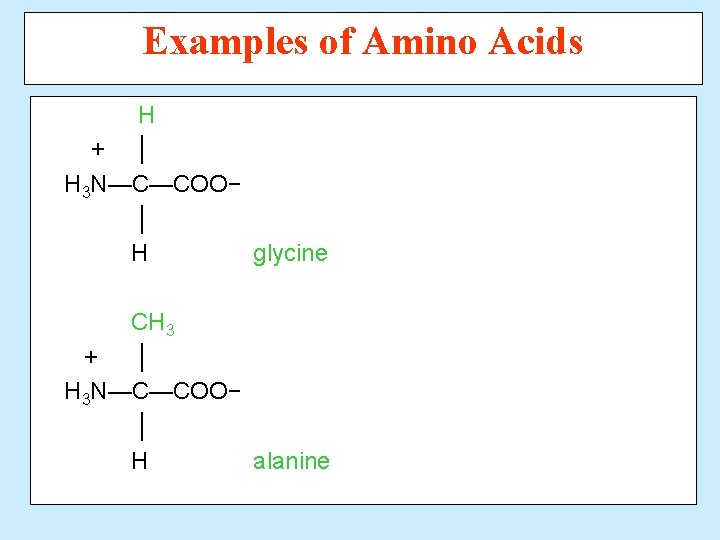
Examples of Amino Acids H + │ H 3 N—C—COO− │ H glycine CH 3 + │ H 3 N—C—COO− │ H alanine

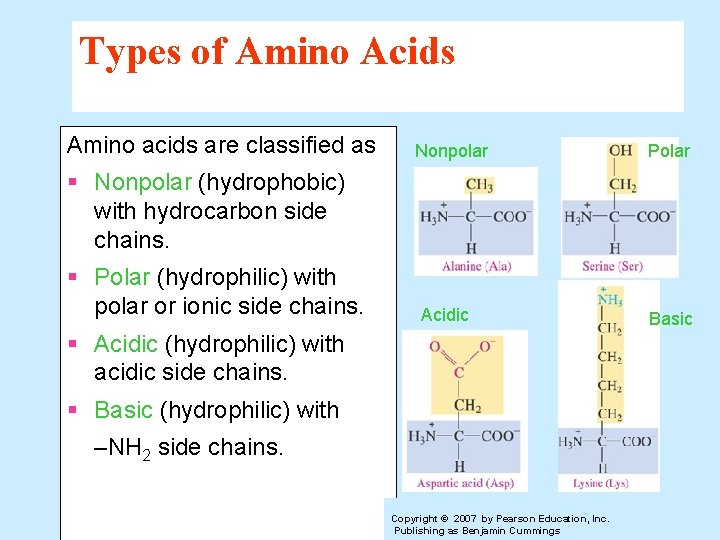
Types of Amino Acids Amino acids are classified as Nonpolar Polar § Nonpolar (hydrophobic) with hydrocarbon side chains. § Polar (hydrophilic) with polar or ionic side chains. Acidic § Acidic (hydrophilic) with acidic side chains. § Basic (hydrophilic) with –NH 2 side chains. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Basic

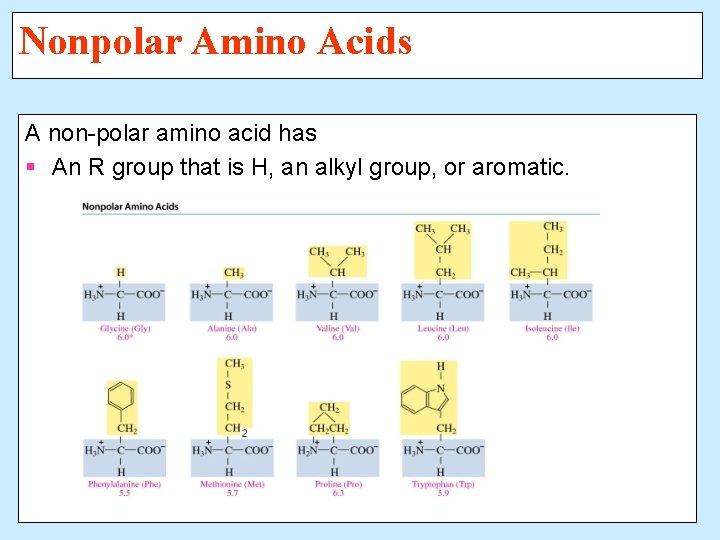
Nonpolar Amino Acids A non-polar amino acid has § An R group that is H, an alkyl group, or aromatic.

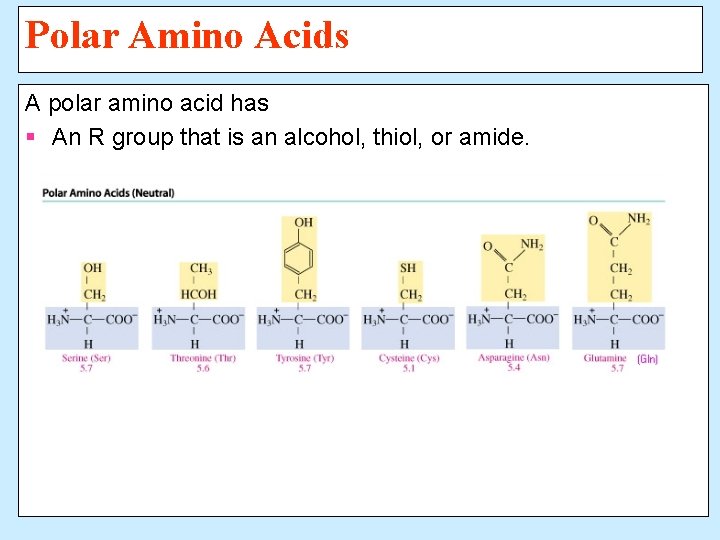
Polar Amino Acids A polar amino acid has § An R group that is an alcohol, thiol, or amide.

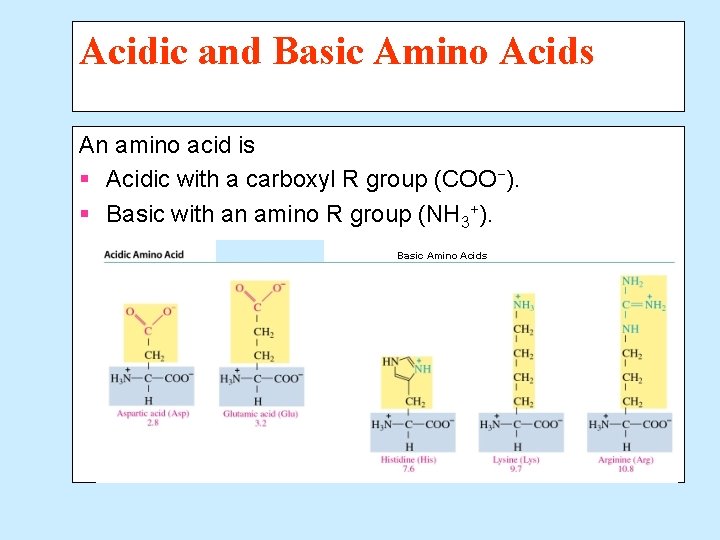
Acidic and Basic Amino Acids An amino acid is § Acidic with a carboxyl R group (COO−). § Basic with an amino R group (NH 3+). Basic Amino Acids

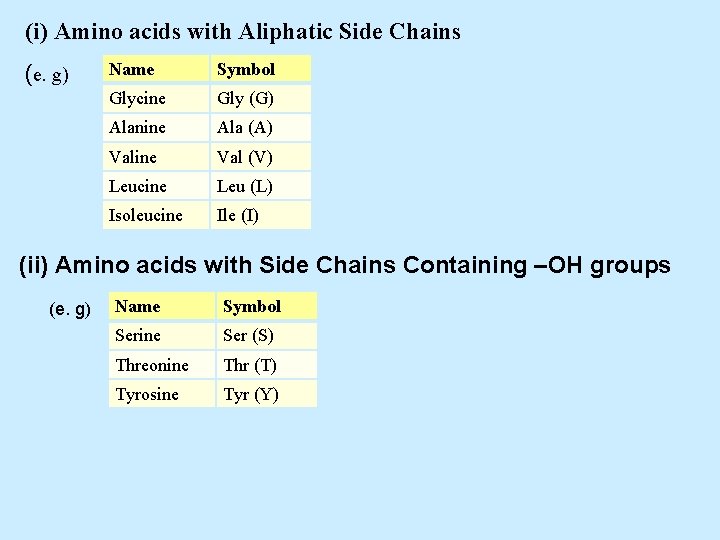
(i) Amino acids with Aliphatic Side Chains (e. g) Name Symbol Glycine Gly (G) Alanine Ala (A) Valine Val (V) Leucine Leu (L) Isoleucine Ile (I) (ii) Amino acids with Side Chains Containing –OH groups (e. g) Name Symbol Serine Ser (S) Threonine Thr (T) Tyrosine Tyr (Y)

(iii) Amino acids with Side Chains Containing Sulfur (e. g) Name Symbol Cysteine Cys (C) Methionine Met (M) (iv) Amino acids with Side Chains Containing Acidic Groups / Amides Name Symbol Aspartic acid Asp (D) Asparagine Asn (N) Glutamic acid Glu (E) Glutamine Gln (Q) (v) Amino acids with side chains containing basic groups Name Symbol Arginine Arg (R) Lysine Lys (K) Histidine His (H)

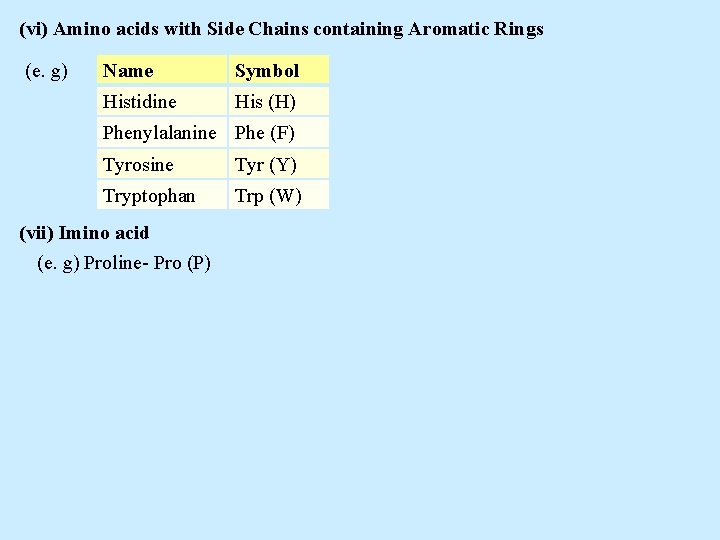
(vi) Amino acids with Side Chains containing Aromatic Rings (e. g) Name Symbol Histidine His (H) Phenylalanine Phe (F) Tyrosine Tyr (Y) Tryptophan Trp (W) (vii) Imino acid (e. g) Proline- Pro (P)

Fischer Projections of Amino Acids Amino acids § Are chiral except for glycine. § Have Fischer projections that are stereoisomers. § That are L are used in proteins. L-alanine D-alanine L-cysteine D-cysteine

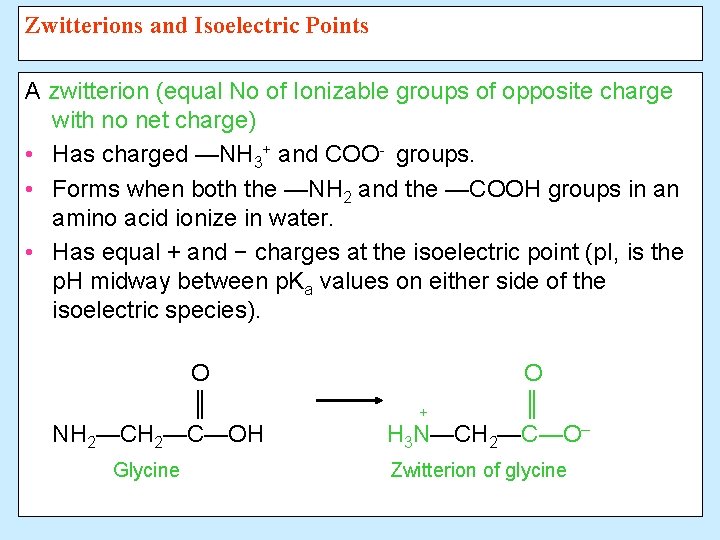
Zwitterions and Isoelectric Points A zwitterion (equal No of Ionizable groups of opposite charge with no net charge) • Has charged —NH 3+ and COO- groups. • Forms when both the —NH 2 and the —COOH groups in an amino acid ionize in water. • Has equal + and − charges at the isoelectric point (p. I, is the p. H midway between p. Ka values on either side of the isoelectric species). O ║ NH 2—C—OH Glycine O ║ + H 3 N—CH 2—C—O– Zwitterion of glycine

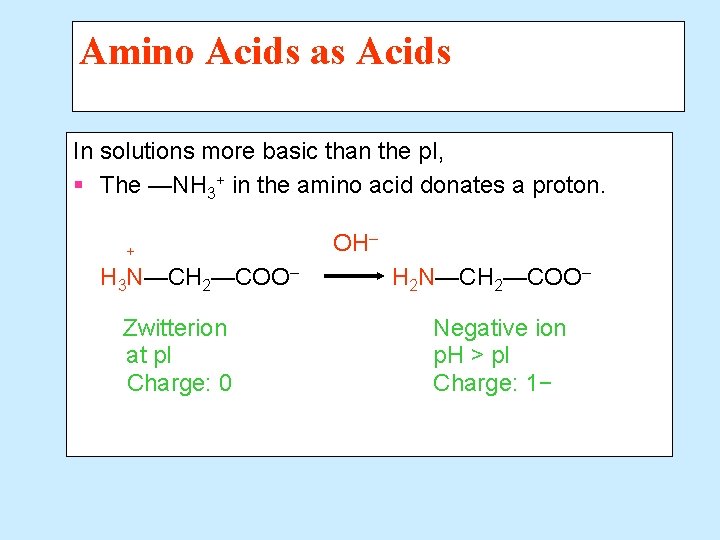
Amino Acids as Acids In solutions more basic than the p. I, § The —NH 3+ in the amino acid donates a proton. + H 3 N—CH 2—COO– Zwitterion at p. I Charge: 0 OH– H 2 N—CH 2—COO– Negative ion p. H > p. I Charge: 1−

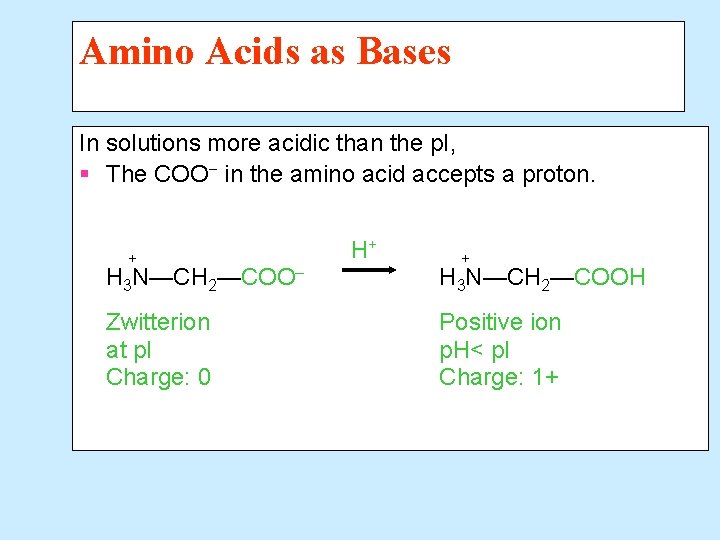
Amino Acids as Bases In solutions more acidic than the p. I, § The COO− in the amino acid accepts a proton. + H+ + H 3 N—CH 2—COO– H 3 N—CH 2—COOH Zwitterion at p. I Charge: 0 Positive ion p. H< p. I Charge: 1+

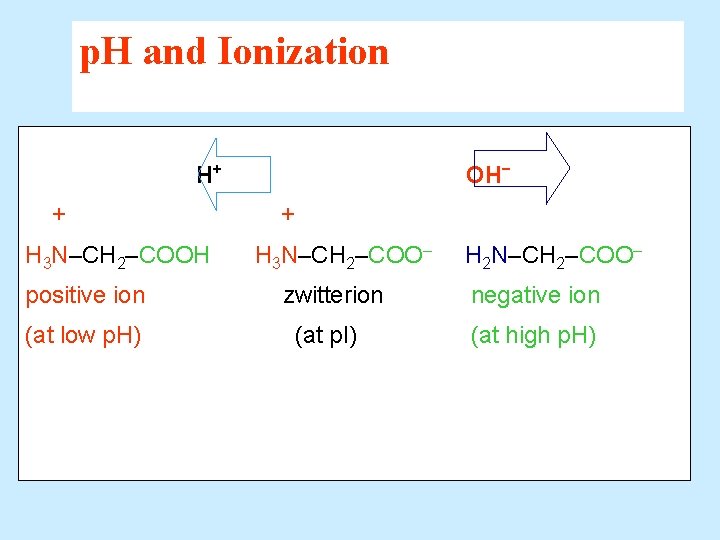
p. H and Ionization H+ + H 3 N–CH 2–COOH positive ion (at low p. H) OH− + H 3 N–CH 2–COO– zwitterion (at p. I) H 2 N–CH 2–COO– negative ion (at high p. H)

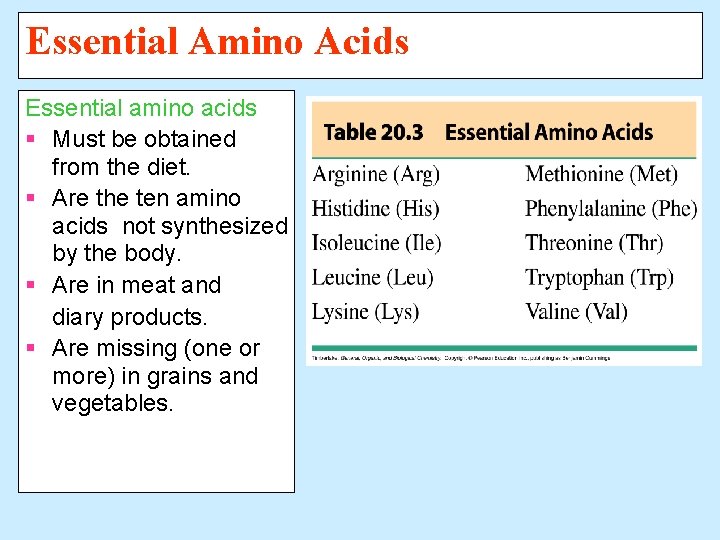
Essential Amino Acids Essential amino acids § Must be obtained from the diet. § Are the ten amino acids not synthesized by the body. § Are in meat and diary products. § Are missing (one or more) in grains and vegetables.

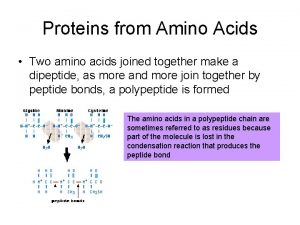
The Peptide Bond A peptide bond § Is an amide bond. § Forms between the carboxyl group of one amino acid and the amino group of the next amino acid. O + || H 3 N—CH 2—C—O– + CH 3 O + | || H 3 N—CH—C—O– O H CH 3 O + || | | || H 3 N—CH 2—C—N—CH—C—O– + H 2 O peptide bond

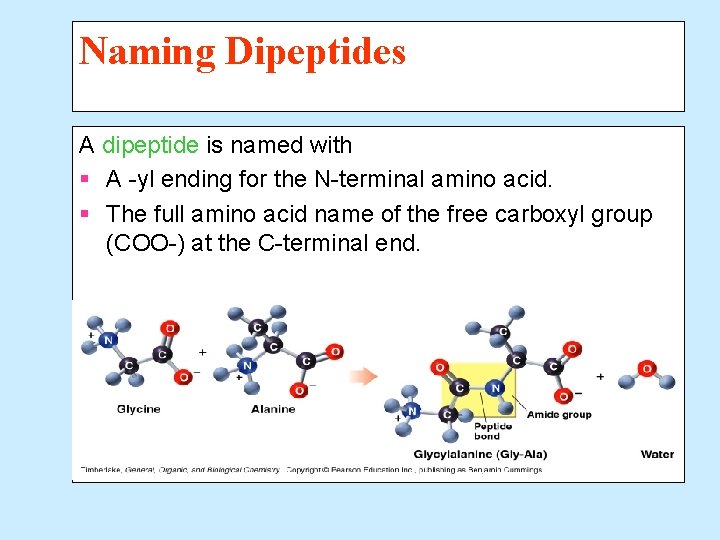
Naming Dipeptides A dipeptide is named with § A -yl ending for the N-terminal amino acid. § The full amino acid name of the free carboxyl group (COO-) at the C-terminal end.

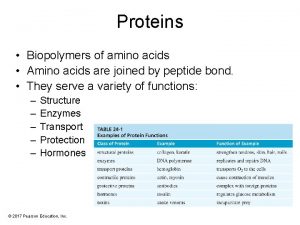
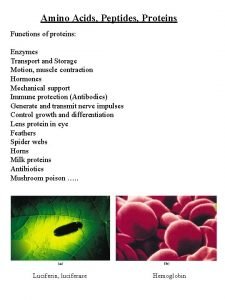
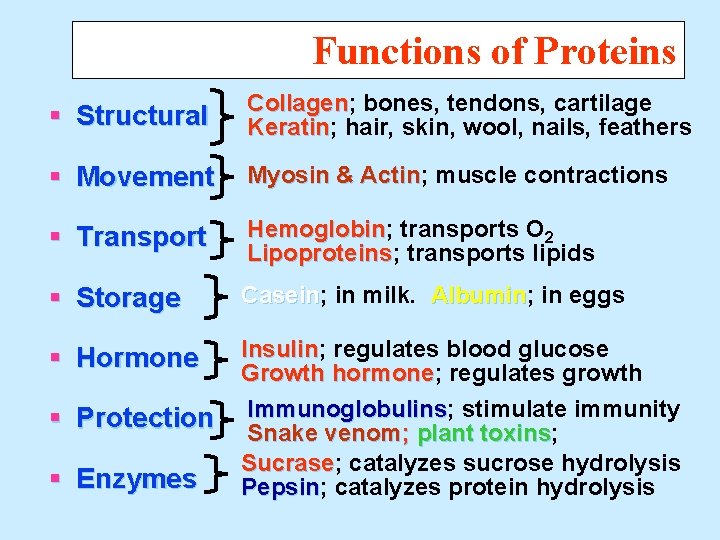
Functions of Proteins § Structural Collagen; Collagen bones, tendons, cartilage Keratin; Keratin hair, skin, wool, nails, feathers § Movement Myosin & Actin; Actin muscle contractions § Transport Hemoglobin; Hemoglobin transports O 2 Lipoproteins; Lipoproteins transports lipids § Storage Casein; Casein in milk. Albumin; Albumin in eggs § Hormone Insulin; Insulin regulates blood glucose Growth hormone; hormone regulates growth Immunoglobulins; Immunoglobulins stimulate immunity Snake venom; plant toxins; toxins Sucrase; Sucrase catalyzes sucrose hydrolysis Pepsin; Pepsin catalyzes protein hydrolysis § Protection § Enzymes

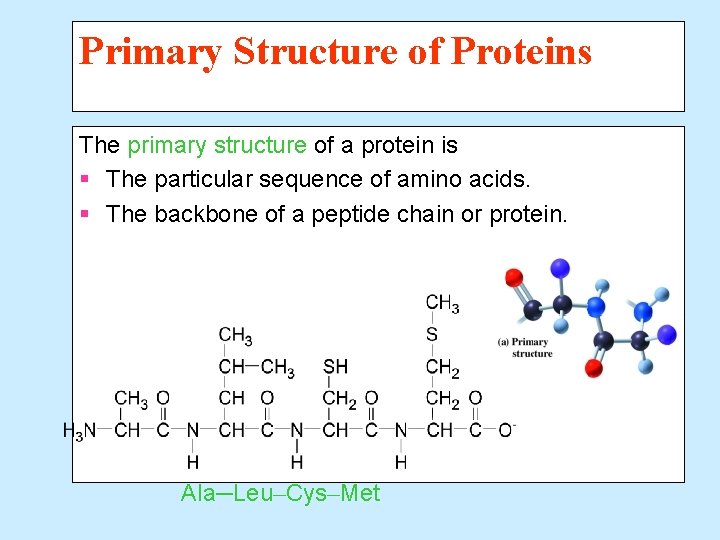
Primary Structure of Proteins The primary structure of a protein is § The particular sequence of amino acids. § The backbone of a peptide chain or protein. Ala─Leu─Cys─Met

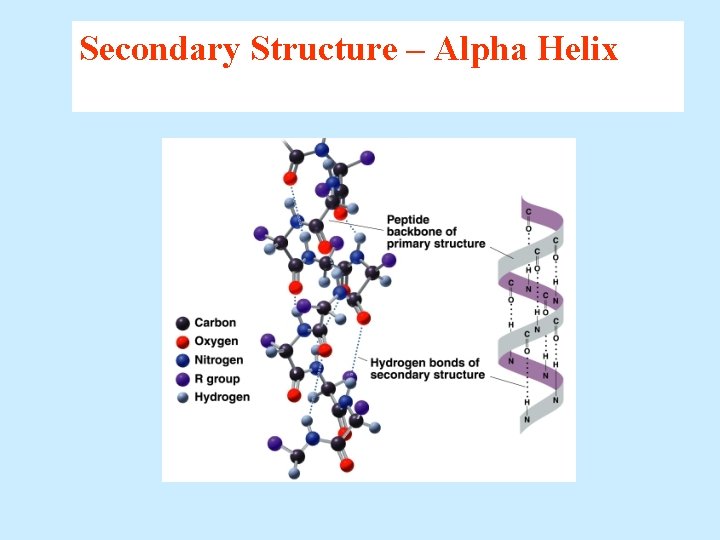
Secondary Structure – Alpha Helix The secondary structures of proteins indicate three-dimensional spatial arrangements of the polypeptide chains. An alpha helix has § A coiled shape held in place by hydrogen bonds between the amide groups and the carbonyl groups of the amino acids along the chain. § Hydrogen bonds between the H of a –N-H group and the O of C=O of the fourth amino acid down the chain.

Secondary Structure – Alpha Helix

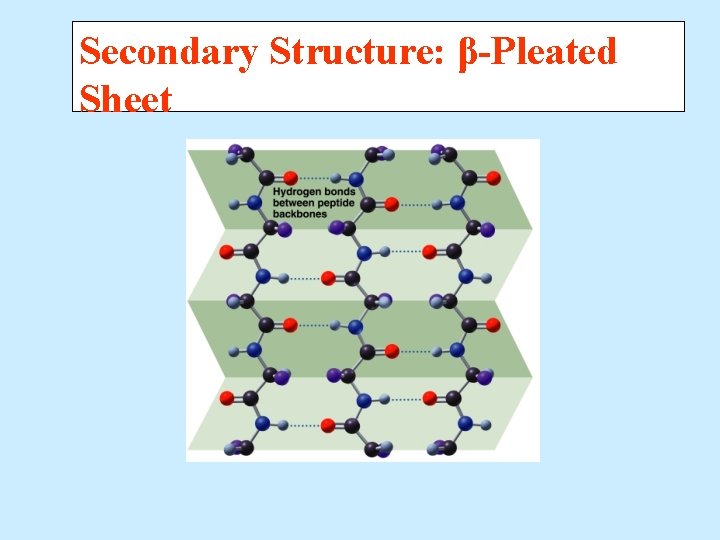
Secondary Structure – Beta Pleated Sheet A beta-pleated sheet is a secondary structure that § Consists of polypeptide chains arranged side by side. § Has hydrogen bonds between chains. § Has R groups above and below the sheet. § Is typical of fibrous proteins such as silk.

Secondary Structure: β-Pleated Sheet

Secondary Structure: Triple Helix A triple helix § Consists of three alpha helix chains woven together. § Contains large amounts glycine, proline, hydroxy proline, and hydroxylysine that contain –OH groups for hydrogen bonding. § Is found in collagen, connective tissue, skin, tendons, and cartilage.

Protein Structure: Tertiary and Quaternary Levels

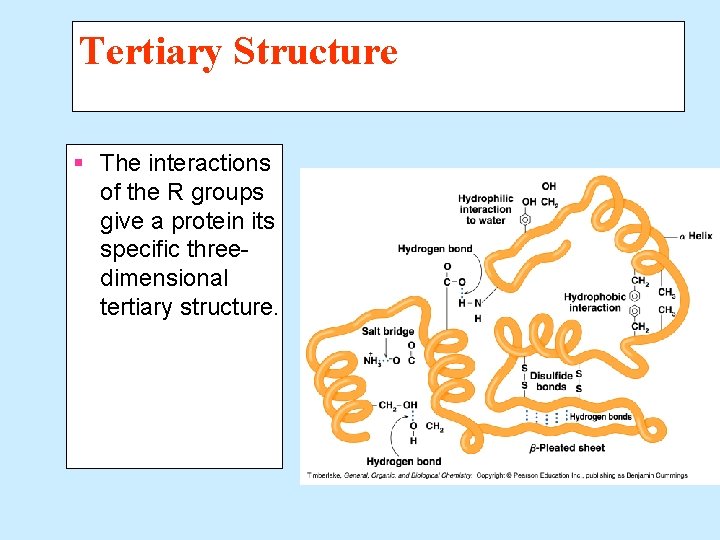
Tertiary Structure The tertiary structure of a protein § Gives a specific three dimensional shape to the polypeptide chain. § Involves interactions and cross links between different parts of the peptide chain. § Is stabilized by Hydrophobic and hydrophilic interactions. Salt bridges. Hydrogen bonds. Disulfide bonds.

Tertiary Structure § The interactions of the R groups give a protein its specific threedimensional tertiary structure.

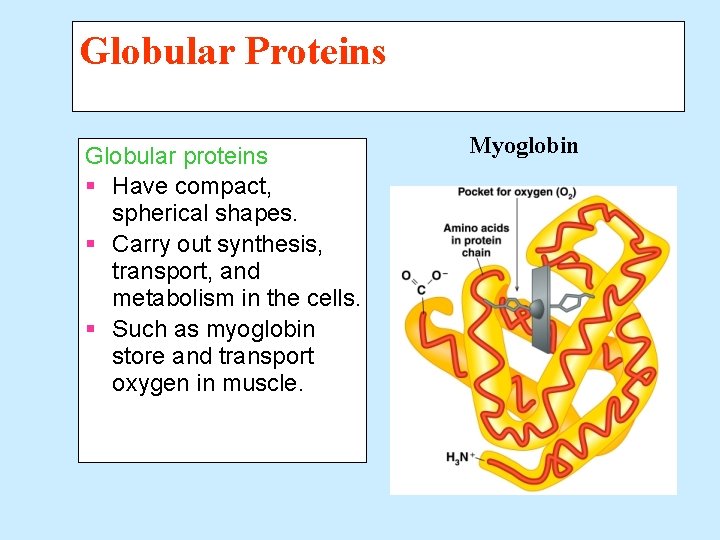
Globular Proteins Globular proteins § Have compact, spherical shapes. § Carry out synthesis, transport, and metabolism in the cells. § Such as myoglobin store and transport oxygen in muscle. Myoglobin

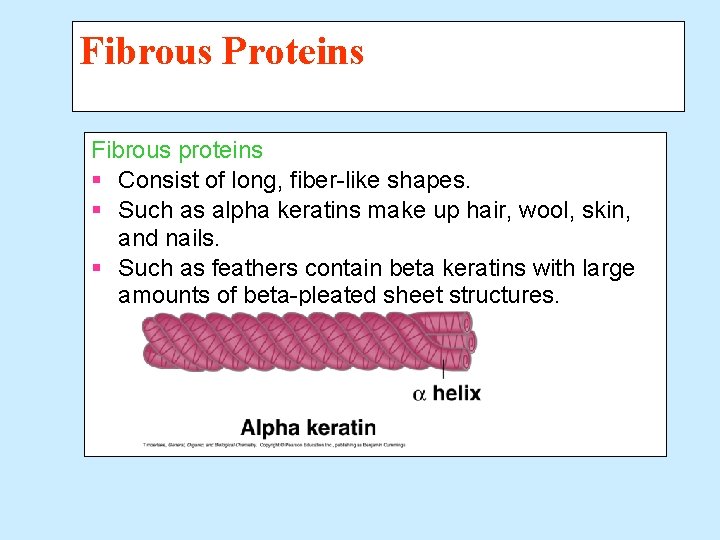
Fibrous Proteins Fibrous proteins § Consist of long, fiber-like shapes. § Such as alpha keratins make up hair, wool, skin, and nails. § Such as feathers contain beta keratins with large amounts of beta-pleated sheet structures.

Quaternary Structure The quaternary structure § Is the combination of two or more tertiary units. § Is stabilized by the same interactions found in tertiary structures. § Of hemoglobin consists of two alpha chains and two beta chains. The heme group in each subunit picks up oxygen for transport in the blood to the tissues. hemoglobin

Summary of Protein Structures

Protein Hydrolysis Protein hydrolysis § Splits the peptide bonds to give smaller peptides and amino acids. § Occurs in the digestion of proteins. § Occurs in cells when amino acids are needed to synthesize new proteins and repair tissues.

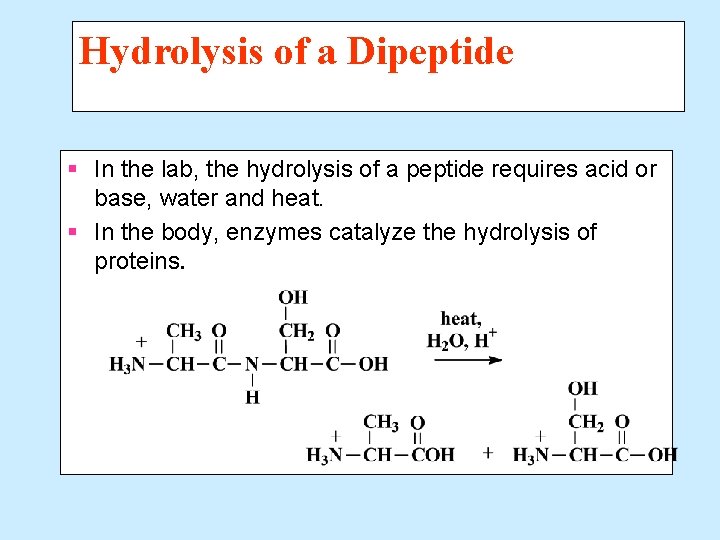
Hydrolysis of a Dipeptide § In the lab, the hydrolysis of a peptide requires acid or base, water and heat. § In the body, enzymes catalyze the hydrolysis of proteins.

Denaturation involves § The disruption of bonds in the secondary, tertiary and quaternary protein structures. § Heat and organic compounds that break apart H bonds and disrupt hydrophobic interactions. § Acids and bases that break H bonds between polar R groups and disrupt ionic bonds. § Heavy metal ions that react with S-S bonds to form solids. § Agitation such as whipping that stretches peptide chains until bonds break.

Applications of Denaturation of protein occurs when § An egg is cooked. § The skin is wiped with alcohol. § Heat is used to cauterize blood vessels. § Instruments are sterilized in autoclaves.
 Antigentest åre
Antigentest åre Amino acids are joined together in proteins by
Amino acids are joined together in proteins by Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Non essential amino acids mnemonics
Non essential amino acids mnemonics Gluconeogenic amino acids
Gluconeogenic amino acids Amino acid r groups
Amino acid r groups Nitrogen balance
Nitrogen balance Deamination of amino acids
Deamination of amino acids 20 amino acids structures
20 amino acids structures Metabolism of proteins
Metabolism of proteins Sp hybridization
Sp hybridization Nitrogen removal from amino acids
Nitrogen removal from amino acids Deamination of amino acids
Deamination of amino acids Milady perm chapter
Milady perm chapter Millon's test negative result
Millon's test negative result Biomedical importance of amino acids
Biomedical importance of amino acids Chymotrypsin cleaves which amino acids
Chymotrypsin cleaves which amino acids Importance of sulphur containing amino acids
Importance of sulphur containing amino acids Alpha carbon amino acid
Alpha carbon amino acid Chirality definition
Chirality definition Chapter 12 dna and rna
Chapter 12 dna and rna How many amino acids are there
How many amino acids are there Adenine thymine cytosine and guanine
Adenine thymine cytosine and guanine Codon wheel
Codon wheel Physical properties of amino acids
Physical properties of amino acids Is glycine polar or nonpolar
Is glycine polar or nonpolar Net charges of amino acids
Net charges of amino acids Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Titration curve for lysine
Titration curve for lysine Purely ketogenic amino acids
Purely ketogenic amino acids Amino acids classification
Amino acids classification Salt bridges in proteins
Salt bridges in proteins The chain of amino acids forms a
The chain of amino acids forms a Kr
Kr Aspartate-argininosuccinate shunt
Aspartate-argininosuccinate shunt Oxidative deamination of amino acids
Oxidative deamination of amino acids Protein amino acids
Protein amino acids