AMINO ACIDS STRUCTURAL UNITS OF PROTEINS AMINO ACIDS






![Non-standard Amino Acids or Uncommon Amino Acids [formed by modification of amino acids] – Non-standard Amino Acids or Uncommon Amino Acids [formed by modification of amino acids] –](https://slidetodoc.com/presentation_image/0d2c6954effdc28ce52266c0e3c0c9f2/image-20.jpg)








- Slides: 38

AMINO ACIDS STRUCTURAL UNITS OF PROTEINS

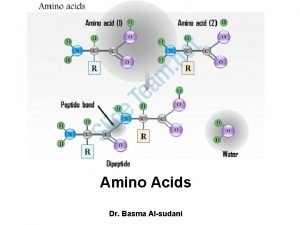
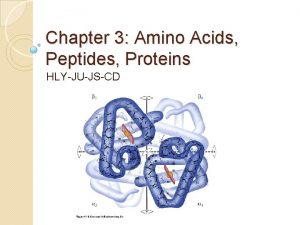
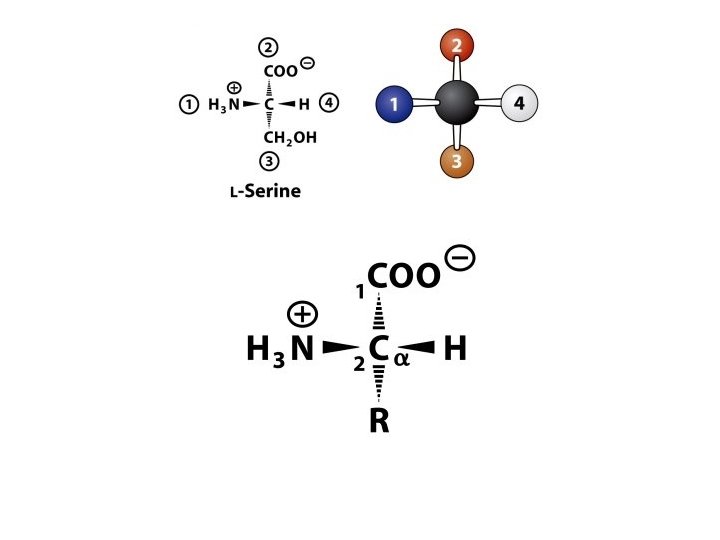
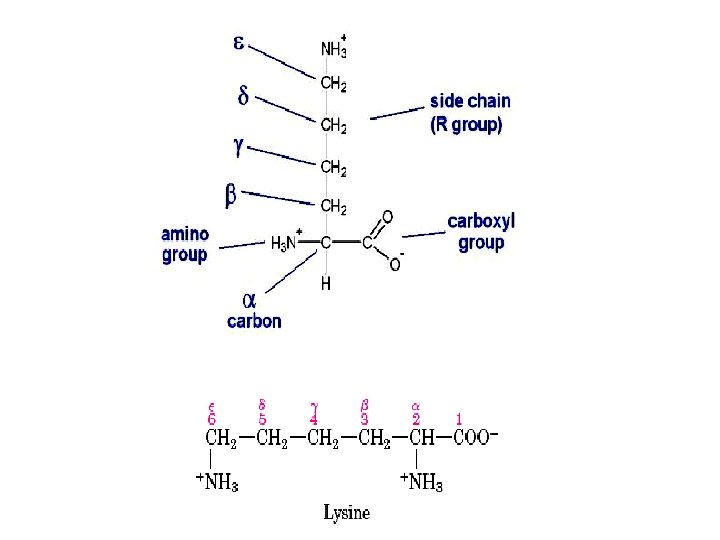
AMINO ACIDS: • These are the building blocks of proteins. • There are 20 amino acids that built up a protein • An a amino acid consists of a central carbon atom called the ‘α’ carbon to which four different groups are attached: 1. an amino group, 2. a carboxylic acid group, 3. a hydrogen atom and 4. a distinctive R group (side chain) is attached [except glycine].

Amino acid

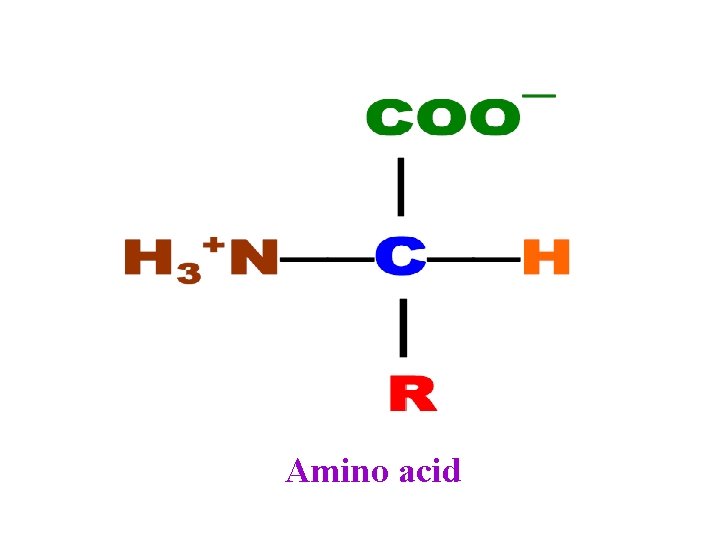
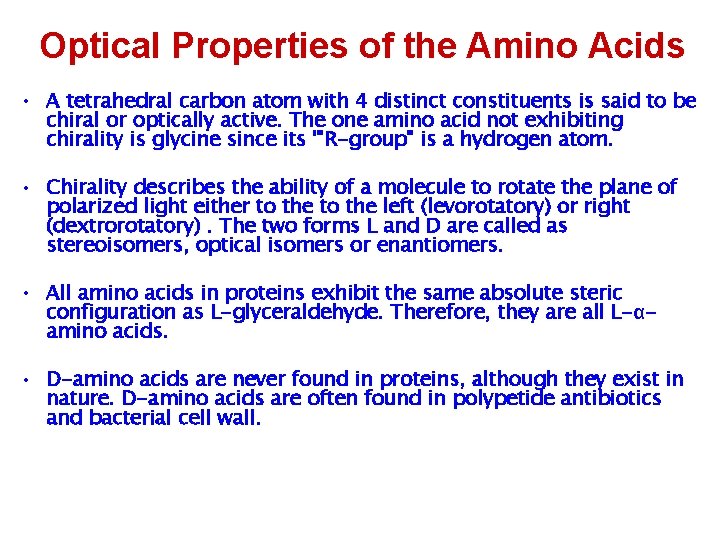
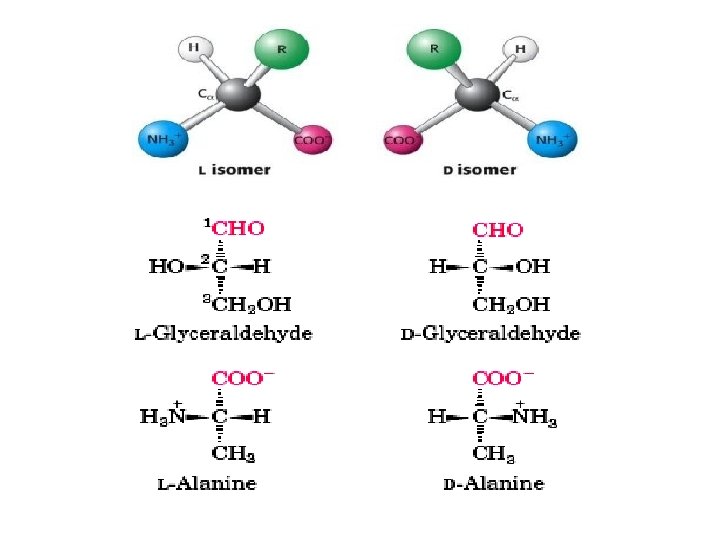
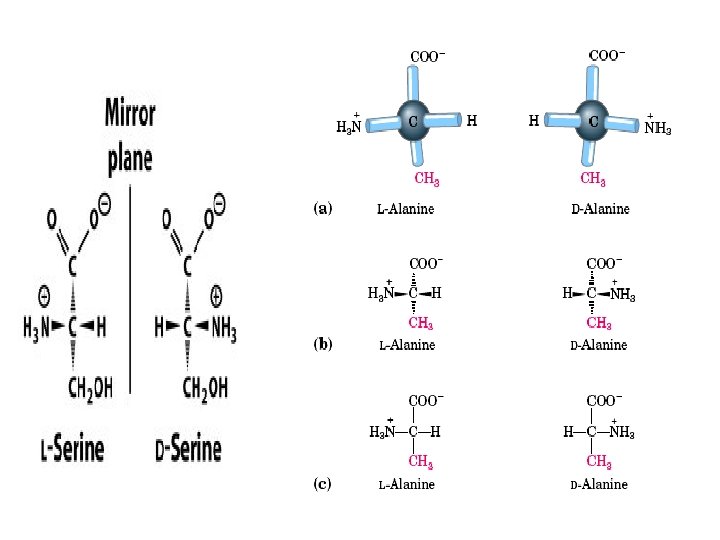
Because of the asymmetric α -carbon, α-amino acids are Chiral and may exist as an L isomer or D isomer. Both the L isomer and D isomer are mirror image of one another and are nonsuperimposable. Only L-Amino Acids Occur in Proteins



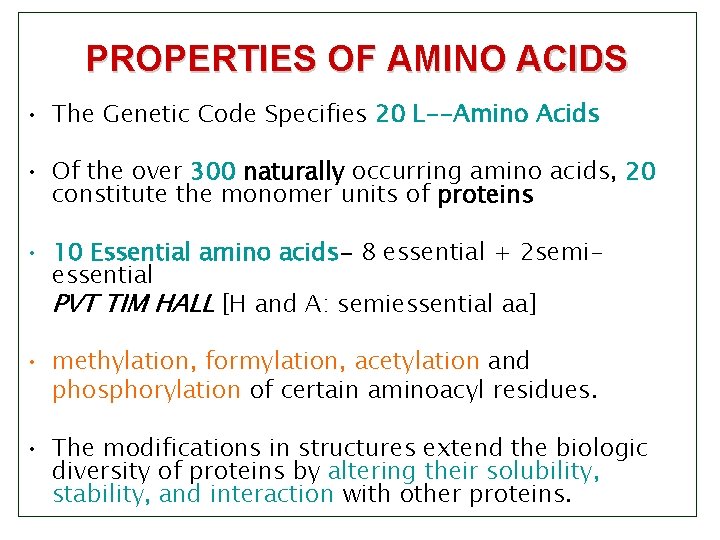
PROPERTIES OF AMINO ACIDS • The Genetic Code Specifies 20 L--Amino Acids • Of the over 300 naturally occurring amino acids, 20 constitute the monomer units of proteins • 10 Essential amino acids- 8 essential + 2 semiessential PVT TIM HALL [H and A: semiessential aa] • methylation, formylation, acetylation and phosphorylation of certain aminoacyl residues. • The modifications in structures extend the biologic diversity of proteins by altering their solubility, stability, and interaction with other proteins.

Optical Properties of the Amino Acids • A tetrahedral carbon atom with 4 distinct constituents is said to be chiral or optically active. The one amino acid not exhibiting chirality is glycine since its '"R-group" is a hydrogen atom. • Chirality describes the ability of a molecule to rotate the plane of polarized light either to the left (levorotatory) or right (dextrorotatory). The two forms L and D are called as stereoisomers, optical isomers or enantiomers. • All amino acids in proteins exhibit the same absolute steric configuration as L-glyceraldehyde. Therefore, they are all L-αamino acids. • D-amino acids are never found in proteins, although they exist in nature. D-amino acids are often found in polypetide antibiotics and bacterial cell wall.



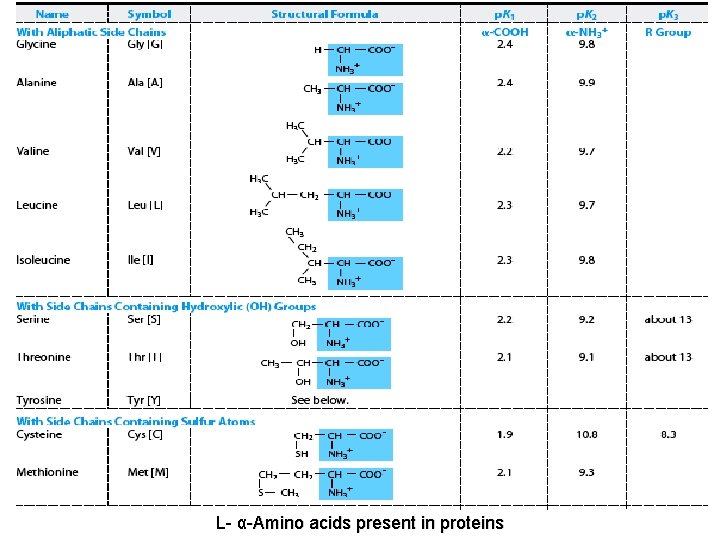
The 20 Standard Amino Acids • Only 20 common or standard amino acids that are used in building proteins R group • The 20 amino acids are distinguished by their R group or side chains • The side chains vary in size, shape charge, hydrogen-bonding capacity, hydrophobic character and chemical reactivity • These properties give rise to the various functions of a protein

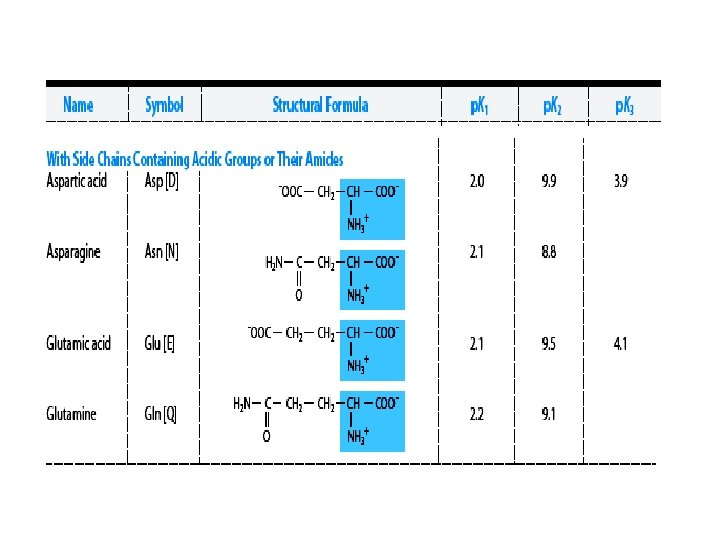
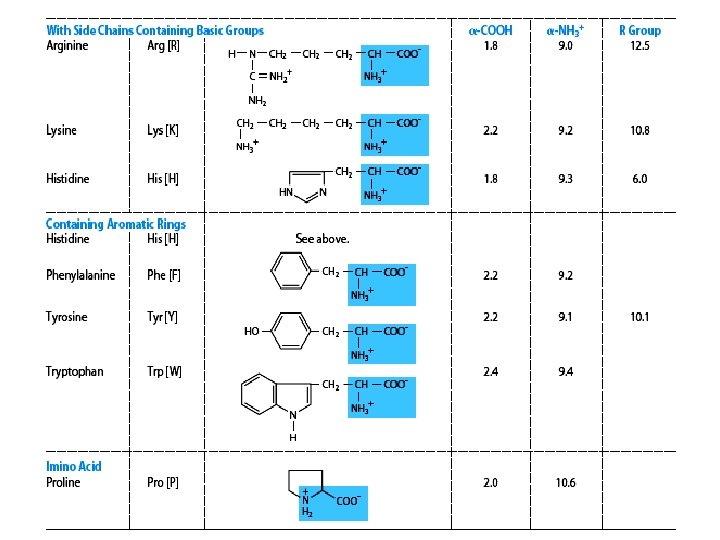
Classification of amino acids (according to the chemical nature) On the basis of R-group 20 common amino acids are categorized into 7 distinct groups – Aliphatics amino acids : G A V L I – OH grp containing amino acids (2+1): S T [Y] – Sulfur containing amino acids (2): C M – Acidic amino acids and Amides (2+2) : D N E Q – Basic amino acids (3) : R K H – Aromatic amino acids (3+1): F Y W [H ] – Imino acid (1) : P

L- α-Amino acids present in proteins



2. Nutritional classification: Essential and non essential amino acids: 10 Essential amino acids 8 essential + 2 semi-essential PVT TIM HALL [H and A: semiessential aa]

3. Classification according to polarity Non-polar: -- hydrophobic in nature no charge on R group: alanine, leucine, isolucine, phenylalanine, tryptophan, valine, methionine, proline Polar: -- hydrophilic in nature a. no charge on R group – glycine, serine, threonine, cysteine, glutamine, asparagine and tyrosine b. With (+)ve R grp – lysine, arginine, histidine

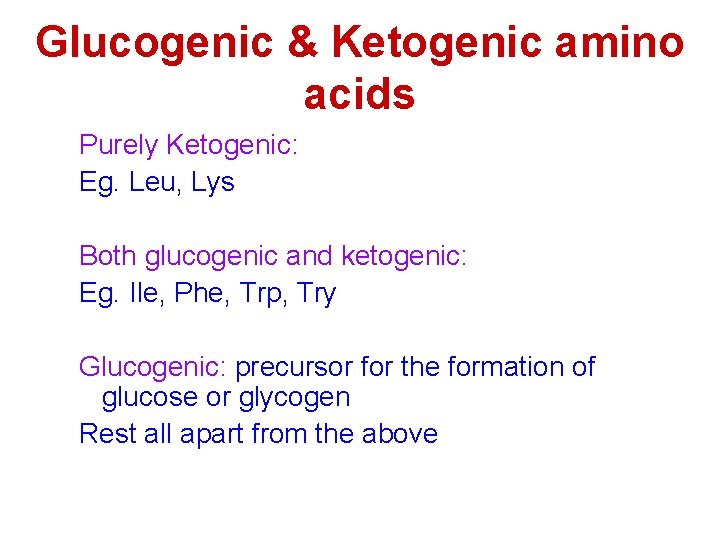
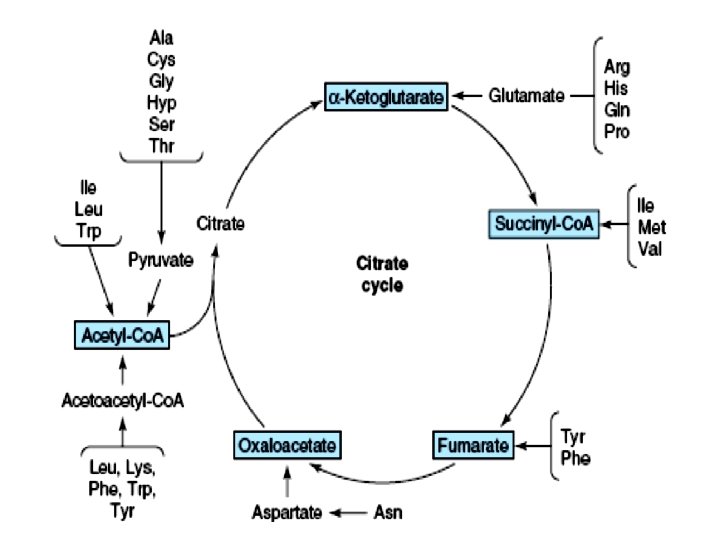
Glucogenic & Ketogenic amino acids Purely Ketogenic: Eg. Leu, Lys Both glucogenic and ketogenic: Eg. Ile, Phe, Trp, Try Glucogenic: precursor for the formation of glucose or glycogen Rest all apart from the above

![Nonstandard Amino Acids or Uncommon Amino Acids formed by modification of amino acids Non-standard Amino Acids or Uncommon Amino Acids [formed by modification of amino acids] –](https://slidetodoc.com/presentation_image/0d2c6954effdc28ce52266c0e3c0c9f2/image-20.jpg)
Non-standard Amino Acids or Uncommon Amino Acids [formed by modification of amino acids] – Homocysteine – ornithine – GABA – histamine

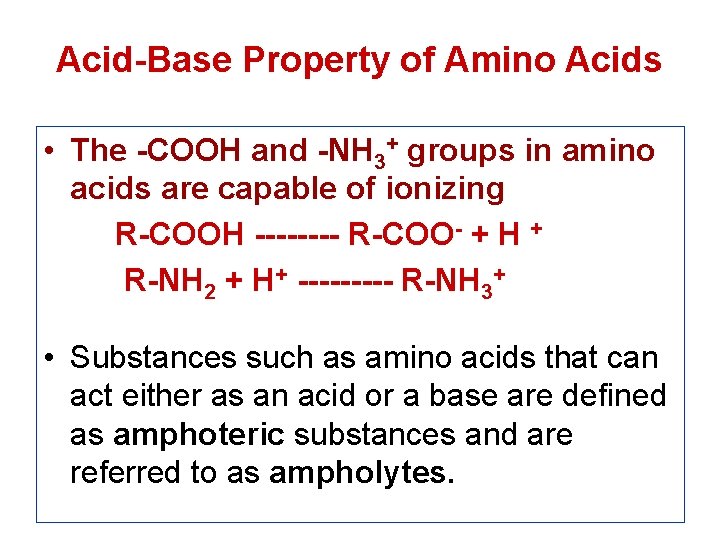
Acid-Base Property of Amino Acids • The -COOH and -NH 3+ groups in amino acids are capable of ionizing R-COOH ---- R-COO- + H + R-NH 2 + H+ ----- R-NH 3+ • Substances such as amino acids that can act either as an acid or a base are defined as amphoteric substances and are referred to as ampholytes.

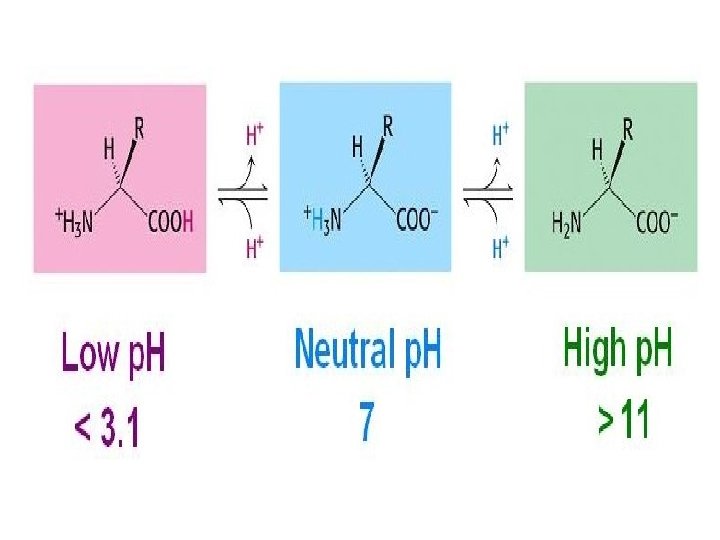
• At physiological p. H (around 7. 4) the carboxyl group will be unprotonated and the amino group will be protonated. An amino acid with no ionizable R-group would be electrically neutral being equal (+)ve & (-)ve charge. This species is termed as zwitterion and bear no net charge. • The acidic strength of the carboxyl, amino and ionizable R-groups in amino acids can be defined by the dissociation constant Ka or more commonly the negative logarithm of Ka i. e. p. Ka. [ ↑Ka → ↑ dissociation → ↑stronger the acid]

• The net charge (the algebraic sum of all the charged groups present) of any amino acid, peptide or protein, will depend upon the p. H of the surrounding aqueous environment. • When the net charge of an amino acid or protein is zero the p. H will be equivalent to the isoelectric point: p. I.


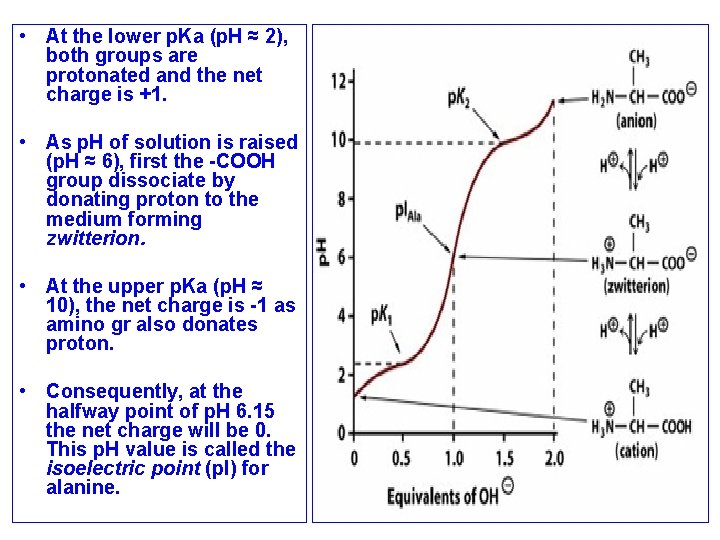
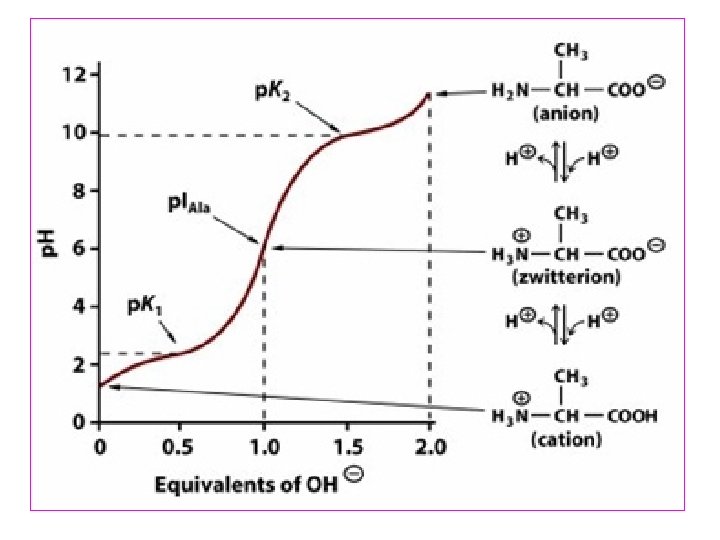
Ionization of Amino Acids • Acids and bases can become ionized by a change in p. H. For individual amino acids, such ionizations occur at least twice: once for the α-carboxyl group and once for the α-amino group • p. Ka value of an acid determines the p. H value at which ionization occurs. For an amino acid such as alanine, we can identify the two p. Ka values from its titration curve

• At the lower p. Ka (p. H ≈ 2), both groups are protonated and the net charge is +1. • As p. H of solution is raised (p. H ≈ 6), first the -COOH group dissociate by donating proton to the medium forming zwitterion. • At the upper p. Ka (p. H ≈ 10), the net charge is -1 as amino gr also donates proton. • Consequently, at the halfway point of p. H 6. 15 the net charge will be 0. This p. H value is called the isoelectric point (p. I) for alanine.


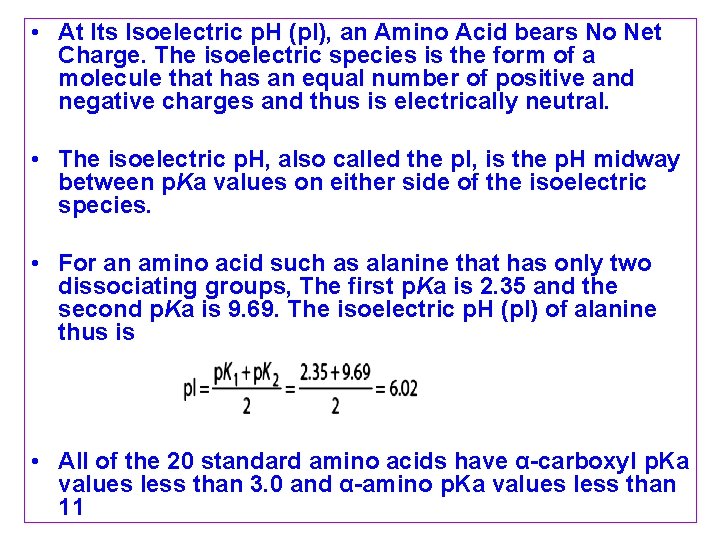
• At Its Isoelectric p. H (p. I), an Amino Acid bears No Net Charge. The isoelectric species is the form of a molecule that has an equal number of positive and negative charges and thus is electrically neutral. • The isoelectric p. H, also called the p. I, is the p. H midway between p. Ka values on either side of the isoelectric species. • For an amino acid such as alanine that has only two dissociating groups, The first p. Ka is 2. 35 and the second p. Ka is 9. 69. The isoelectric p. H (p. I) of alanine thus is • All of the 20 standard amino acids have α-carboxyl p. Ka values less than 3. 0 and α-amino p. Ka values less than 11

Protonic equilibria of aspartic acid

Physical properties of amino acids: 1. Solubility: - most aa are water soluble and insoluble in organic solvants 2. Melting point: - higher temp above 2000 C 3. Taste: - may be different sweet- Gly, Ala, Val bitter- Arg, Ile (Monosodium glutamate [MSG]-salt of glutamic acid) Amino acids as drugs: D-penicillamine- Wilson’s disaese N-Acetylcysteine- cystic fibrosis, antioxidant Gabapentin- anticonvulsant

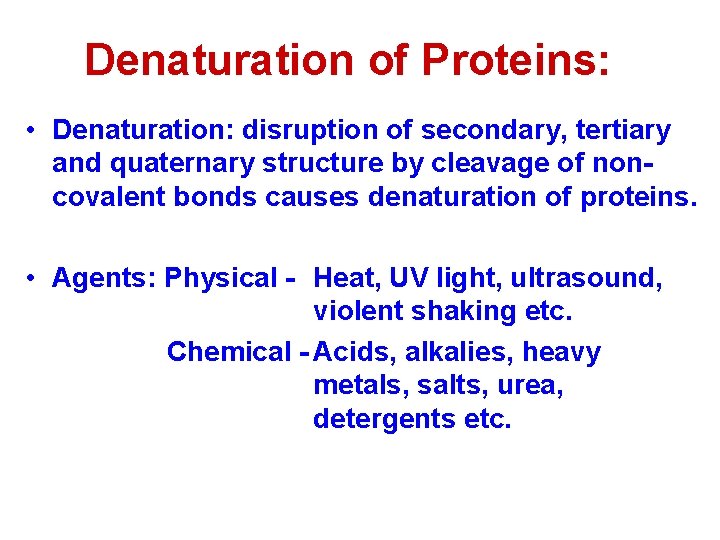
Denaturation of Proteins: • Denaturation: disruption of secondary, tertiary and quaternary structure by cleavage of noncovalent bonds causes denaturation of proteins. • Agents: Physical - Heat, UV light, ultrasound, violent shaking etc. Chemical - Acids, alkalies, heavy metals, salts, urea, detergents etc.

• Precipitation: separation of protein part from the medium. eg. Isoelectric precipitation, precipitation by salting out, heavy metals, organic solvents etc. • Coagulation: irreversible denaturation of protein by heat. egg coagulation by heat • Flocculation: precipitation of proteins at isoelectric point eg. milk caesin precipitation at ph 4. 6

Salting in & salting out • Salting in: - if protein solublizes in solvent after adding small amount of salt then it is known as salting in. • Salting out: - the process of protein precipitation by the addition of neutral salts such as ammonium sulfate or sodium sulfate is known as salting out. Small size of protein (albumin) requires full saturation and large size of protein (globulin) requires half saturation to precipitate it.

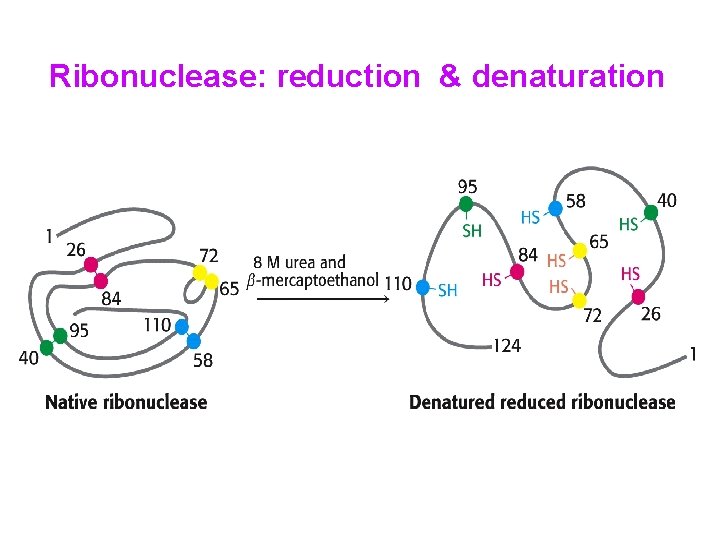
Ribonuclease: reduction & denaturation

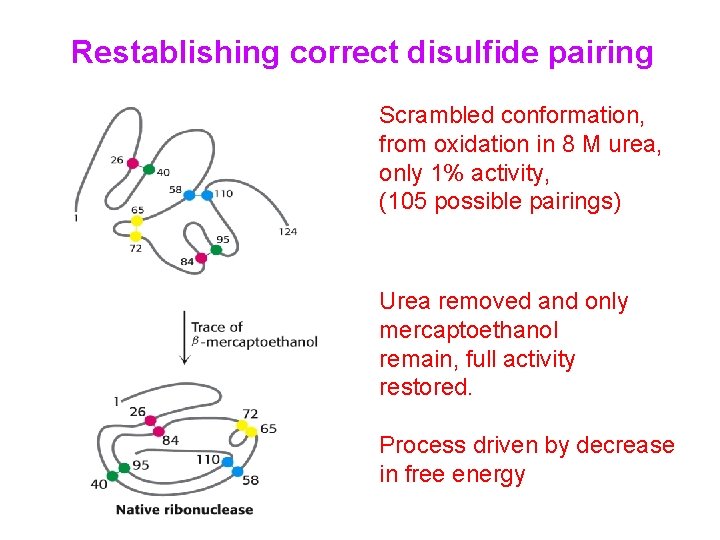
Restablishing correct disulfide pairing Scrambled conformation, from oxidation in 8 M urea, only 1% activity, (105 possible pairings) Urea removed and only mercaptoethanol remain, full activity restored. Process driven by decrease in free energy

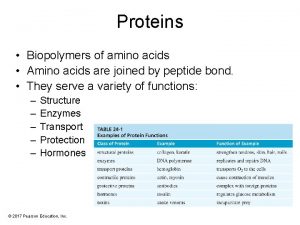
Some biologically important peptides • Glutathione: tripeptide of glutamic acid, cysteine and glycine (GSH) Functions: 1. prevents oxidation of sulfhydryl group of proteins 2. maintains RBC membrane structure and integrity 3. prevents hemoglobin from getting oxidised 4. helps in amino acid absorption 5. helps in detoxification of xenobiotics 6. help to scavenge free radicals. • Thyrotropin releasing hormone (TRH): tripeptide secreted by hypothalamus which stimulates pituitary gland to release thyrotropic hormone • Oxytocin: nonapeptide hormone secreted by posterior pituitary, helps in contraction of uterus.

• Vasopressin (ADH): nonapeptide secreted by posterior pituitary which helps to retain water in kidneys and increase of blood pressure. • Angiotensins: decapeptide which has hypertensive effect. • Bradikinin: nona & decapeptide and act as vasodilators. • Enkephalin: pentapeptide found in brain. It inhibits sense of pain. • Gastrointestinal hormones: gastrin, secretin etc • Aspartame: dipeptide of aspartic acid and phenylalanine. It is 200 times sweeter than sucrose and used as a low calorie artificial sweetener.

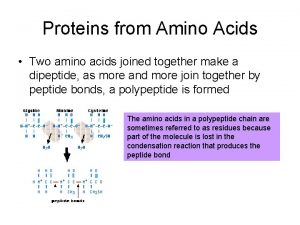
 Amino acids are joined together in proteins by
Amino acids are joined together in proteins by Structural proteins function
Structural proteins function Function of muscular tissue
Function of muscular tissue Structural role of proteins
Structural role of proteins Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Importance of sulphur containing amino acids
Importance of sulphur containing amino acids Basic amino acids
Basic amino acids Lysine titration curve
Lysine titration curve Salt bridge amino acids
Salt bridge amino acids Histidine catabolism
Histidine catabolism 20 amino acids structures
20 amino acids structures Amino acids groups
Amino acids groups Are amino acids negatively charged
Are amino acids negatively charged Utritional
Utritional Milady chemical texture services test
Milady chemical texture services test Serylglycyltyrosylalanylleucine
Serylglycyltyrosylalanylleucine Acid base properties of amino acids
Acid base properties of amino acids Is valine ketogenic or glucogenic
Is valine ketogenic or glucogenic Transdeamination of amino acids
Transdeamination of amino acids Chapter 12 dna and rna
Chapter 12 dna and rna Quantitative estimation of amino acids by ninhydrin
Quantitative estimation of amino acids by ninhydrin Properties of amino acids
Properties of amino acids Classification of amino acids
Classification of amino acids Net charges of amino acids
Net charges of amino acids Aromatic amino acids
Aromatic amino acids Deamination of amino acids
Deamination of amino acids Dehydration synthesis of amino acids
Dehydration synthesis of amino acids What are enzymes made of
What are enzymes made of Amino acids 2 chemsheets answers
Amino acids 2 chemsheets answers What is made of amino acids
What is made of amino acids Pvt tim hall
Pvt tim hall Kr
Kr Difference between hydrophobic and hydrophilic amino acids
Difference between hydrophobic and hydrophilic amino acids Francis crick
Francis crick Chirality definition
Chirality definition Conditionally essential amino acids
Conditionally essential amino acids Amino acid to urea
Amino acid to urea Which amino acids have ionizable side chains
Which amino acids have ionizable side chains Translation
Translation