Amino acids Essential Amino Acids 10 amino acids
















- Slides: 32

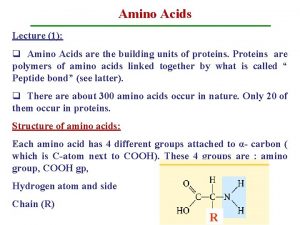
Amino acids

Essential Amino Acids • 10 amino acids not synthesized by the body • arg, his, ile, leu, lys, met, phe, thr, trp, val • Must obtain from the diet • All in diary products • 1 or more missing in grains and vegetables

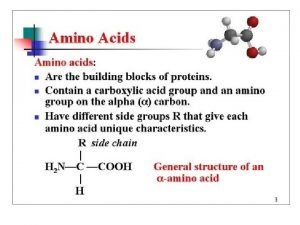
• All proteins are composed of the 20 standard amino acids. They are referred to as α-amino acids with the exception of proline

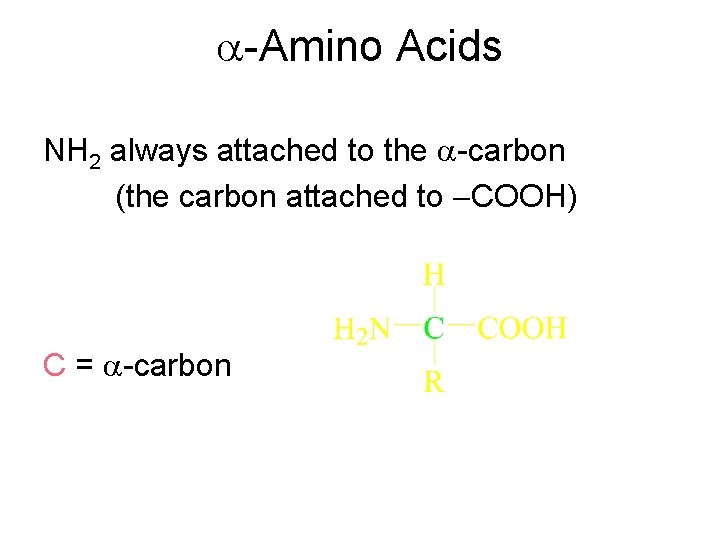
-Amino Acids NH 2 always attached to the -carbon (the carbon attached to COOH) C = -carbon

• In the physiological p. H range, both the carboxylic acid group and the amino groups of the α-amino acids ionize. An Amino acid can therefore act as both an acid and a base. • This property is called amphoteric, and they are referred to as ampholytes.

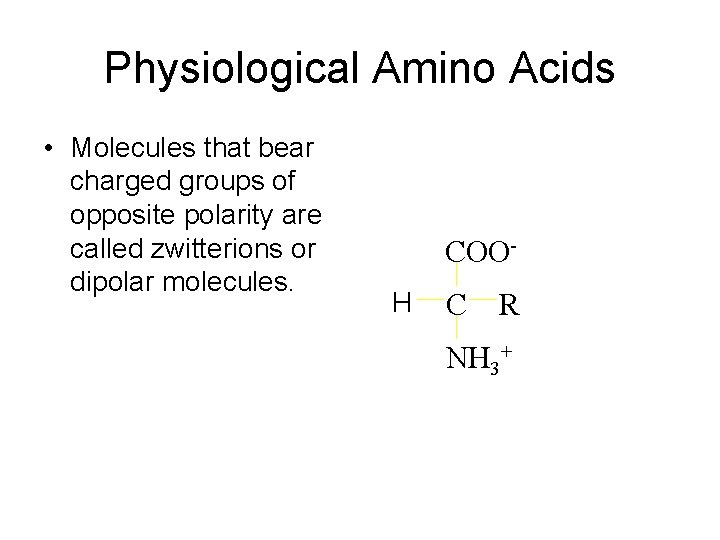
Physiological Amino Acids • Molecules that bear charged groups of opposite polarity are called zwitterions or dipolar molecules. COOH C R NH 3+

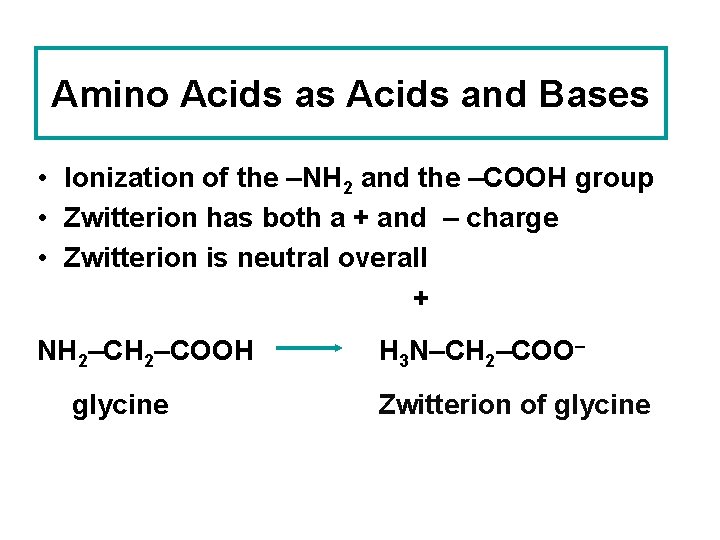
Amino Acids as Acids and Bases • Ionization of the –NH 2 and the –COOH group • Zwitterion has both a + and – charge • Zwitterion is neutral overall + NH 2–COOH glycine H 3 N–CH 2–COO– Zwitterion of glycine

• Because they are dipoles, they have physical properties characteristic of ionic substances. • This makes amino acids more soluble in polar solvents than in nonpolar ones. • Most α-amino acids are very soluble in water but largely insoluble in most organic solvents

p. H and ionization H+ + OH– + H 3 N–CH 2–COOH H 3 N–CH 2–COO– H 2 N–CH 2–COO– Positive ion zwitterion Negative ion Low p. H neutral p. H High p. H

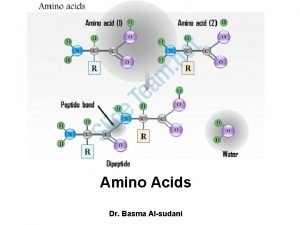
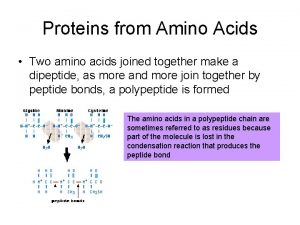
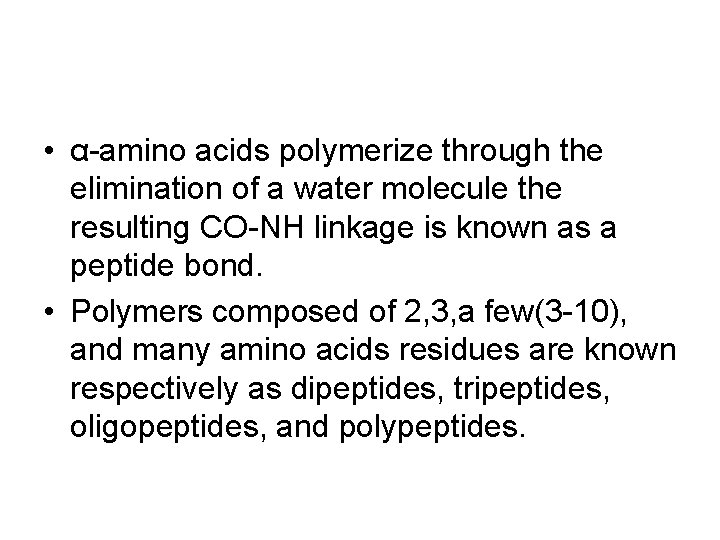
• α-amino acids polymerize through the elimination of a water molecule the resulting CO-NH linkage is known as a peptide bond. • Polymers composed of 2, 3, a few(3 -10), and many amino acids residues are known respectively as dipeptides, tripeptides, oligopeptides, and polypeptides.

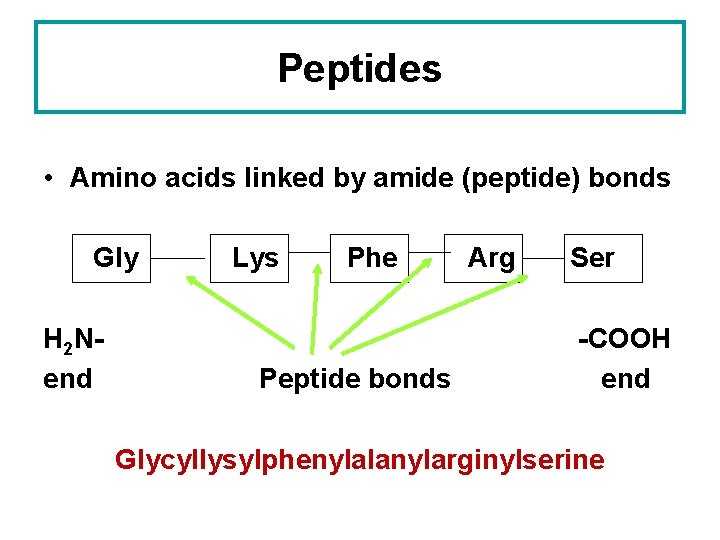
Peptides • Amino acids linked by amide (peptide) bonds Gly H 2 Nend Lys Phe Peptide bonds Arg Ser -COOH end Glycyllysylphenylalanylarginylserine

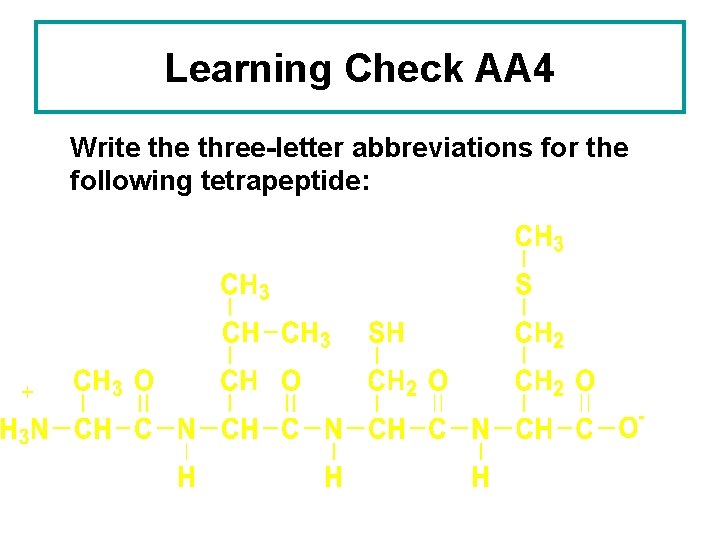
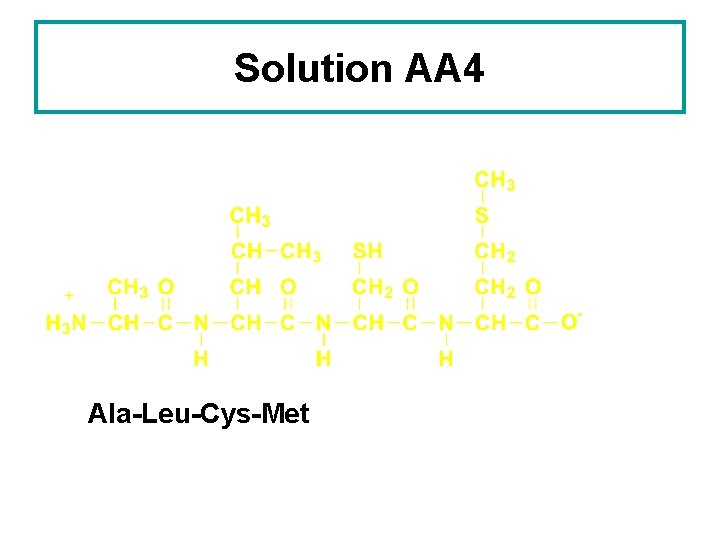
Learning Check AA 4 Write three-letter abbreviations for the following tetrapeptide:

Solution AA 4 Ala-Leu-Cys-Met

The Peptide Bond Amide bond formed by the –COOH of an amino acid and the –NH 2 of the next amino acid + O || CH 3 + | NH 3–CH 2–COH O + || + H 3 N–CH–COO– CH 3 | NH 3–CH 2–C – N–CH–COO– | H peptide bond

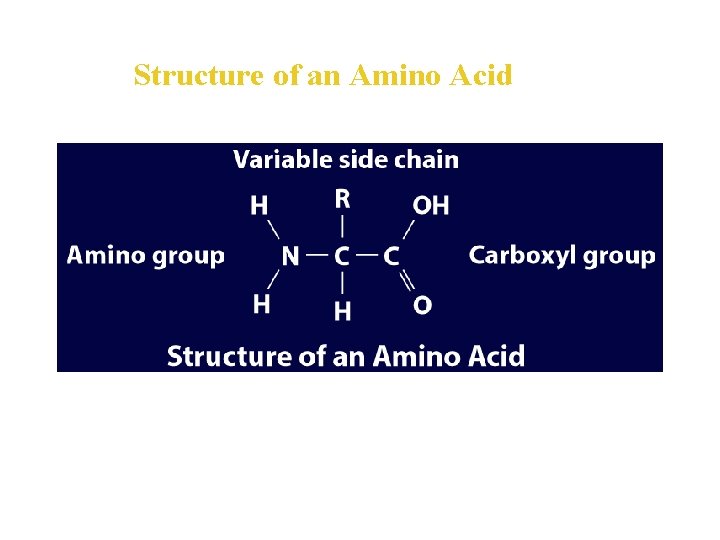
Proteins • A protein is an organic polymer composed of amino acids bonded together in one or more chains. • An amino acid has a central carbon atom, to which are bonded a carboxyl group, an amino group, a hydrogen atom, and a variable side chain designated as R, as shown in the following structural formula.

Structure of an Amino Acid

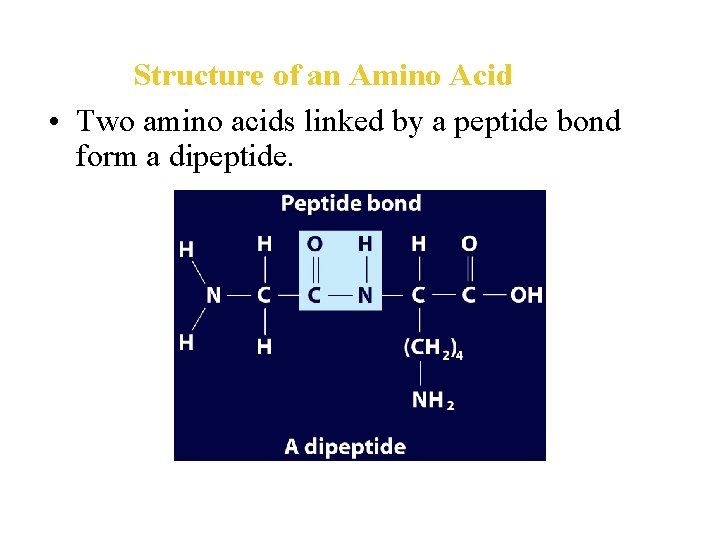
Structure of an Amino Acid • Amino acids bond to each other by forming a peptide bond, an amide group formed by a condensation reaction between the carboxyl group of one amino acid and the amino group of another.

Structure of an Amino Acid • Two amino acids linked by a peptide bond form a dipeptide.

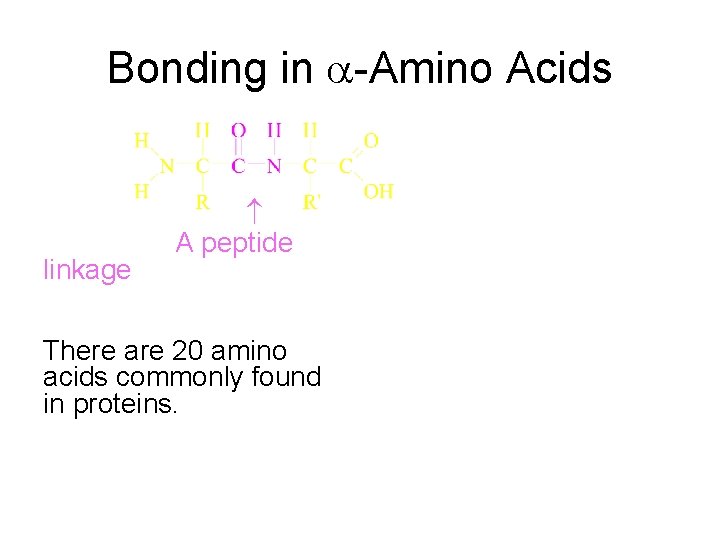
Bonding in -Amino Acids A peptide linkage There are 20 amino acids commonly found in proteins.

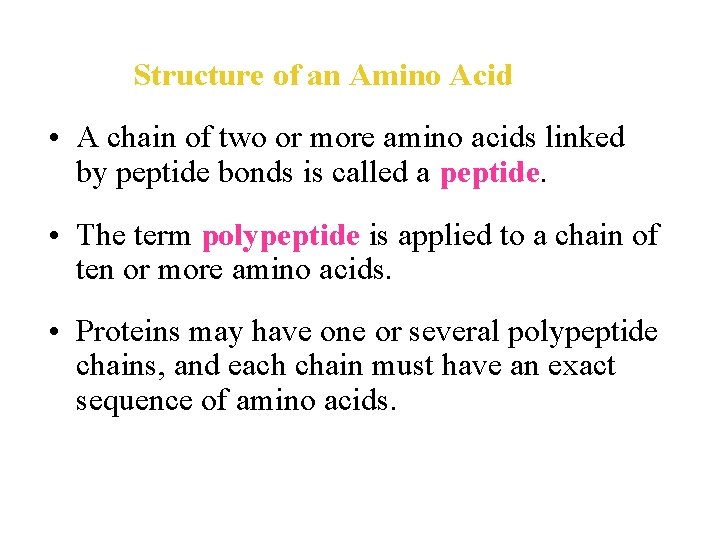
Structure of an Amino Acid • A chain of two or more amino acids linked by peptide bonds is called a peptide. • The term polypeptide is applied to a chain of ten or more amino acids. • Proteins may have one or several polypeptide chains, and each chain must have an exact sequence of amino acids.

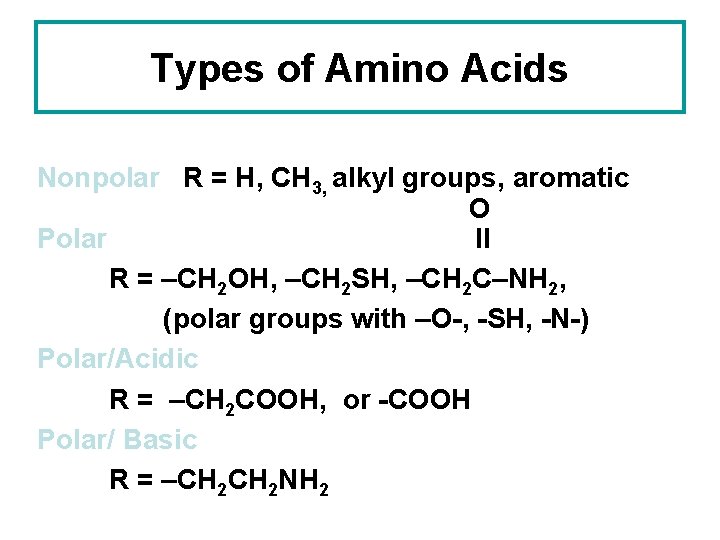
Classification of Amino Acids • Classified according to their side chains polarity. (R groups) • This is because in their native conformations, largely in response to the tendency to remove their hydrophobic side chains from contact with water and to solvate their hydrophilic side chains.

• According to this classification scheme, there are three major groups. • 1. those w/nonpolar R groups • 2. Uncharged polar R groups • 3. charged polar R groups

Types of Amino Acids Nonpolar R = H, CH 3, alkyl groups, aromatic O Polar ll R = –CH 2 OH, –CH 2 SH, –CH 2 C–NH 2, (polar groups with –O-, -SH, -N-) Polar/Acidic R = –CH 2 COOH, or -COOH Polar/ Basic R = –CH 2 NH 2

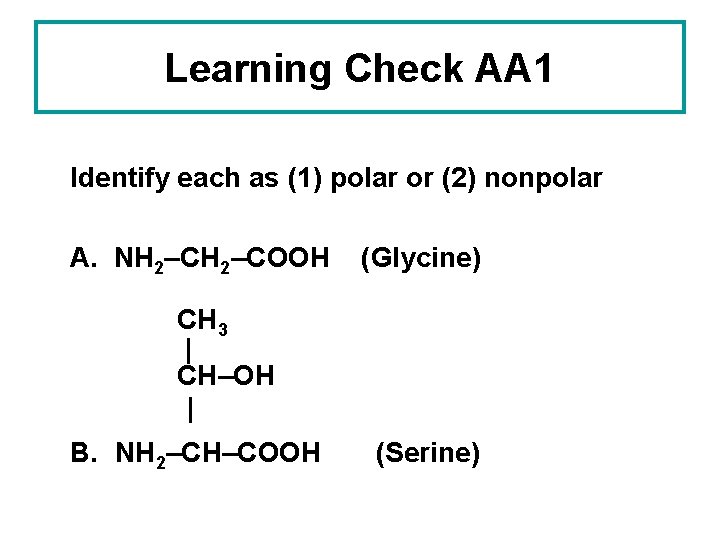
Learning Check AA 1 Identify each as (1) polar or (2) nonpolar A. NH 2–COOH (Glycine) CH 3 | CH–OH | B. NH 2–CH–COOH (Serine)

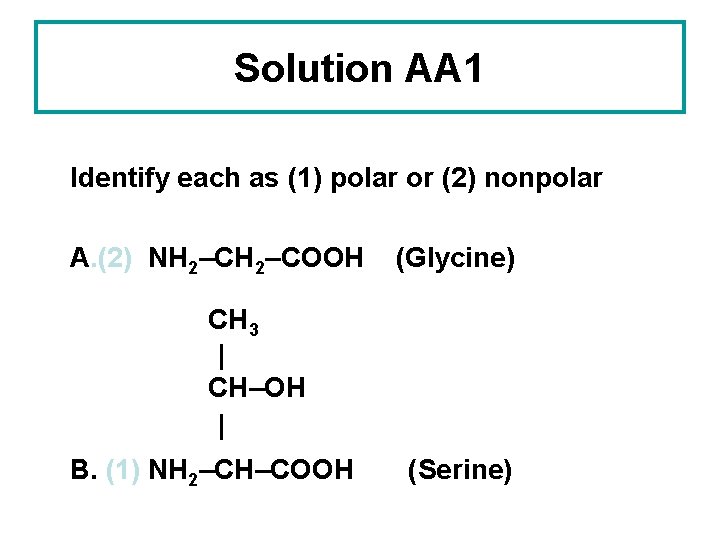
Solution AA 1 Identify each as (1) polar or (2) nonpolar A. (2) NH 2–COOH (Glycine) CH 3 | CH–OH | B. (1) NH 2–CH–COOH (Serine)

Non polar amino acids ( hydrophobic ) • (9) • Glycine, Alanine, valine, leucine, isoleucine, methionine, proline, phenylalanine, and tryptophan

Uncharged polar side chains ( hydrophilic) • (6) these have Hydroxyl, Amide, or Thiol groups • Serine, threonine, asparagine, glutamine, tyrosine, cysteine.

Charged polar side chains ( ionic) • Can be positively or negatively charged. • The basic amino acids are (+) charged at Physiological p. H values. • Lysine, arginine, and histidine. • The acidic amino acids are (-) charged above p. H 3 • Aspartic acid, glutamic acid

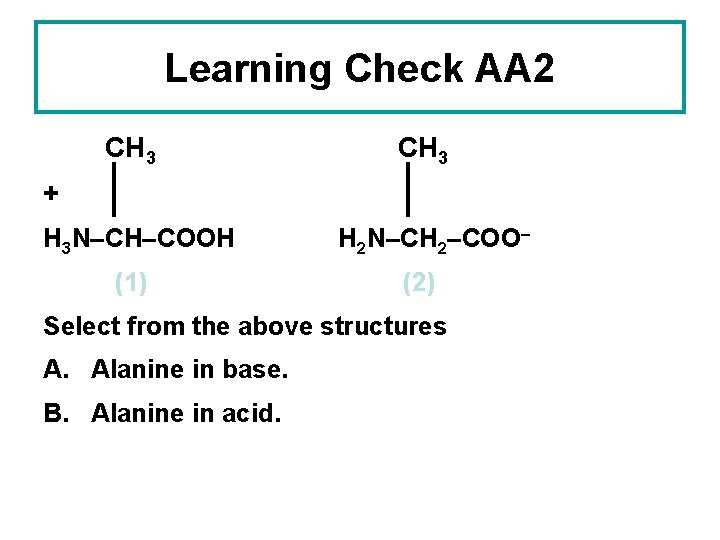
Learning Check AA 2 CH 3 + H 3 N–CH–COOH (1) H 2 N–CH 2–COO– (2) Select from the above structures A. Alanine in base. B. Alanine in acid.

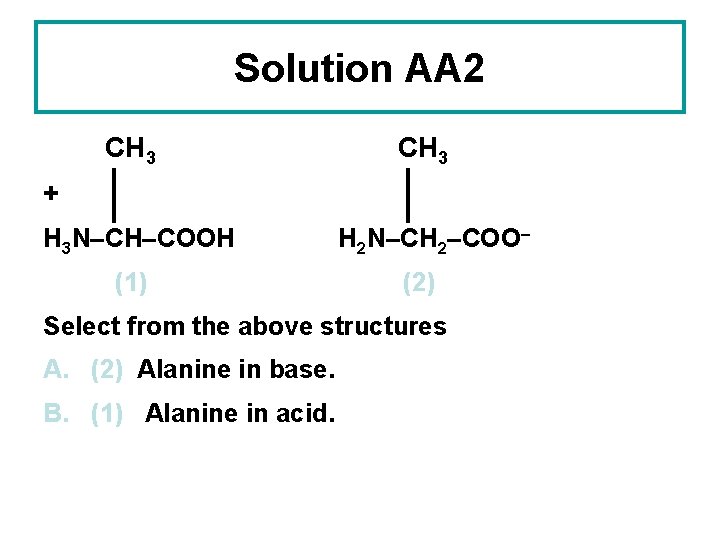
Solution AA 2 CH 3 + H 3 N–CH–COOH (1) H 2 N–CH 2–COO– (2) Select from the above structures A. (2) Alanine in base. B. (1) Alanine in acid.

• The 20 amino acids vary considerably in their physical and chemical properties such as polarity, acidity, basicity, aromaticity, flexibility, ability to cross link, ability to hydrogen bond, and chemical reactivity. These characteristics, several that are interrelated contribute to proteins great range of properties.

Non standard amino acids • The 20 common amino acids are by no means the only amino acids that occur in biological systems. Non standard amino acid residues are often important constituents in proteins. The changes comes from a conformation rearrangement in their 3 -D structures. These are called D-amino acids and are quite prevalent in many antibiotics.
 Plamatic acid
Plamatic acid Non essential amino acids mnemonics
Non essential amino acids mnemonics Non essential amino acids mnemonics
Non essential amino acids mnemonics Conditionally essential amino acids
Conditionally essential amino acids Essential amino acids mnemonics
Essential amino acids mnemonics Non essential amino acids in food
Non essential amino acids in food Amino acid r groups
Amino acid r groups Essential amino acid
Essential amino acid Non-essential nutrients
Non-essential nutrients Omega 3 beyin gelişimi
Omega 3 beyin gelişimi Non essential fatty acids
Non essential fatty acids Esansiyel yağ asitleri
Esansiyel yağ asitleri Unsaturated fat chain
Unsaturated fat chain Importance of sulphur containing amino acids
Importance of sulphur containing amino acids Pi of alanine
Pi of alanine Titration of amino acids
Titration of amino acids Salt bridges in proteins
Salt bridges in proteins 20 amino acids structures
20 amino acids structures Oxidative deamination of glutamate
Oxidative deamination of glutamate What is protien
What is protien Mrna amino acid chart
Mrna amino acid chart Amino acid wheel chart
Amino acid wheel chart Dextro amino acid
Dextro amino acid Utritional
Utritional Neutral amino acids
Neutral amino acids Safety precautions for permanent waving
Safety precautions for permanent waving Oxidative deamination of amino acids
Oxidative deamination of amino acids Amino acid name
Amino acid name Transdeamination of amino acids
Transdeamination of amino acids Qualitative tests for amino acids
Qualitative tests for amino acids Properties of amino acids slideshare
Properties of amino acids slideshare Are amino acids negatively charged
Are amino acids negatively charged Dehydration synthesis of amino acids
Dehydration synthesis of amino acids