Chapter 3 Amino Acids Peptides Proteins HLYJUJSCD Amino









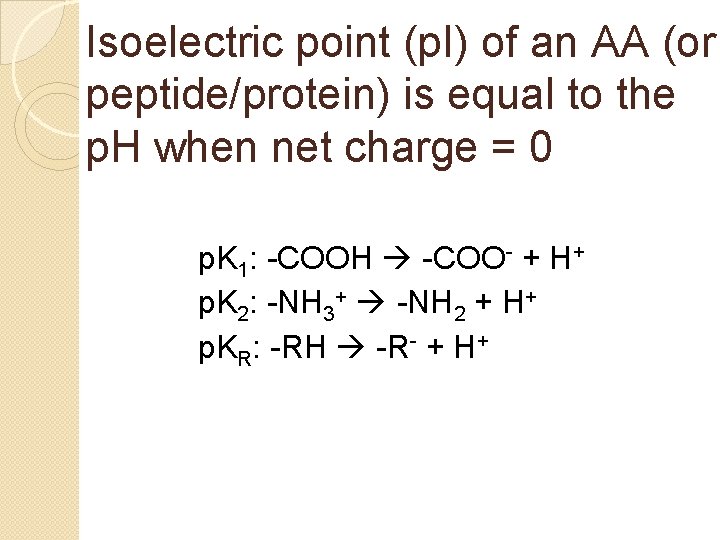




- Slides: 34

Chapter 3: Amino Acids, Peptides, Proteins HLY-JU-JS-CD

Amino Acids (AA) are the building blocks of peptides and proteins Peptides generally contain 2 -10 AA Polypeptides contain 10 -100 AA Proteins contain >100 AA General structure of AA: at p. H ~7. 4:

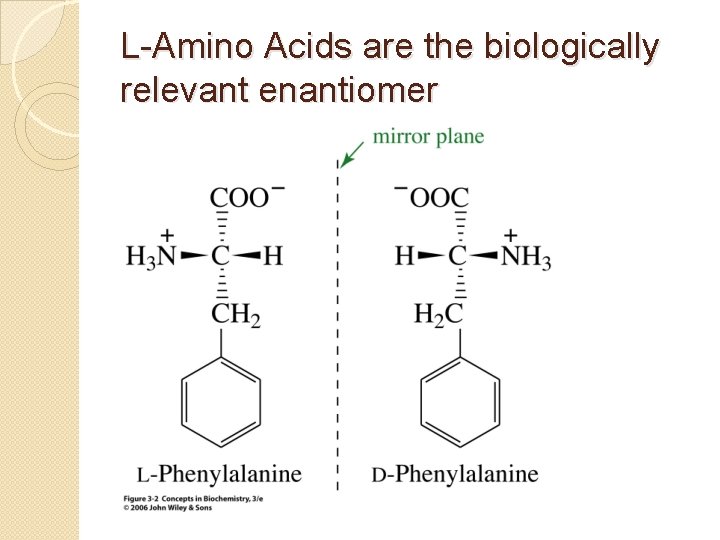
L-Amino Acids are the biologically relevant enantiomer

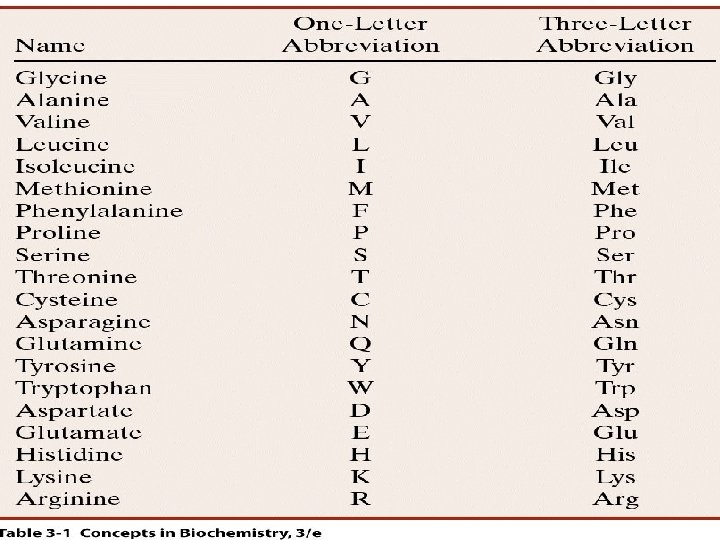
Of the 20 common AA, 10 of them are considered essential see page 67 of your book


Of the 20 common AA, 10 of them are considered essential see page 67 of your book Mnemonics: MILK FTW RHV “ESSENTIAL” = cannot be produced de novo by the body Some AA are conditionally essential Note though that there are now more than 20 AA! (but we will only focus on the 20)

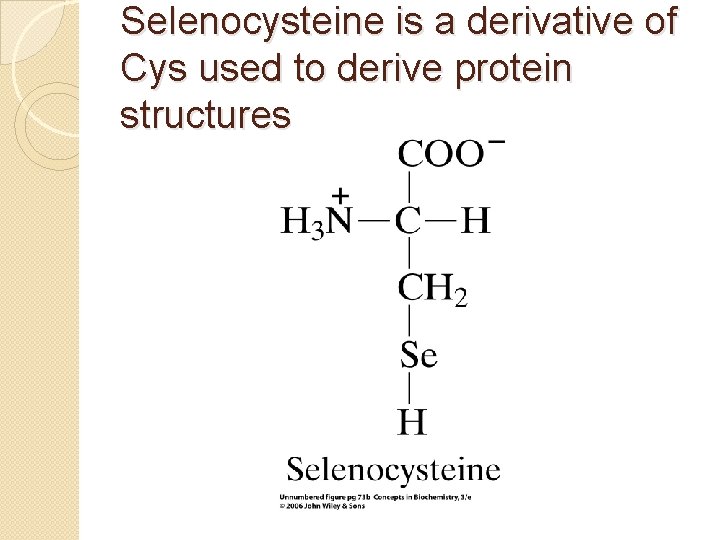
Selenocysteine is a derivative of Cys used to derive protein structures

The 20 AA can be grouped according to functional classes Aliphatic (GAVLIMP) Aromatic (WYF) Polar, uncharged (CHNQST) Polar, charged – acidic (DE) Polar, charged – basic (RK) *Histidine is basic but uncharged See structures on page 70 of your book.

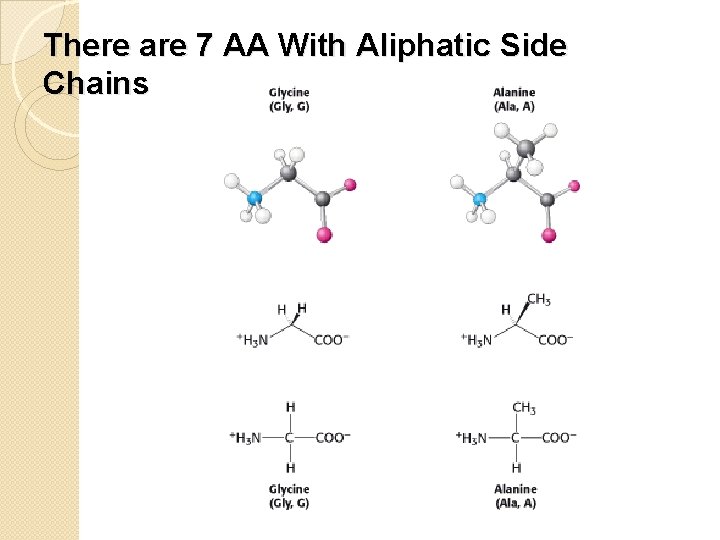
There are 7 AA With Aliphatic Side Chains


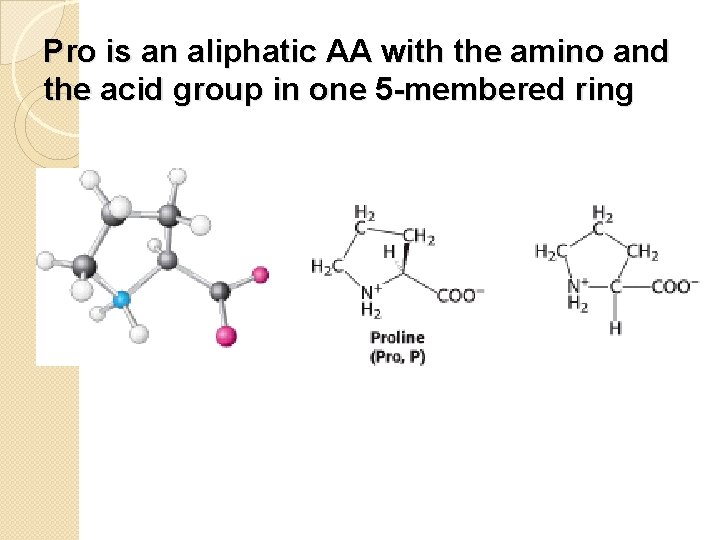
Pro is an aliphatic AA with the amino and the acid group in one 5 -membered ring

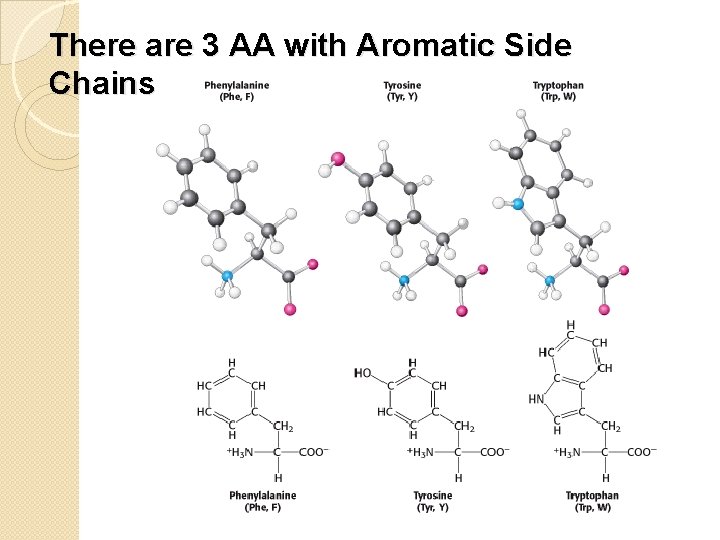
There are 3 AA with Aromatic Side Chains

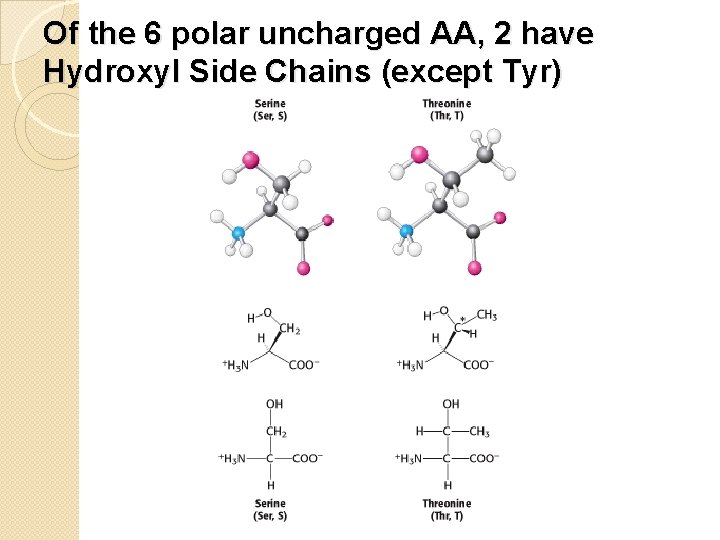
Of the 6 polar uncharged AA, 2 have Hydroxyl Side Chains (except Tyr)

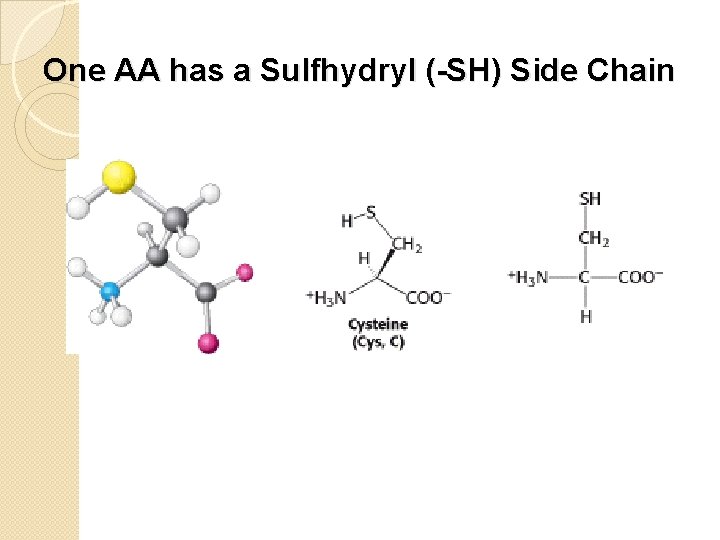
One AA has a Sulfhydryl (-SH) Side Chain

There are 3 AA With Basic Side Chains, 2 of them ccharged (RK)

Two AA have acidic side chains (DE). Their amide counterparts (NQ) are polar, uncharged

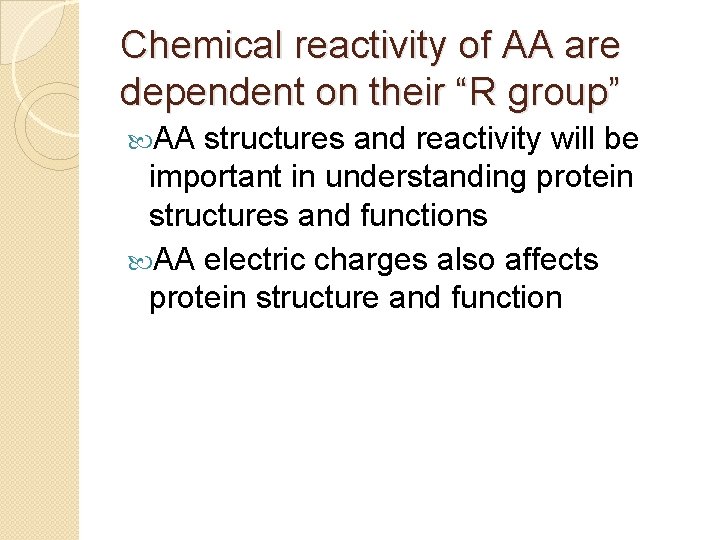
Chemical reactivity of AA are dependent on their “R group” AA structures and reactivity will be important in understanding protein structures and functions AA electric charges also affects protein structure and function

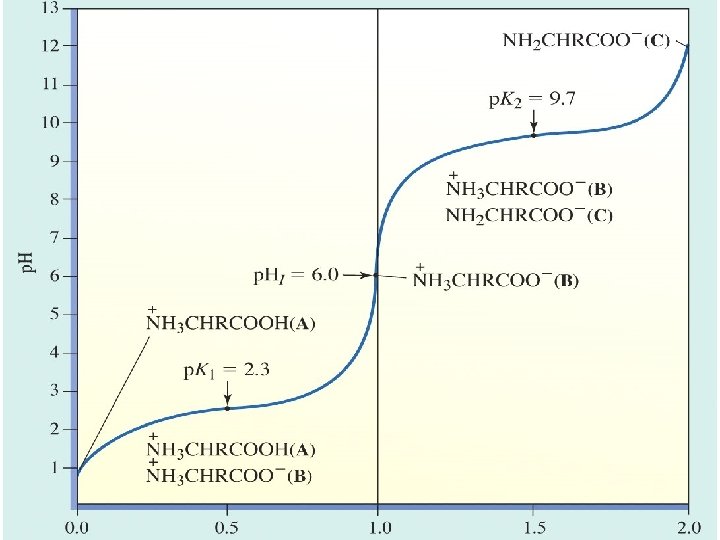
Isoelectric point (p. I) of an AA (or peptide/protein) is equal to the p. H when net charge = 0

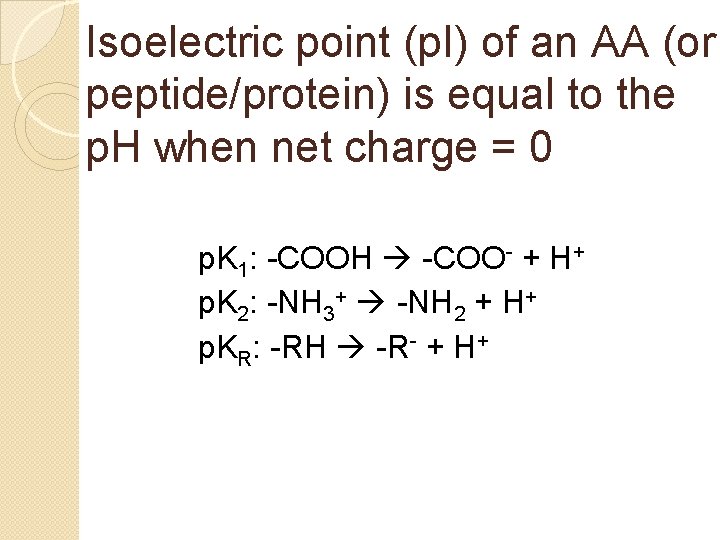
Isoelectric point (p. I) of an AA (or peptide/protein) is equal to the p. H when net charge = 0 p. K 1: -COOH -COO- + H+ p. K 2: -NH 3+ -NH 2 + H+ p. KR: -RH -R- + H+

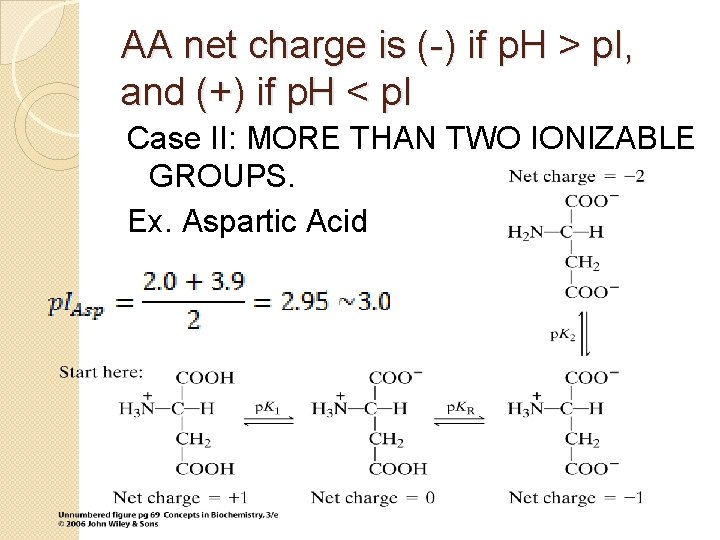
AA net charge is (-) if p. H > p. I, and (+) if p. H < p. I is estimated to be the AVERAGE of the two p. K values representing neutral species.

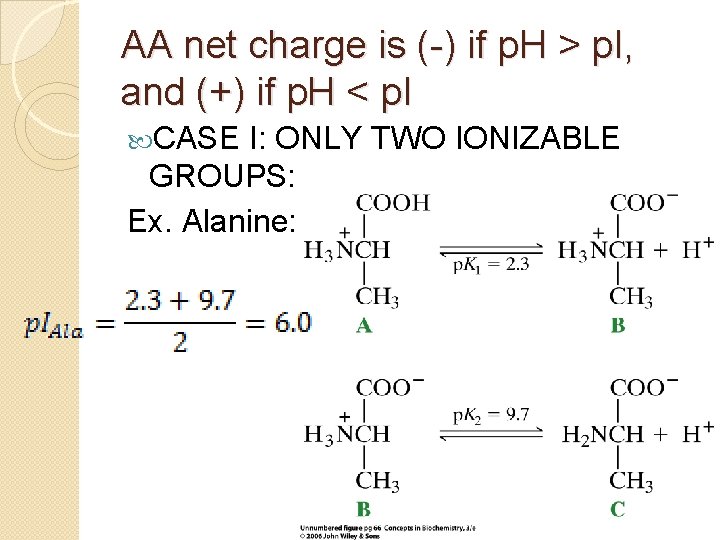
AA net charge is (-) if p. H > p. I, and (+) if p. H < p. I CASE I: ONLY TWO IONIZABLE GROUPS: Ex. Alanine:


AA net charge is (-) if p. H > p. I, and (+) if p. H < p. I Case II: MORE THAN TWO IONIZABLE GROUPS. Ex. Aspartic Acid


Activity, open book/notes BUT no talking (20 points) Determine the p. I of Lysine. Show ALL conformations and the net charges at different p. H’s. 2. Draw the titration curve for Lysine. 3. Determine the inflection points, and draw the structure/s of Lysine at each interval (i. e. , before p. K 1, at p. K 1, after p. K 1 but before p. K 2, etc. ) 1.

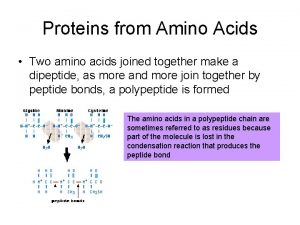
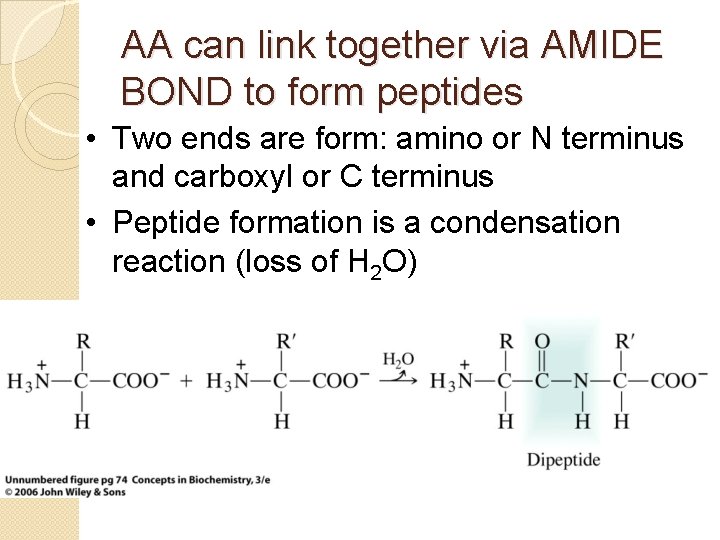
AA can link together via AMIDE BOND to form peptides • Two ends are form: amino or N terminus and carboxyl or C terminus • Peptide formation is a condensation reaction (loss of H 2 O)

AA can link together via AMIDE BOND to form peptides VIDEO!

Peptides are cleaved via hydrolysis Acids, bases or enzymes can be used to facilitate the hydrolysis In our stomach or intestine, peptidases or proteases are present Enzymes specific to some AA are used for protein analysis (more of this later )

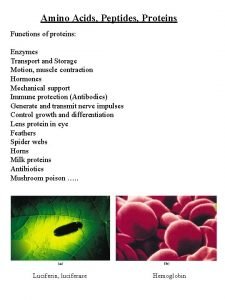
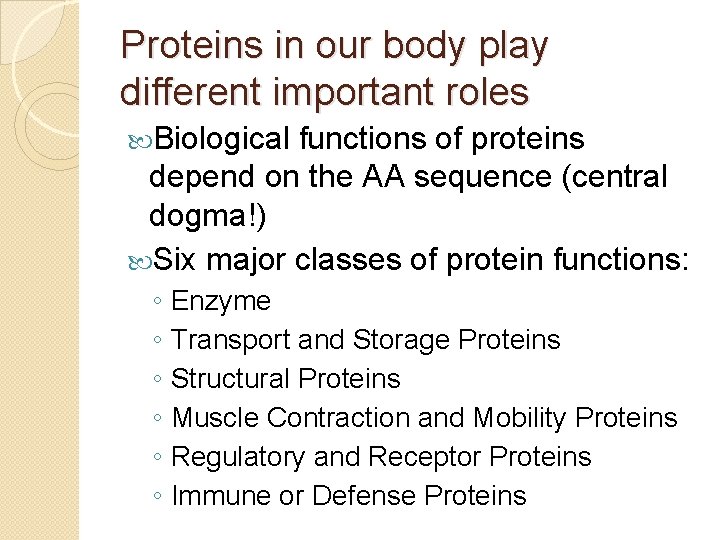
Proteins in our body play different important roles Biological functions of proteins depend on the AA sequence (central dogma!) Six major classes of protein functions: ◦ Enzyme ◦ Transport and Storage Proteins ◦ Structural Proteins ◦ Muscle Contraction and Mobility Proteins ◦ Regulatory and Receptor Proteins ◦ Immune or Defense Proteins

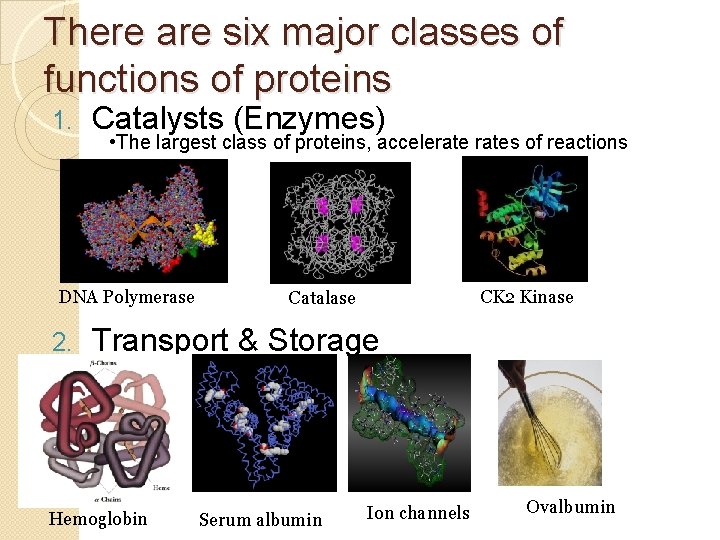
There are six major classes of functions of proteins 1. Catalysts (Enzymes) • The largest class of proteins, accelerates of reactions DNA Polymerase 2. CK 2 Kinase Catalase Transport & Storage Hemoglobin Serum albumin Ion channels Ovalbumin

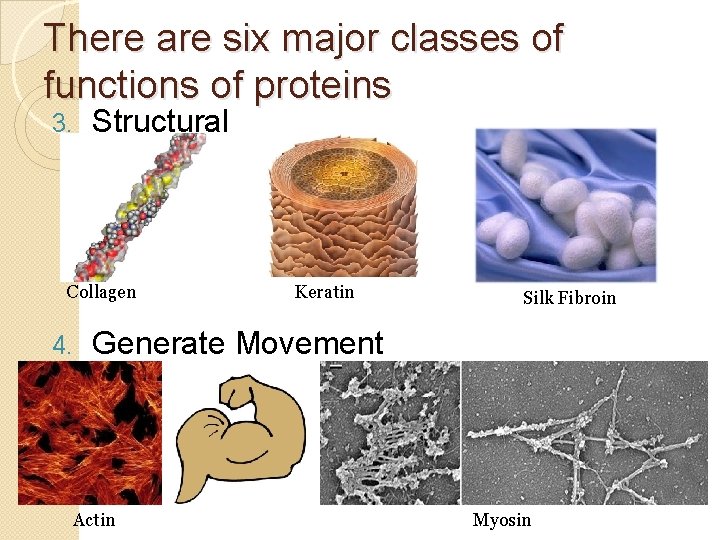
There are six major classes of functions of proteins 3. Structural Collagen 4. Keratin Silk Fibroin Generate Movement Actin Myosin

There are six major classes of functions of proteins 5. Regulation of Metabolism and Gene Expression Lac repressor Insulin 6. Protection Immunoglobulin Thrombin and Fibrinogen Venom Proteins

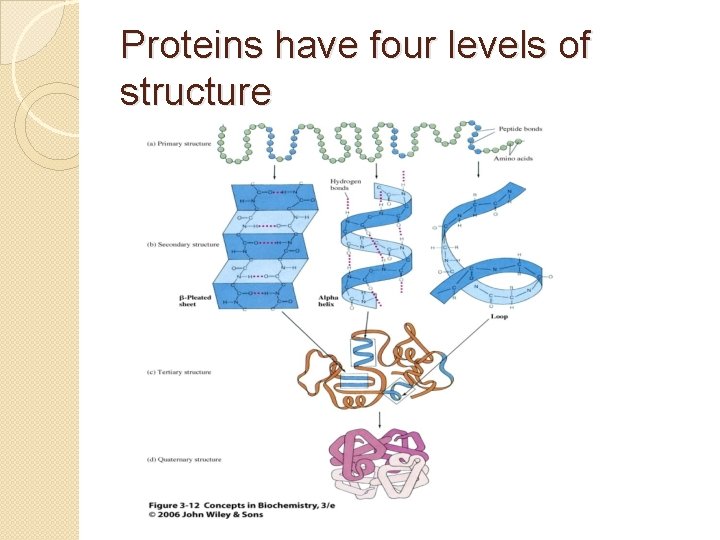
Proteins have four levels of structure
 Peptides and proteins
Peptides and proteins Peptides and proteins
Peptides and proteins Amino acids are joined together in proteins by
Amino acids are joined together in proteins by Ppgbqa
Ppgbqa Image
Image Bromocripine
Bromocripine Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Amino acid wheel chart
Amino acid wheel chart Titration curve of arginine
Titration curve of arginine Titration curve of amino acids
Titration curve of amino acids Deamination of amino acids
Deamination of amino acids 2 amino acids joined together
2 amino acids joined together Deamination of glutamine
Deamination of glutamine Amino acids groups
Amino acids groups Right handed amino acids
Right handed amino acids Non essential amino acids in food
Non essential amino acids in food Titration plot
Titration plot Pyruvate to lactate enzyme
Pyruvate to lactate enzyme Isoleucine ketogenic glucogenic
Isoleucine ketogenic glucogenic Pvt tim hall
Pvt tim hall Neutral amino acids
Neutral amino acids Amino acids classification
Amino acids classification Amino acids 2 chemsheets answers
Amino acids 2 chemsheets answers Optical properties of amino acids
Optical properties of amino acids Chymotrypsin cleaves which amino acids
Chymotrypsin cleaves which amino acids Positive charged amino acids
Positive charged amino acids Which amino acids have ionizable side chains
Which amino acids have ionizable side chains Non essential amino acids mnemonics
Non essential amino acids mnemonics Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Aromatic amino acids
Aromatic amino acids Phenol containing amino acids
Phenol containing amino acids Millon's test positive result
Millon's test positive result Peptide bond dehydration synthesis
Peptide bond dehydration synthesis Properties of amino acids
Properties of amino acids Biomedical importance of amino acids
Biomedical importance of amino acids