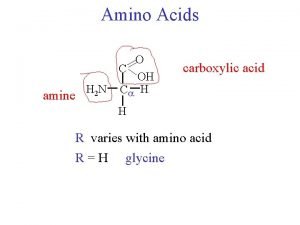
The general ways of amino acids degradation Transamination






- Slides: 18


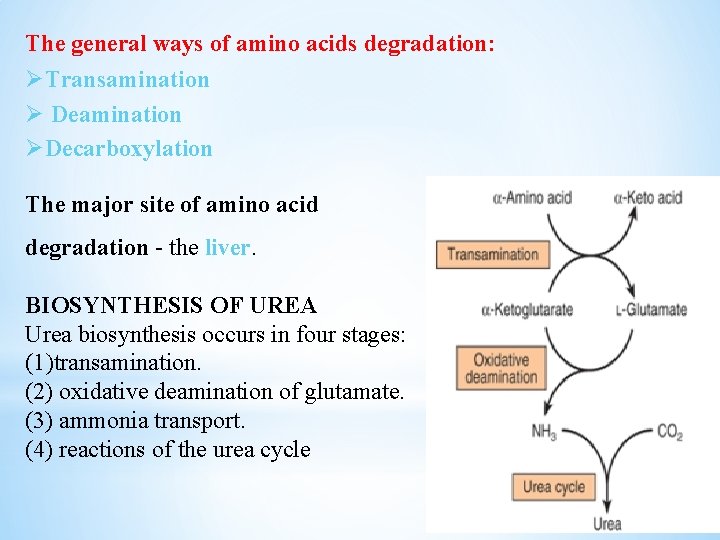
The general ways of amino acids degradation: ØTransamination Ø Deamination ØDecarboxylation The major site of amino acid degradation - the liver. BIOSYNTHESIS OF UREA Urea biosynthesis occurs in four stages: (1)transamination. (2) oxidative deamination of glutamate. (3) ammonia transport. (4) reactions of the urea cycle

Removal of nitrogen from amino acids is essential for producing energy from amino acids. It is an obligatory step in the catabolism of amino acids. Once removed this N can be incorporated into other compounds or excreted. Glutamate and glutamine play especially critical roles in nitrogen metabolism, acting as a kind of general collection point for amino groups In the cytosol of hepatocytes, amino groups from most amino acids are transferred to -ketoglutarate to form glutamate, which enters mitochondria and gives up its amino group to form NH 4

Excess ammonia generated in most other tissues is converted to the amide nitrogen of glutamine, which passes to the liver, then into liver mitochondria Glutamine or glutamate or both are present in higher concentrations than other amino acids in most tissues. In skeletal muscle, excess amino groups are generally transferred to pyruvate to form alanine, another important molecule in the transport of amino groups to the liver. Two reactions are important: 1. Transamination. 2. Oxidative deamination

A. Transamination The first step in the catabolism of most amino acids is the transfer of their α-amino group to α-ketoglutarate. The products are an α-keto acid and glutamate. α-Ketoglutarate plays a unique role in amino acid metabolism by accepting the amino groups from other amino acids, thus becoming glutamate. Glutamate produced by transamination can be: 1 - oxidatively deaminated. 2 - used as an amino group donor in the synthesis of nonessential amino acids. This transfer of amino groups from one carbon skeleton to another is catalyzed by a family of enzymes called aminotransferases These enzymes are found in the cytosol of cells throughout the

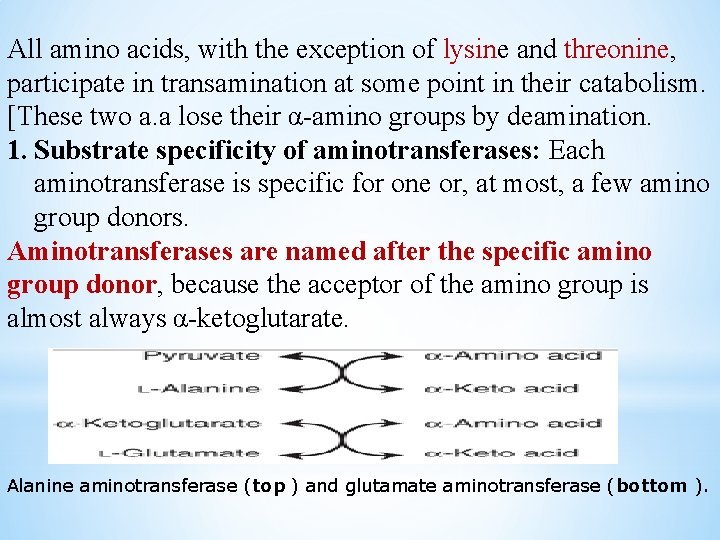
All amino acids, with the exception of lysine and threonine, participate in transamination at some point in their catabolism. [These two a. a lose their α-amino groups by deamination. 1. Substrate specificity of aminotransferases: Each aminotransferase is specific for one or, at most, a few amino group donors. Aminotransferases are named after the specific amino group donor, because the acceptor of the amino group is almost always α-ketoglutarate. Alanine aminotransferase (top ) and glutamate aminotransferase (bottom ).

The two most important aminotransferase reactions are catalyzed by alanine aminotransferase and aspartate aminotransferase a. Alanine aminotransferase (ALT), formerly called glutamate-pyruvate transaminase {GPT). -The enzyme catalyzes the transfer of the amino group of alanine to α-ketoglutarate, resulting in the formation of pyruvate and glutamate. -The reaction is readily reversible. This enzyme (like most aminotransferases) functions in the direction of glutamate synthesis. Thus, glutamate, in effect, acts as a "collector" of nitrogen from alanine. Alanine is the principal amino acid released from muscle tissue during starvation. It is an important substrate for hepatic gluconeogenesis

Mechanism of action of aminotransferases: All aminotransferases require the coenzyme pyridoxal phosphate (a derivative of vitamin. B 6) Ping-pong kinetic mechanism First step: the amino group of amino acid is transferred to pyridoxal phosphate, forming pyridoxamine phosphate and releasing keto acid. Second step: -ketoglutarate reacts with pyridoxamine phosphate forming glutamate

b. Aspartate aminotransferase (AST), formerly called glutsmate-oxaloacetate transaminase (GOT), is an exception to the rule that aminotransferases funnel amino groups to form glutamate. During amino acid catabolism, AST transfers amino groups from glutamate to oxaloacetate, forming aspartate, which is used as a source of nitrogen in the urea cycle.

Diagnostic value of plasma aminotransferases: Aminotransferases are normally intracellular enzymes, with the low levels found in the plasma. The presence of elevated plasma levels of aminotransferases indicates damage to cells rich in these enzymes (physical trauma or a disease process ) can cause cell lysis, resulting in release of intracellular enzymes into the blood. AST and ALT are of particular diagnostic value when they are found in the plasma. a. Liver disease: Plasma AST and ALT are elevated in nearly all liver diseases, such as severe viral hepatitis, toxic injury. b. Non hepatic disease: Aminotransferases may be elevated in non hepatic disease, such as myocardial infarction and muscle disorders.

Deamination of amino acids Deamination - elimination of amino group from amino acid with ammonia formation. Four types of deamination: - oxidative (the most important for higher animals - Reduction deamination: R-CH(NH 2)-COOH + 2 H+ R-CH 2 -COOH + NH 3 amino acid fatty acid Hydrolytic deamination: R-CH(NH 2)-COOH + H 2 O R-CH(OH)-COOH + NH 3 amino acid hydroxyacid Intramolecular deamination: R-CH(NH 2)-COOH R-CH-CH-COOH + NH 3 amino acid unsaturated fatty acid

The oxidative deamination of amino acids oxidative deamination by Glutamate dehydrogenase results in the liberation of the amino group as free ammonia. These reactions occur primarily in the liver and kidney. They provide α-keto acids that can enter the central pathway of energy metabolism, and ammonia, which is a source of nitrogen in urea synthesis. Glutamate dehydrogenase: the amino groups of most amino acids are ultimately funneled to glutamate by means of transamination with α-ketoglutarate. Glutamate is unique in that it is the only amino acid that undergoes rapid oxidative deamination a reaction catalyzed by glutamate dehydrogenase. In hepatocytes, glutamate is transported from the cytosol into mitochondria, where it undergoes oxidative deamination catalyzed by L glutamate

Therefore, the sequential action of transamination and the subsequent oxidative deamination of that glutamate provide a pathway whereby the amino groups of most amino acids can be released as ammonia.

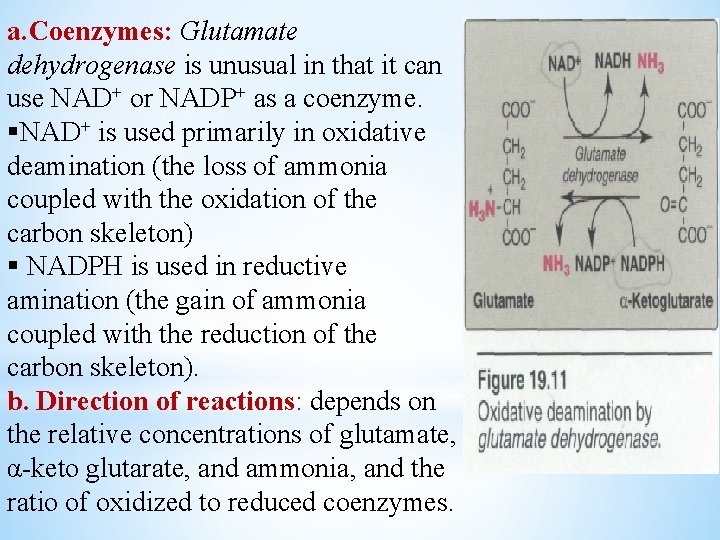
a. Coenzymes: Glutamate dehydrogenase is unusual in that it can use NAD+ or NADP+ as a coenzyme. §NAD+ is used primarily in oxidative deamination (the loss of ammonia coupled with the oxidation of the carbon skeleton) § NADPH is used in reductive amination (the gain of ammonia coupled with the reduction of the carbon skeleton). b. Direction of reactions: depends on the relative concentrations of glutamate, α-keto glutarate, and ammonia, and the ratio of oxidized to reduced coenzymes.

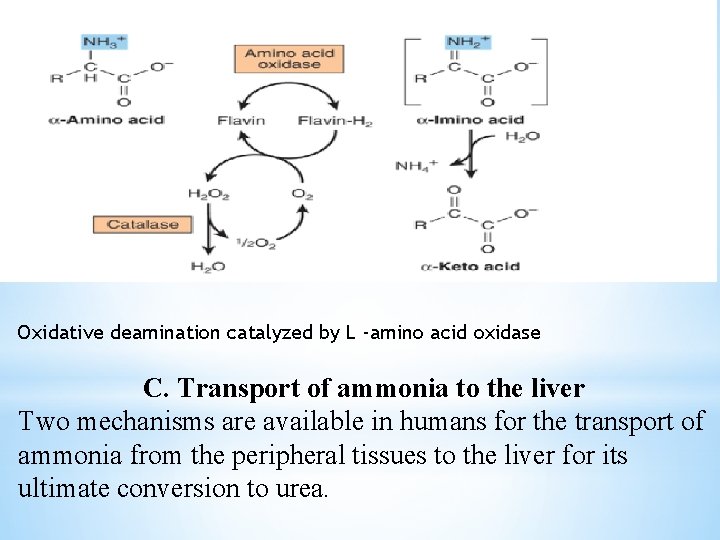
c. Allosteric regulators: ATP and GTP are allosteric inhibitors of the enzyme. ADP and GDP are activators of the enzyme. The combined action of an aminotransferase and glutamate dehydrogenase is referred to as Transdeamination. 2. Amino Acid Oxidases Also Remove Nitrogen as Ammonia L -amino acid oxidases of liver and kidney convert an amino acid to an α-imino acid that decomposes to an α -keto acid with release of ammonium ion (Figure 9). The reduced flavin is reoxidized by molecular oxygen, forming hydrogen peroxide (H 2 O 2 ), which then is split to O 2 and H 2 O by catalase

Oxidative deamination catalyzed by L -amino acid oxidase C. Transport of ammonia to the liver Two mechanisms are available in humans for the transport of ammonia from the peripheral tissues to the liver for its ultimate conversion to urea.

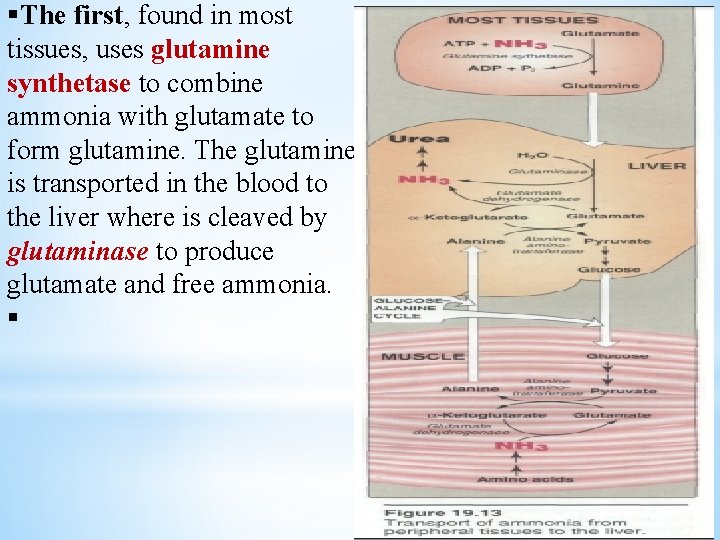
§The first, found in most tissues, uses glutamine synthetase to combine ammonia with glutamate to form glutamine. The glutamine is transported in the blood to the liver where is cleaved by glutaminase to produce glutamate and free ammonia. §

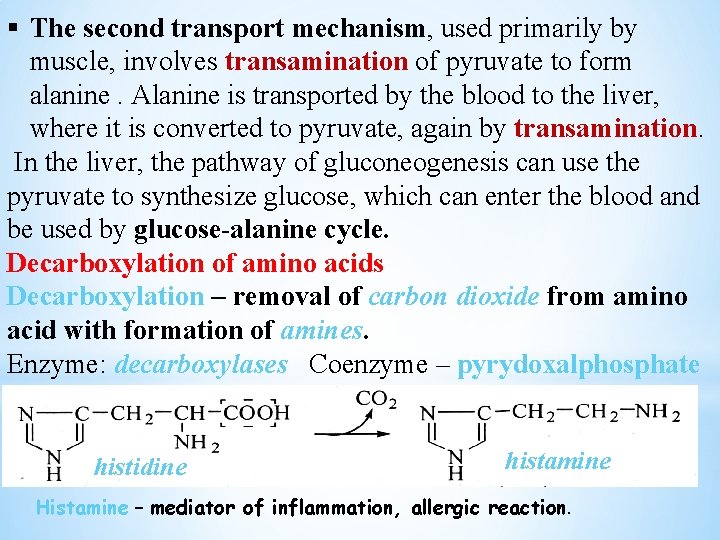
§ The second transport mechanism, used primarily by muscle, involves transamination of pyruvate to form alanine. Alanine is transported by the blood to the liver, where it is converted to pyruvate, again by transamination. In the liver, the pathway of gluconeogenesis can use the pyruvate to synthesize glucose, which can enter the blood and be used by glucose-alanine cycle. Decarboxylation of amino acids Decarboxylation – removal of carbon dioxide from amino acid with formation of amines. Enzyme: decarboxylases Coenzyme – pyrydoxalphosphate histidine histamine Histamine – mediator of inflammation, allergic reaction.
 Transamination of amino acids
Transamination of amino acids Acid base chemistry of amino acids
Acid base chemistry of amino acids Non essential amino acids mnemonics
Non essential amino acids mnemonics Perm wraps begin with sectioning the hair into
Perm wraps begin with sectioning the hair into Titration curves of all 20 amino acids
Titration curves of all 20 amino acids Asparagine titration curve
Asparagine titration curve Transdeamination of amino acids
Transdeamination of amino acids Salt bridges in proteins
Salt bridges in proteins 20 amino acids structures
20 amino acids structures Pvt tim hall
Pvt tim hall Jana novotn
Jana novotn Properties of amino acids slideshare
Properties of amino acids slideshare Ninhydrin test for amino acids
Ninhydrin test for amino acids Deamination of glutamine
Deamination of glutamine Nitrogen removal from amino acids
Nitrogen removal from amino acids Epsilon amino
Epsilon amino What are enzymes made of
What are enzymes made of Amino acid name
Amino acid name Quantitative estimation of amino acids by ninhydrin
Quantitative estimation of amino acids by ninhydrin