Amino Acids Proteins and Enzymes Types of Proteins















- Slides: 62

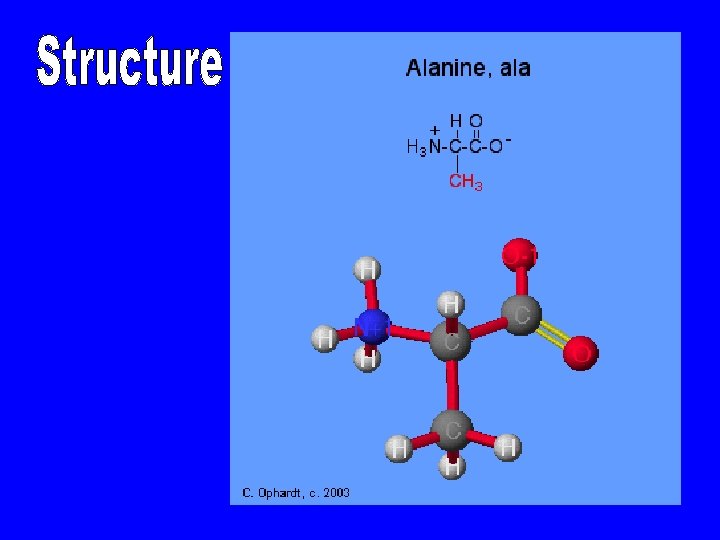
Amino Acids Proteins, and Enzymes Types of Proteins Amino Acids The Peptide Bond 1

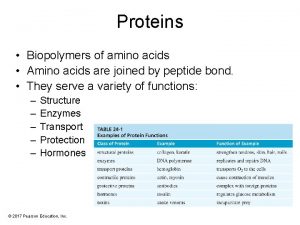
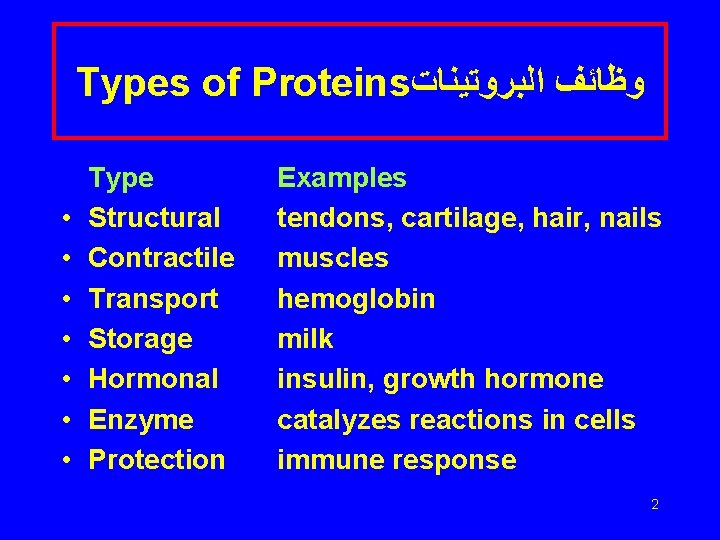
Types of Proteins ﻭﻇﺎﺋﻒ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ • • Type Structural Contractile Transport Storage Hormonal Enzyme Protection Examples tendons, cartilage, hair, nails muscles hemoglobin milk insulin, growth hormone catalyzes reactions in cells immune response 2

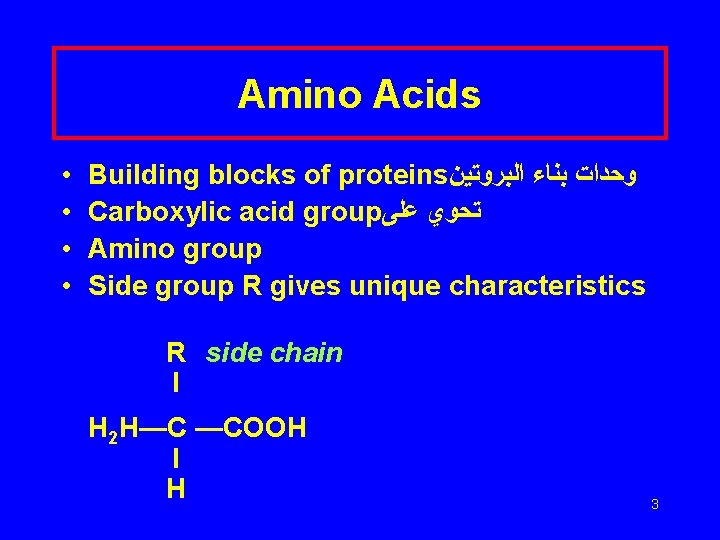
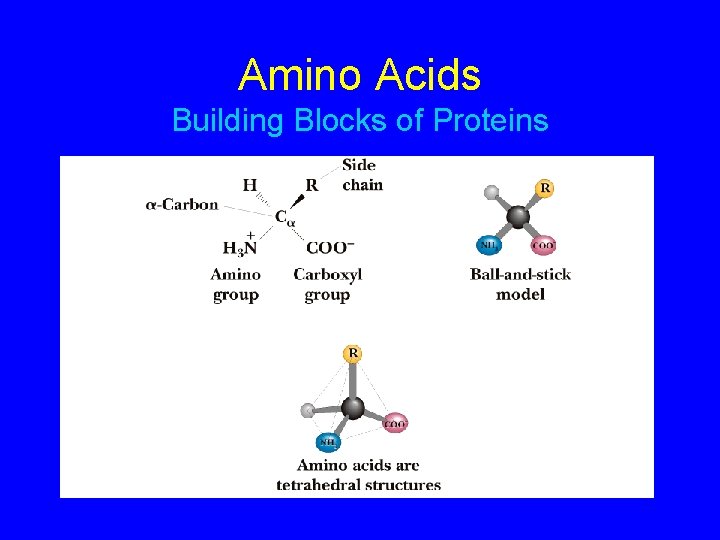
Amino Acids • • Building blocks of proteins ﻭﺣﺪﺍﺕ ﺑﻨﺎﺀ ﺍﻟﺒﺮﻭﺗﻴﻦ Carboxylic acid group ﺗﺤﻮﻱ ﻋﻠﻰ Amino group Side group R gives unique characteristics R side chain I H 2 H—C —COOH I H 3

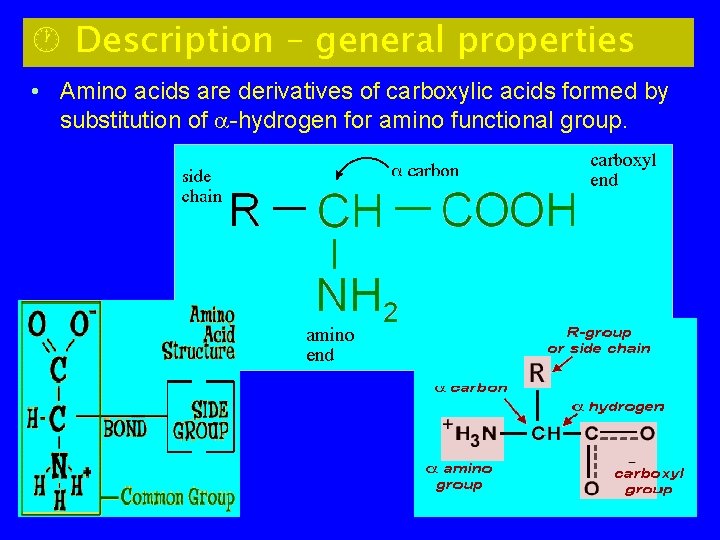
· Description – general properties • Amino acids are derivatives of carboxylic acids formed by substitution of -hydrogen for amino functional group.

5

-carbon

Amino Acids Building Blocks of Proteins

Amino Acids • Amino acids are the structural building blocks (monomers) of proteins. ﺍﻷﺤﻤﺎﺽ ﻣﻦ ﺃﻮﻟﻴﺔ ﻭﺣﺪﺍﺕ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ ﻟﺒﻨﺎﺀ ﺍﻻﻣﻴﻨﻴﺔ • There are twenty different kinds of amino acids used in proteins. ﺑﻨﺎﺀ ﻓﻲ ﺍﻣﻴﻨﻲ ﺣﺎﻣﺾ ﻋﺸﺮﻳﻦ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ • Proteins are referred to as heteropolymers due the variety of amino acids involved in their structure. ﻋﻠﻰ ﻳﺤﺘﻮﻱ ﻣﺘﻌﺪﺩ ﺑﻮﻟﻴﻤﺮ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ ﺍﻟﺘﺮﻛﻴﺐ ﻣﺨﺘﻠﻔﺔ ﺍﻣﻴﻨﻴﺔ ﺃﺤﻤﺎﺽ

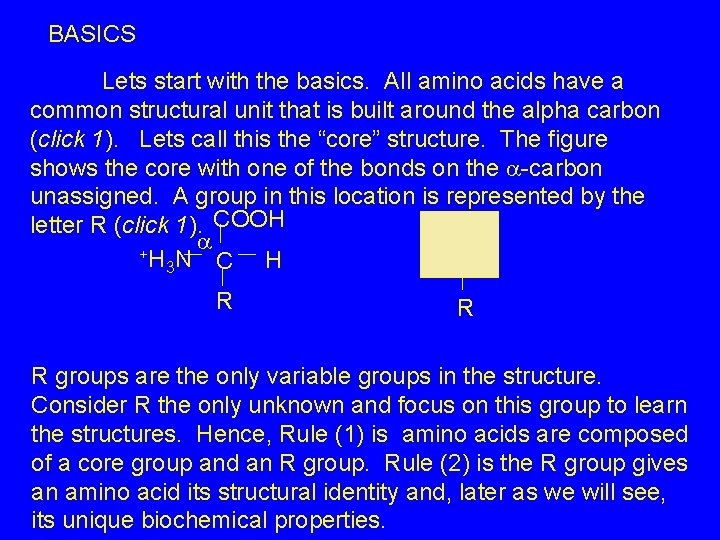
BASICS Lets start with the basics. All amino acids have a common structural unit that is built around the alpha carbon (click 1). Lets call this the “core” structure. The figure shows the core with one of the bonds on the -carbon unassigned. A group in this location is represented by the letter R (click 1). COOH +H N C H 3 R R R groups are the only variable groups in the structure. Consider R the only unknown and focus on this group to learn the structures. Hence, Rule (1) is amino acids are composed of a core group and an R group. Rule (2) is the R group gives an amino acid its structural identity and, later as we will see, its unique biochemical properties.

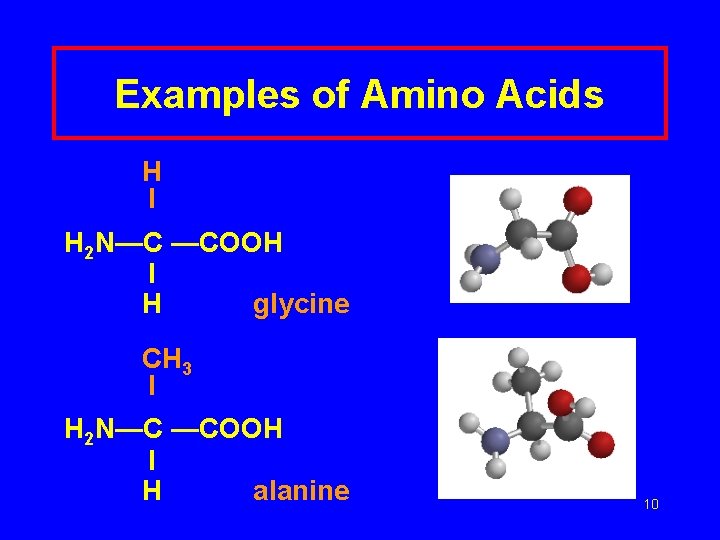
Examples of Amino Acids H I H 2 N—C —COOH I H glycine CH 3 I H 2 N—C —COOH I H alanine 10

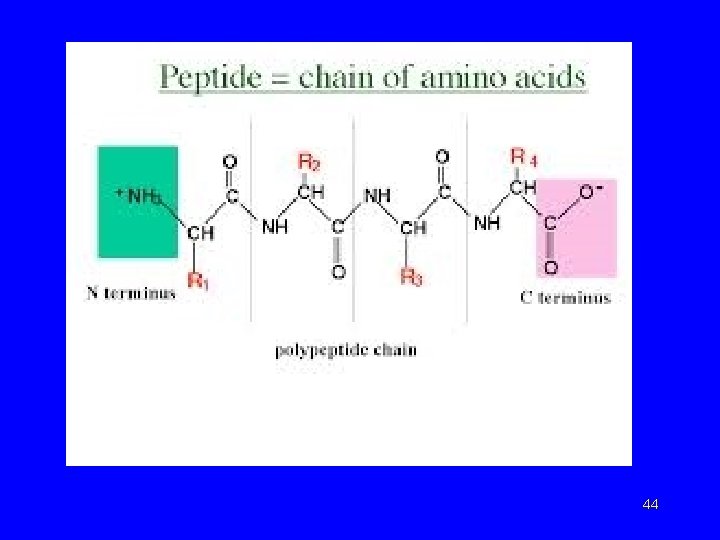
Amino Acids (cont’d. ) • The repeating sequence of atoms along a proteins is referred to as the polypeptide backbone. Attached to this repetitive chain are the different amino acid side chains (Rgroups) which are not involved in the peptide bond but which give each amino acid its unique property.

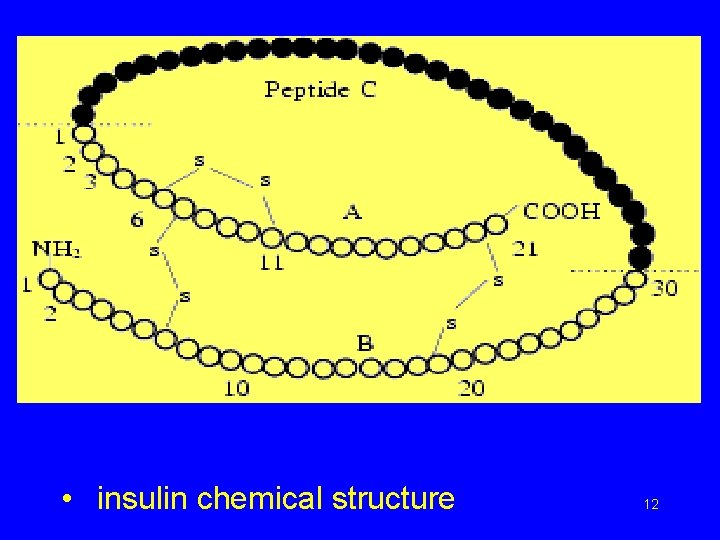
• insulin chemical structure 12

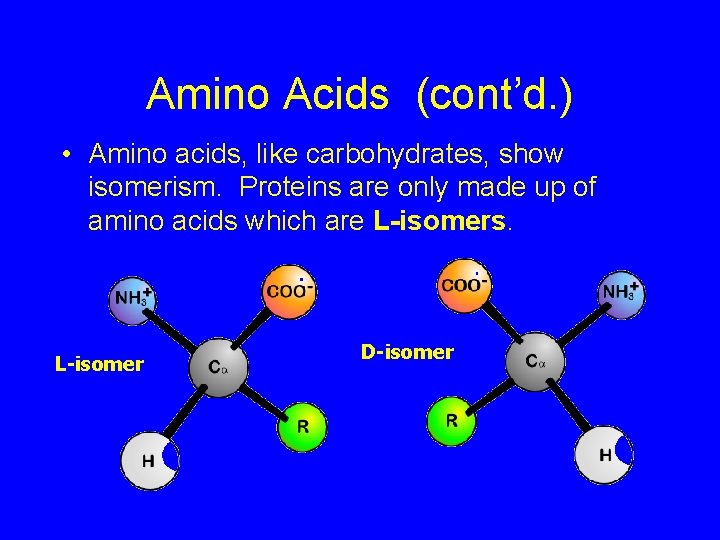
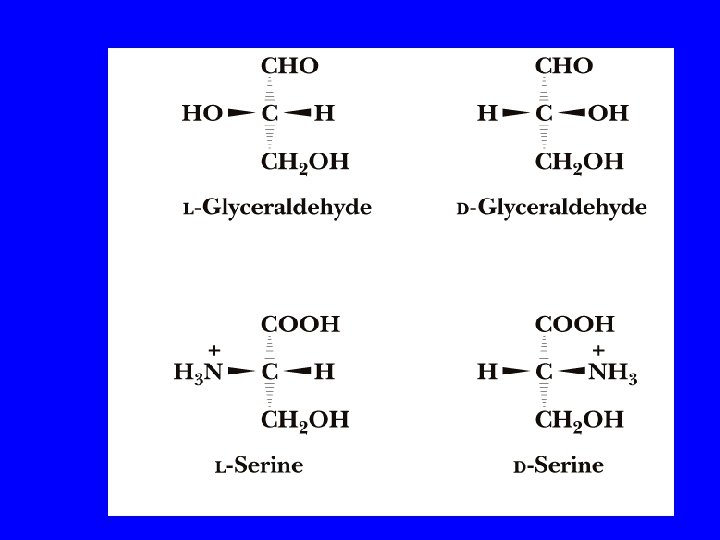
Amino Acids (cont’d. ) • Amino acids, like carbohydrates, show isomerism. Proteins are only made up of amino acids which are L-isomers. L-isomer D-isomer

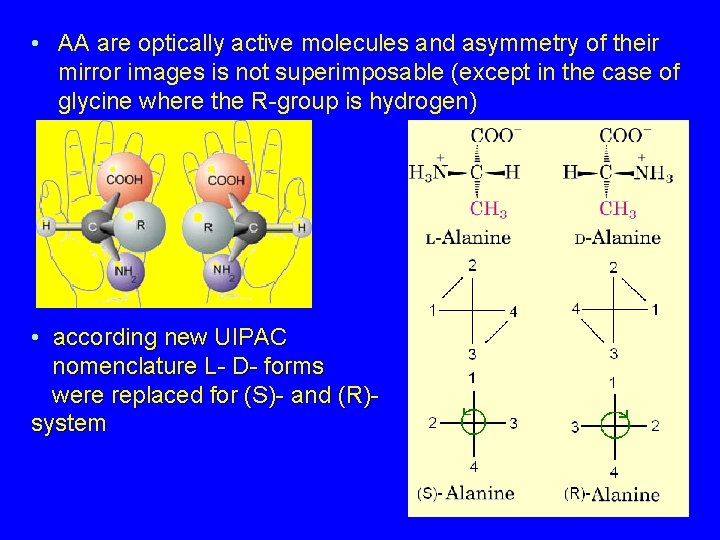
• AA are optically active molecules and asymmetry of their mirror images is not superimposable (except in the case of glycine where the R-group is hydrogen) • according new UIPAC nomenclature L- D- forms were replaced for (S)- and (R)- system


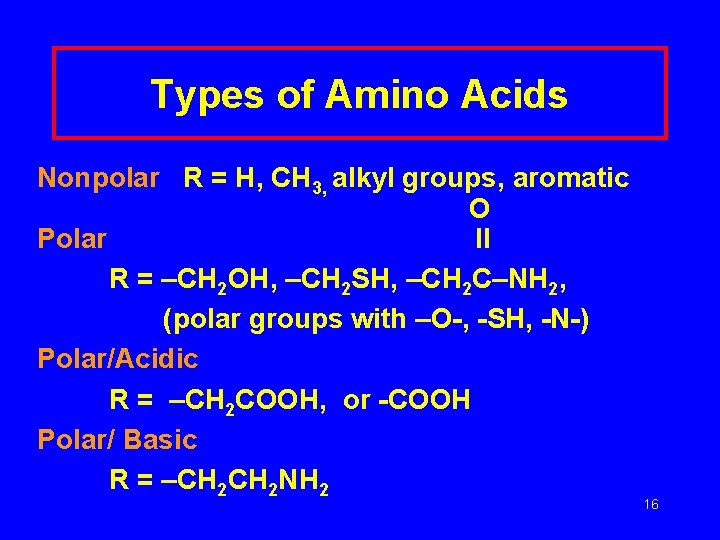
Types of Amino Acids Nonpolar R = H, CH 3, alkyl groups, aromatic O Polar ll R = –CH 2 OH, –CH 2 SH, –CH 2 C–NH 2, (polar groups with –O-, -SH, -N-) Polar/Acidic R = –CH 2 COOH, or -COOH Polar/ Basic R = –CH 2 NH 2 16

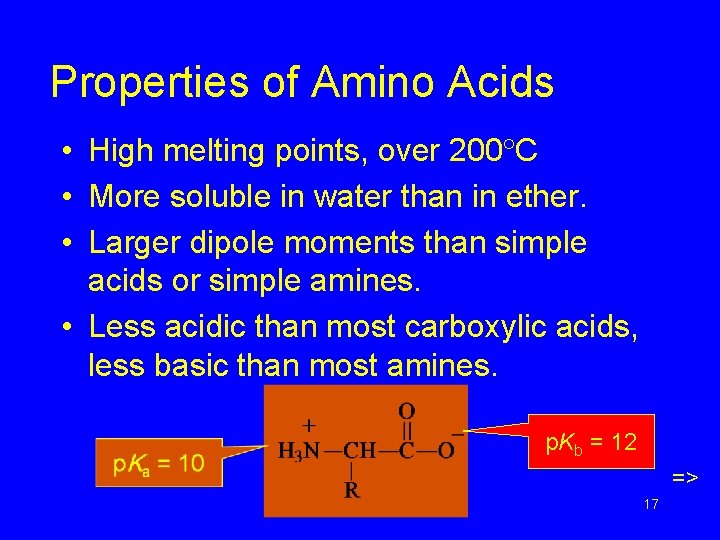
Properties of Amino Acids • High melting points, over 200 C • More soluble in water than in ether. • Larger dipole moments than simple acids or simple amines. • Less acidic than most carboxylic acids, less basic than most amines. p. Kb = 12 => Chapter 24 17

Complete Proteins • • Provide all the essential amino acids. Examples: those in meat, fish, milk, eggs. Plant proteins are generally incomplete. Vegetarians should eat many different kinds of plants, or supplement diet with milk or eggs. ﻣﺼﺎﺩ ﺍﻟﻐﺬﺍﺋﻴﺔ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ ﺭ => Chapter 24 18

19

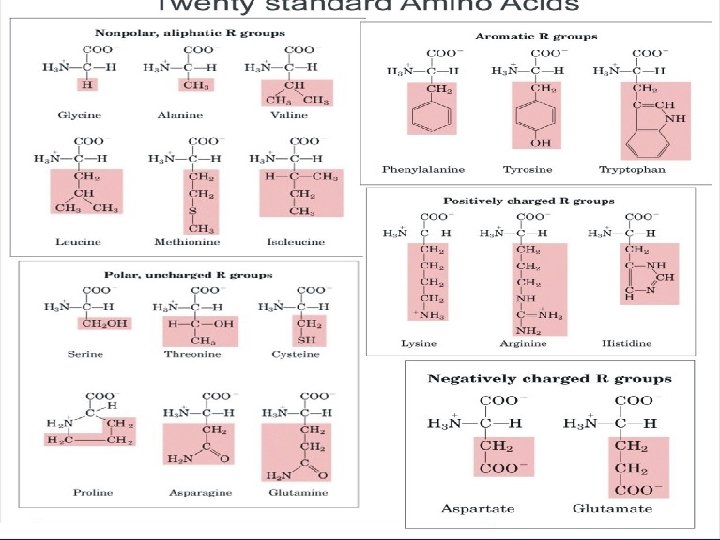
20 Common Amino Acids You should know names, structures, p. Ka values, 3 -letter and 1 -letter codes • Non-polar amino acids • Polar, uncharged amino acids • Acidic amino acids • Basic amino acids

Amino Acids (cont’d. ) • Amino acids are grouped according to whether their side chains are: • acidic • basic • uncharged polar • non polar

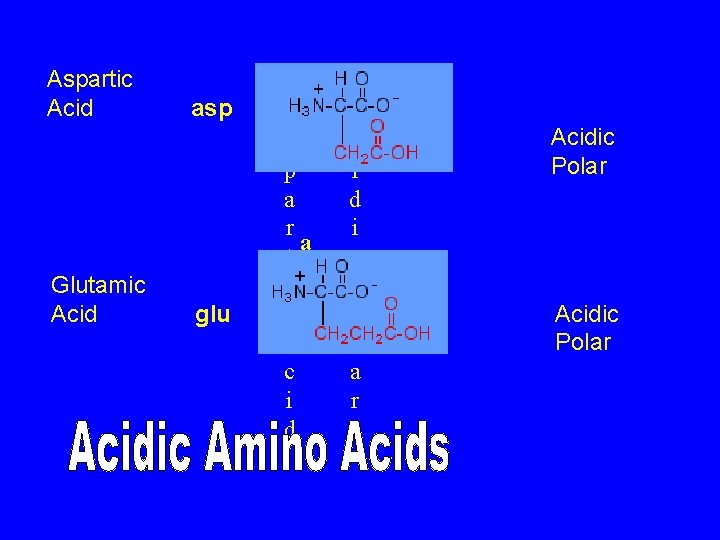
Aspartic Acid Glutamic Acid asp glu A A c s i p d a i r a t c s i D P p c o l A a c r i d Acidic Polar

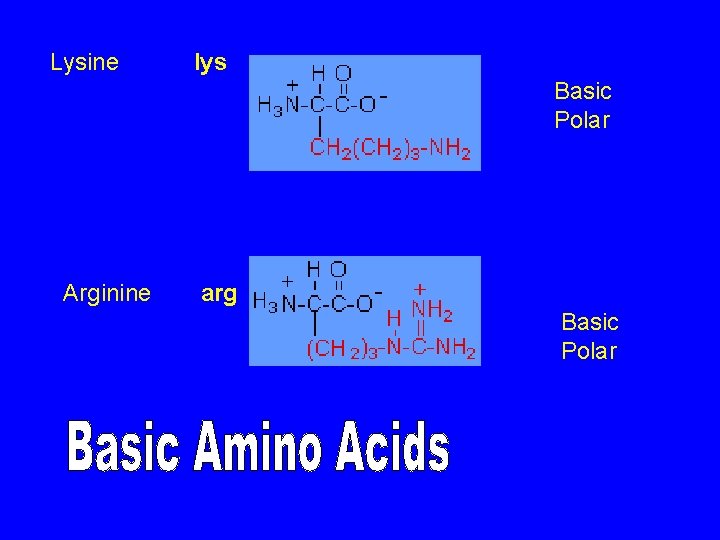
Lysine lys Arginine arg Basic Polar

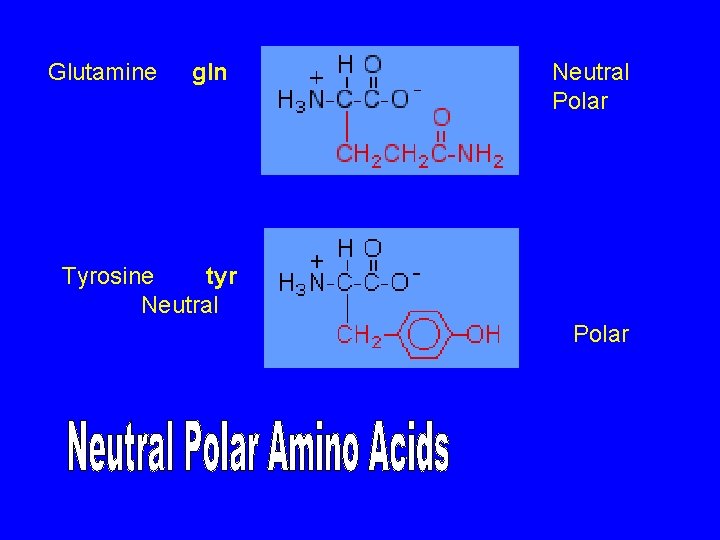
Glutamine gln Neutral Polar Tyrosine tyr Neutral Polar

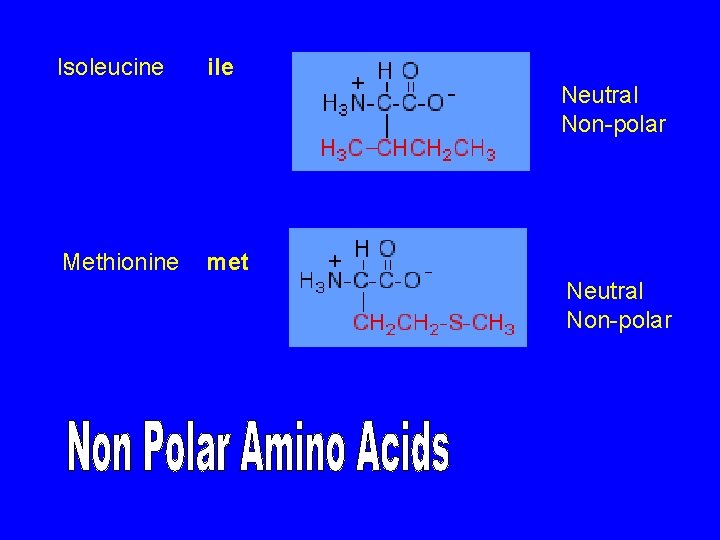
Isoleucine ile Neutral Non-polar Methionine met Neutral Non-polar

Amino Acids (cont’d. ) • The type of side chain is very important as it affects the solubility of the amino acid. • Hydrophobic features include long non-polar (uncharged) chains or complex aromatic rings. • Hydrophilic features include additional carboxyl groups or amino groups not involved in peptide bonding which are ionised in solution.

Essential Amino Acids • 10 amino acids not synthesized by the body • arg, his, ile, leu, lys, met, phe, thr, trp, val • Must obtain from the diet • All in diary products • 1 or more missing in grains and vegetables 27

Essential Amino Acids • • • Arginine (Arg) Threonine (Thr) Lysine (Lys) Valine (Val) Phenylalanine (Phe) • • • Tryptophan (Trp) Methionine (Met) Histidine (His) Leucine (Leu) Isoleucine (Ile) => Chapter 24 28

Amino Acids (cont’d. ) • At neutral p. H’s amino acids exist in an ionised form and have both acidic and basic properties. This is because the carboxylic group donates hydrogen ions to the solution (acidic) whereas the amino group (NH 2) attracts hydrogen ions from the solution.

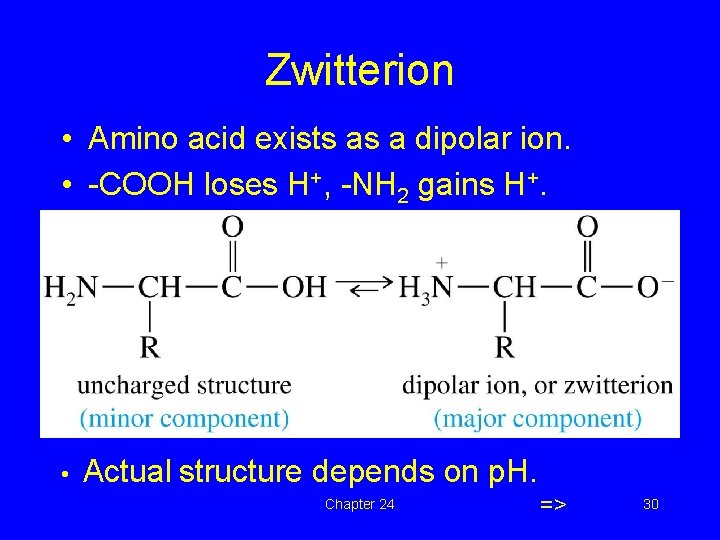
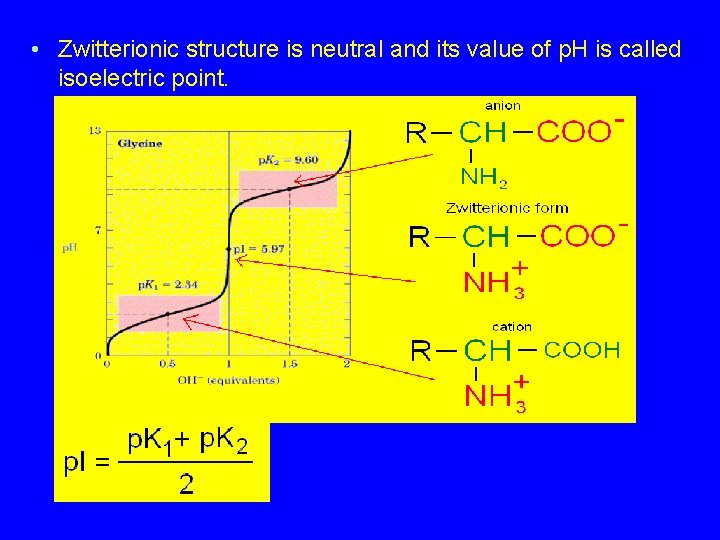
Zwitterion • Amino acid exists as a dipolar ion. • -COOH loses H+, -NH 2 gains H+. • Actual structure depends on p. H. Chapter 24 => 30

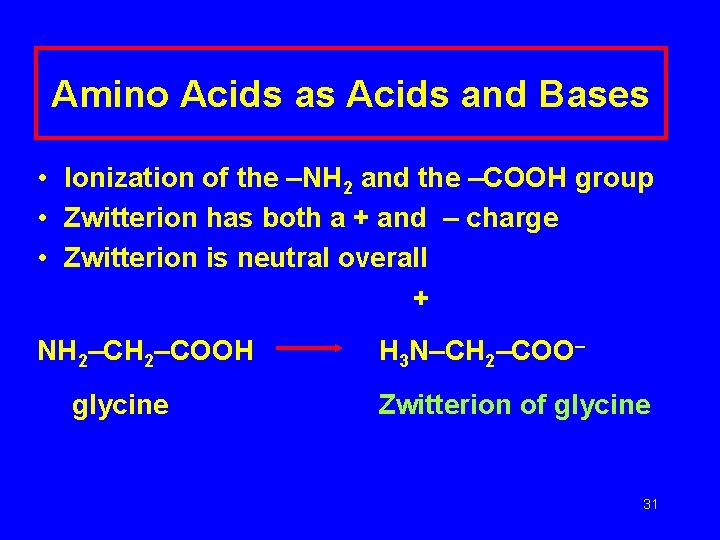
Amino Acids as Acids and Bases • Ionization of the –NH 2 and the –COOH group • Zwitterion has both a + and – charge • Zwitterion is neutral overall + NH 2–COOH glycine H 3 N–CH 2–COO– Zwitterion of glycine 31

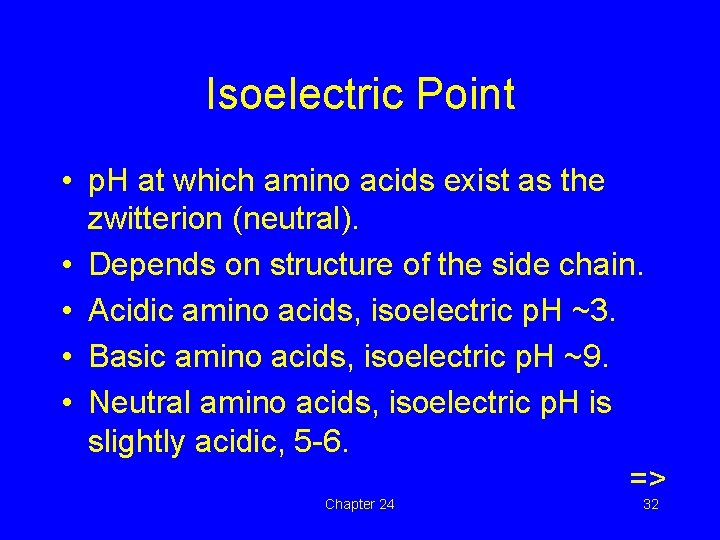
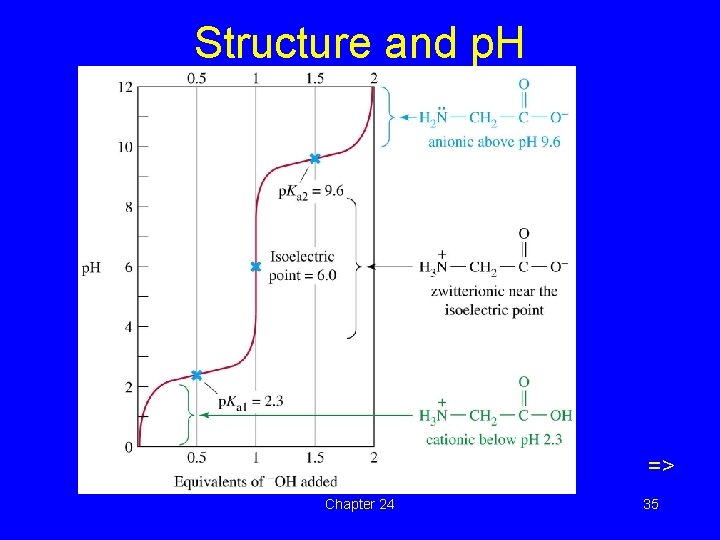
Isoelectric Point • p. H at which amino acids exist as the zwitterion (neutral). • Depends on structure of the side chain. • Acidic amino acids, isoelectric p. H ~3. • Basic amino acids, isoelectric p. H ~9. • Neutral amino acids, isoelectric p. H is slightly acidic, 5 -6. => Chapter 24 32

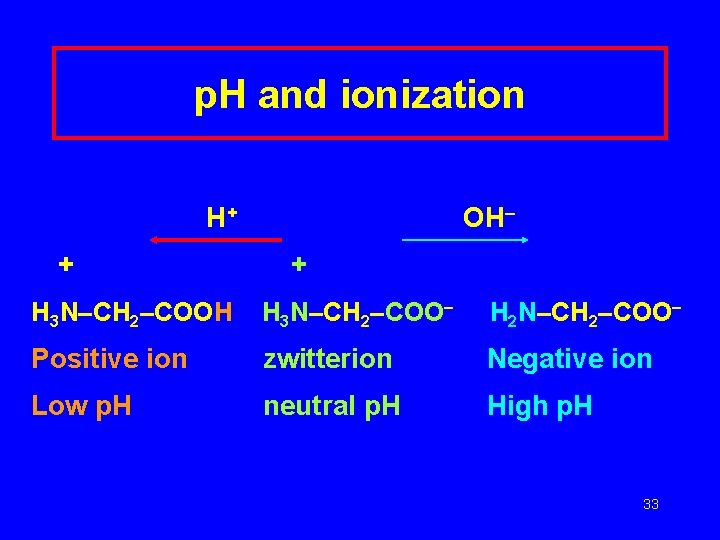
p. H and ionization H+ + OH– + H 3 N–CH 2–COOH H 3 N–CH 2–COO– H 2 N–CH 2–COO– Positive ion zwitterion Negative ion Low p. H neutral p. H High p. H 33

• Zwitterionic structure is neutral and its value of p. H is called isoelectric point.

Structure and p. H => Chapter 24 35

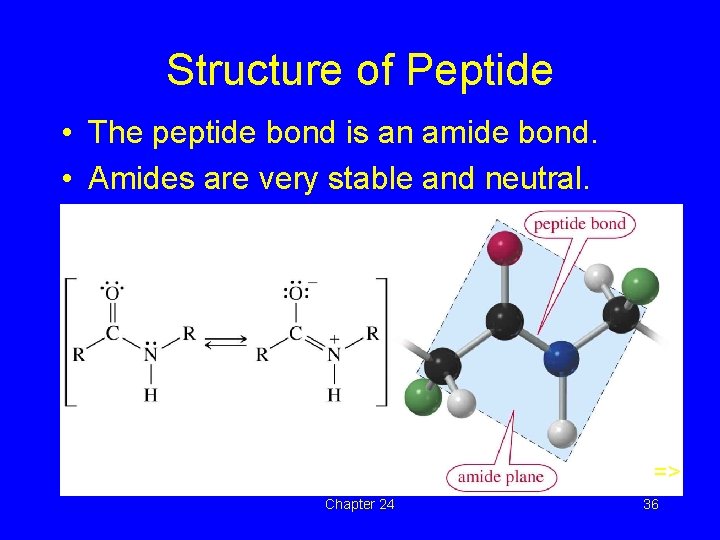
Structure of Peptide • The peptide bond is an amide bond. • Amides are very stable and neutral. => Chapter 24 36

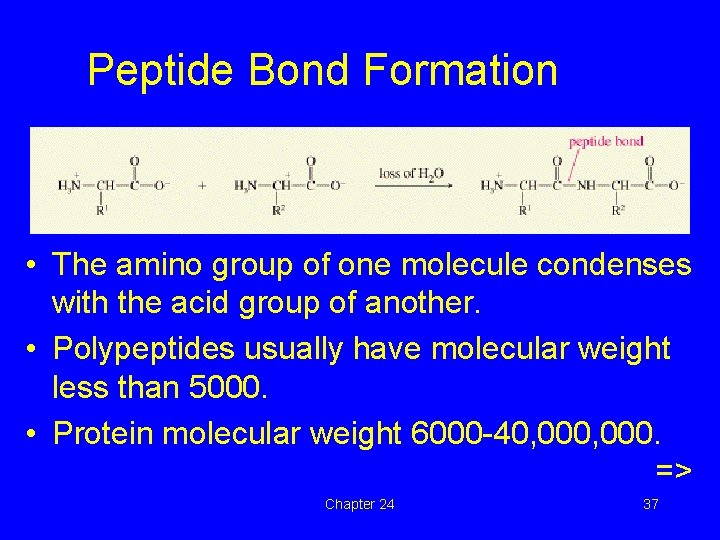
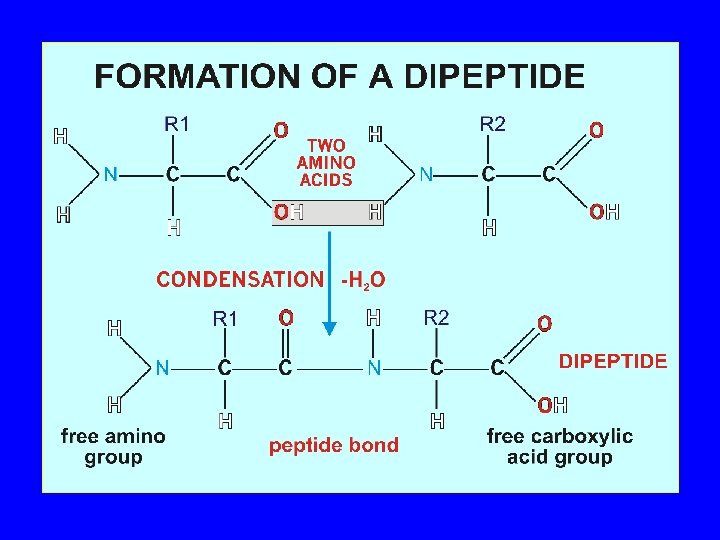
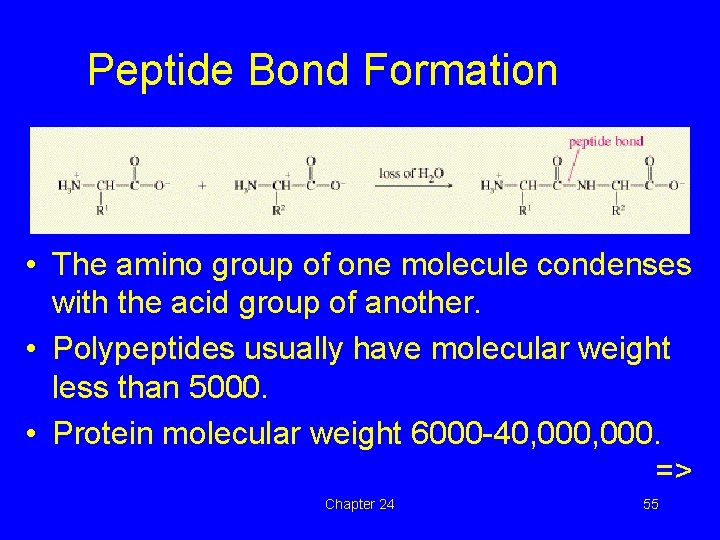
Peptide Bond Formation • The amino group of one molecule condenses with the acid group of another. • Polypeptides usually have molecular weight less than 5000. • Protein molecular weight 6000 -40, 000. => Chapter 24 37

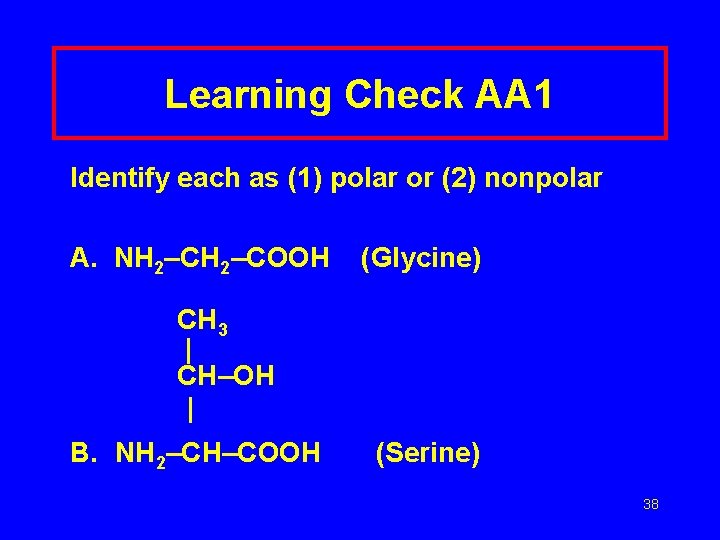
Learning Check AA 1 Identify each as (1) polar or (2) nonpolar A. NH 2–COOH (Glycine) CH 3 | CH–OH | B. NH 2–CH–COOH (Serine) 38

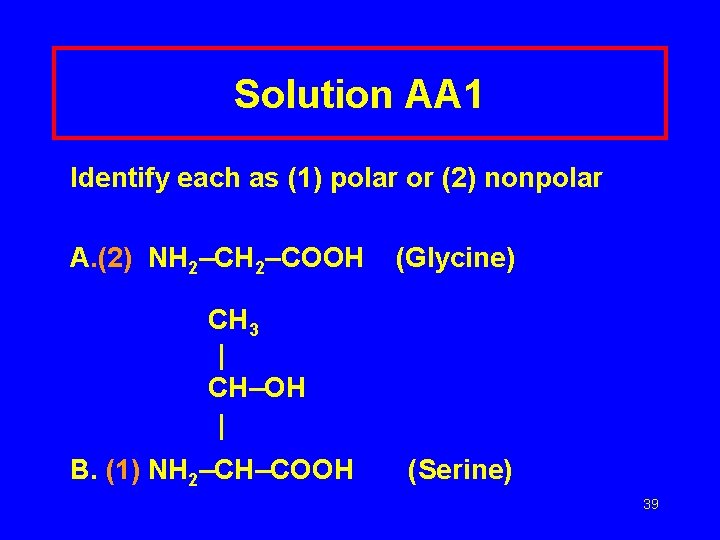
Solution AA 1 Identify each as (1) polar or (2) nonpolar A. (2) NH 2–COOH (Glycine) CH 3 | CH–OH | B. (1) NH 2–CH–COOH (Serine) 39

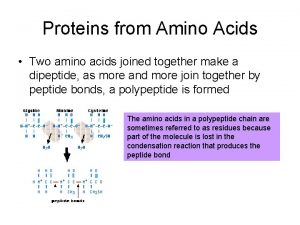
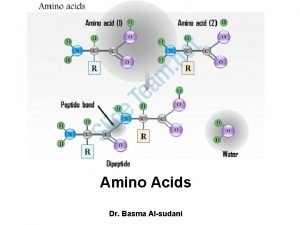
Amino Acids (cont’d. ) • Amino acids link together by covalent peptide bonds. This involves a condensation /dehydration reaction. These bonds are very strong. When this takes place the charged amino and carboxylic groups disappear.

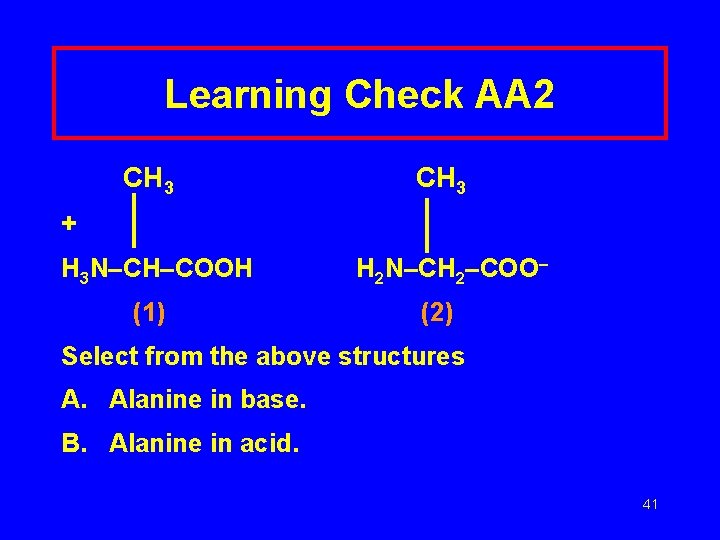
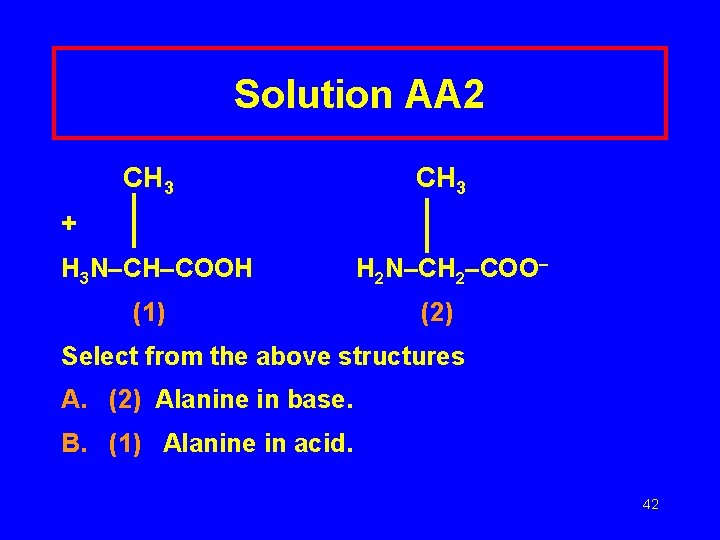
Learning Check AA 2 CH 3 + H 3 N–CH–COOH (1) H 2 N–CH 2–COO– (2) Select from the above structures A. Alanine in base. B. Alanine in acid. 41

Solution AA 2 CH 3 + H 3 N–CH–COOH (1) H 2 N–CH 2–COO– (2) Select from the above structures A. (2) Alanine in base. B. (1) Alanine in acid. 42

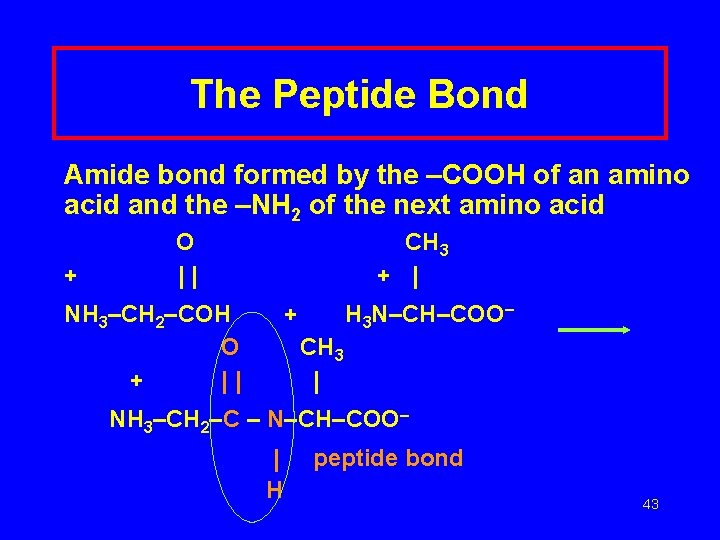
The Peptide Bond Amide bond formed by the –COOH of an amino acid and the –NH 2 of the next amino acid + O || CH 3 + | NH 3–CH 2–COH O + || + H 3 N–CH–COO– CH 3 | NH 3–CH 2–C – N–CH–COO– | H peptide bond 43

44

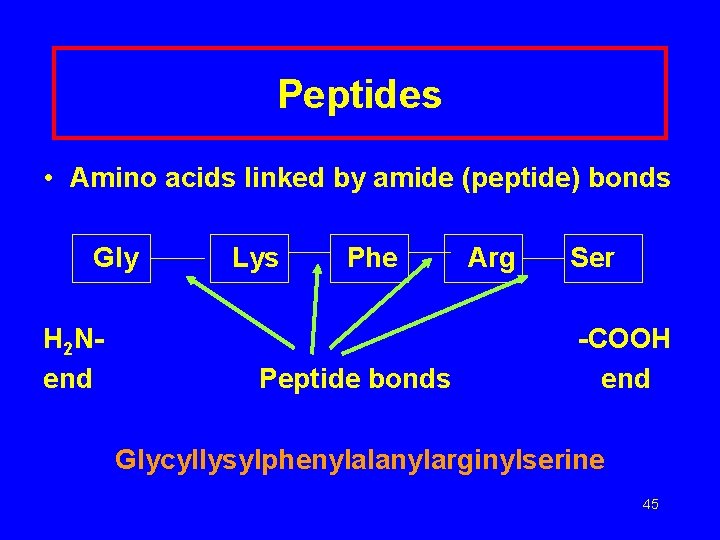
Peptides • Amino acids linked by amide (peptide) bonds Gly H 2 Nend Lys Phe Peptide bonds Arg Ser -COOH end Glycyllysylphenylalanylarginylserine 45

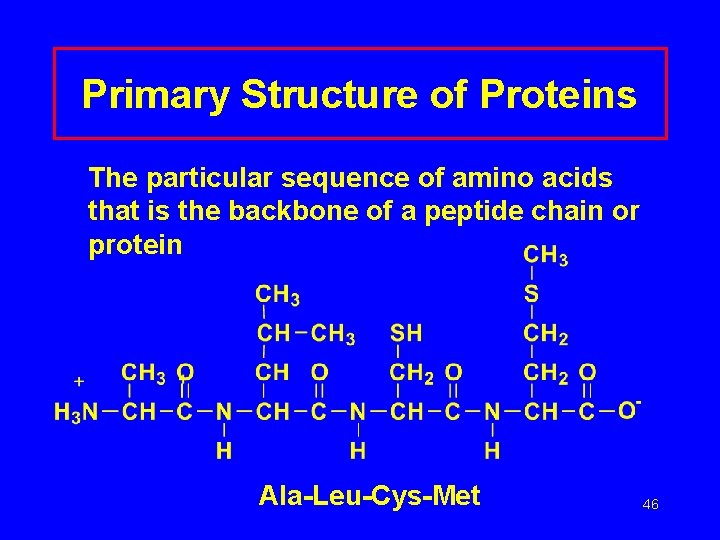
Primary Structure of Proteins The particular sequence of amino acids that is the backbone of a peptide chain or protein Ala-Leu-Cys-Met 46

Learning Check AA 3 What are the possible tripeptides formed from one each of leucine, glycine, and alanine? 47

Solution AA 3 Tripeptides possible from one each of leucine, glycine, and alanine Leu-Gly-Ala Leu-Ala-Gly Ala-Leu-Gly Ala-Gly-Leu Gly-Ala-Leu Gly-Leu-Ala 48

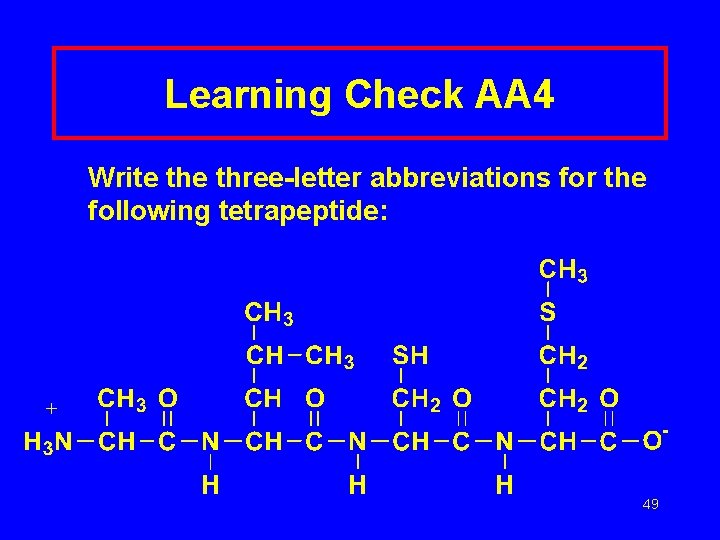
Learning Check AA 4 Write three-letter abbreviations for the following tetrapeptide: 49

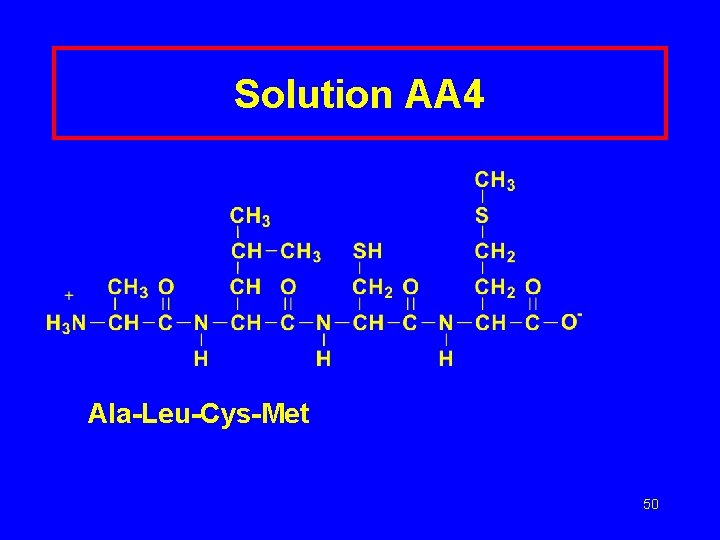
Solution AA 4 Ala-Leu-Cys-Met 50

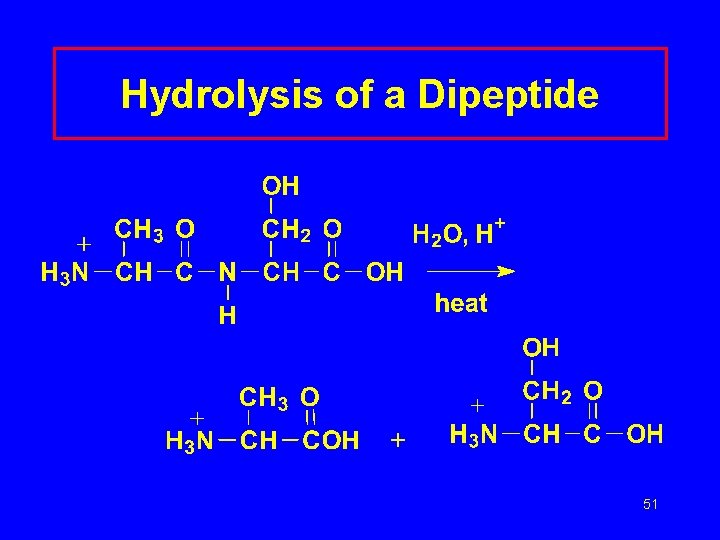
Hydrolysis of a Dipeptide 51

Learning Check P 4 What are the products of the complete hydrolysis of Ala-Ser-Val? 52

Solution P 4 The products of the complete hydrolysis of Ala-Ser-Val are alanine serine valine 53

Test Your Knowledge. Click to see the answer. Q: What amino acid has the shortest carbon chain in its R group? A: Glycine. It has no carbon in its R group. Q: What structural feature is common to alanine, serine and cyst A: All three have a single carbon in their R groups. Q: Which amino acid has the longest straight chain of carbons in its R group? A: Lysine. It has 4. Leucine and isoleucine have 4 but their chains are branched Q: What R group structural feature is common to phenylalanine, tyrosine, tryptophan, and histidine? A: All four have rings that are attached to the core via a –CH 2 Q: What structural feature is common to isoleucine and threonine A: Both have an asymmetric carbon in their R group

Peptide Bond Formation • The amino group of one molecule condenses with the acid group of another. • Polypeptides usually have molecular weight less than 5000. • Protein molecular weight 6000 -40, 000. => Chapter 24 55


Classification of Proteins • Simple: hydrolyze to amino acids only. • Conjugated: bonded to a nonprotein group, such as sugar, nucleic acid, or lipid. • Fibrous: long, stringy filaments, insoluble in water, function as structure. • Globular: folded into spherical shape, function as enzymes, hormones, or transport proteins. => Chapter 24 57

Levels of Protein Structure • Primary: the sequence of the amino acids in the chain and the disulfide links. • Secondary: structure formed by hydrogen bonding. Examples are helix and pleated sheet. • Tertiary: complete 3 -D conformation. • Quaternary: association of two or more peptide chains to form protein. => Chapter 24 58

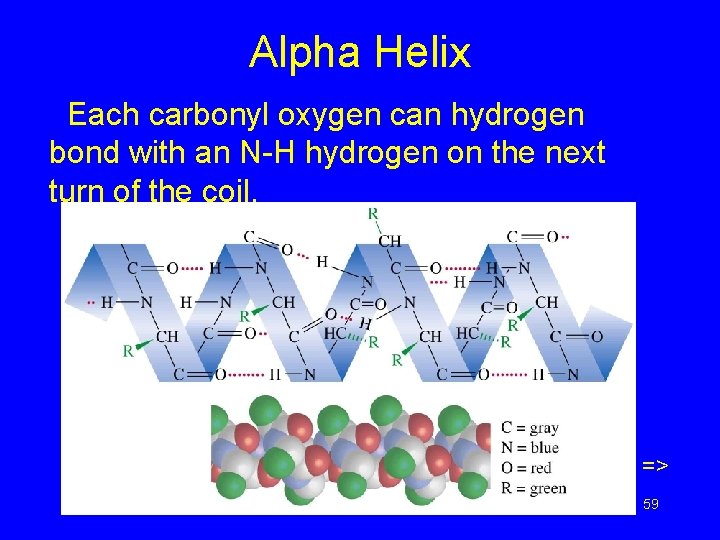
Alpha Helix Each carbonyl oxygen can hydrogen bond with an N-H hydrogen on the next turn of the coil. => Chapter 24 59

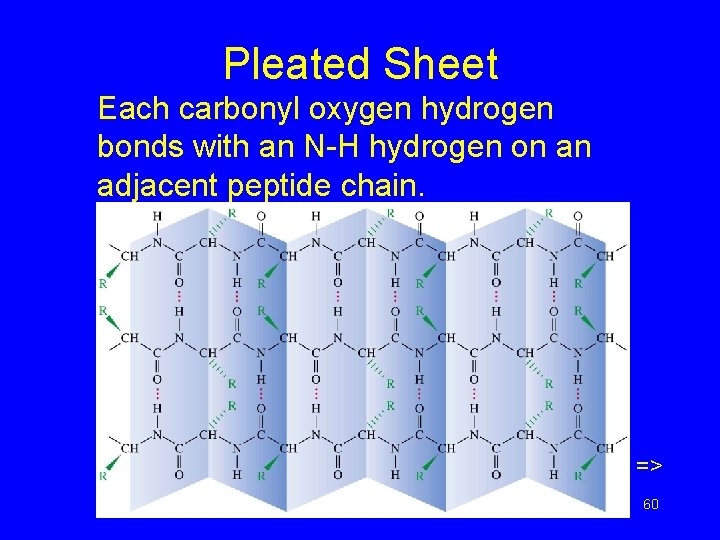
Pleated Sheet Each carbonyl oxygen hydrogen bonds with an N-H hydrogen on an adjacent peptide chain. => Chapter 24 60

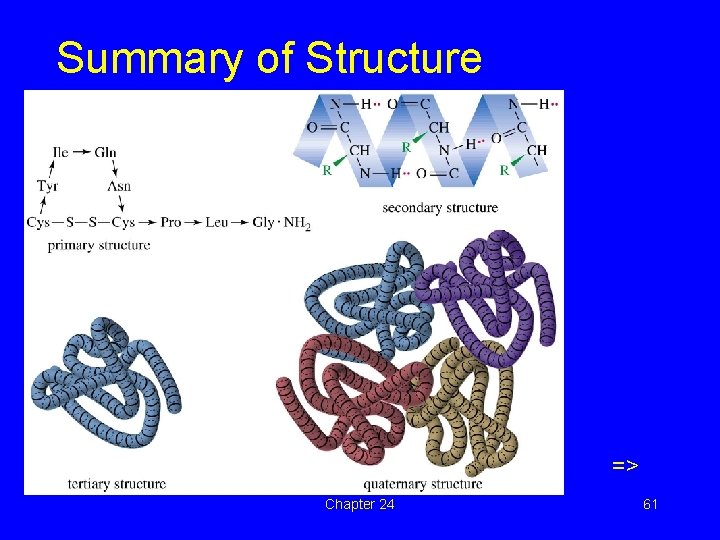
Summary of Structure => Chapter 24 61

Denaturation • Disruption of the normal structure of a protein, such that it loses biological activity. • Usually caused by heat or changes in p. H. • Usually irreversible. A cooked egg cannot be “uncooked. ” => Chapter 24 62
 Amino acids are joined together in proteins by
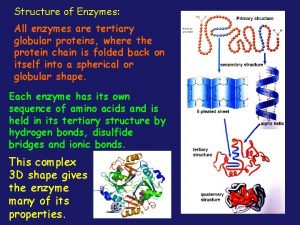
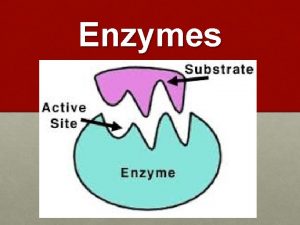
Amino acids are joined together in proteins by All enzymes are globular proteins
All enzymes are globular proteins Not all enzymes are proteins
Not all enzymes are proteins Ketogenic amino acids
Ketogenic amino acids Why is lysine basic
Why is lysine basic Amino acid classification
Amino acid classification Difference between hydrophobic and hydrophilic amino acids
Difference between hydrophobic and hydrophilic amino acids Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Titration curve for amino acids
Titration curve for amino acids Titration curve of amino acids
Titration curve of amino acids Deamination of amino acids
Deamination of amino acids Two amino acids joined
Two amino acids joined Deamination of glutamine
Deamination of glutamine 20 amino acid structure
20 amino acid structure Chirality definition
Chirality definition N
N Glycine titration curve
Glycine titration curve Amino acids gluconeogenesis
Amino acids gluconeogenesis Plp mechanism transamination
Plp mechanism transamination What is the r group in amino acids
What is the r group in amino acids Solubility of amino acids in water
Solubility of amino acids in water Chemsheets
Chemsheets Properties of amino acids
Properties of amino acids Chymotrypsin cleaves which amino acids
Chymotrypsin cleaves which amino acids Positively charged amino acids
Positively charged amino acids Which amino acids have ionizable side chains
Which amino acids have ionizable side chains Non essential amino acids mnemonics
Non essential amino acids mnemonics Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Aromatic amino acids
Aromatic amino acids Phenol containing amino acids
Phenol containing amino acids Xanthoproteic test positive result
Xanthoproteic test positive result Peptide bond dehydration synthesis
Peptide bond dehydration synthesis Properties of amino acids
Properties of amino acids Biomedical importance of amino acids
Biomedical importance of amino acids Are amino acids negatively charged
Are amino acids negatively charged Conditionally essential amino acids
Conditionally essential amino acids Essential aa mnemonic
Essential aa mnemonic Tyrosine letter code
Tyrosine letter code Amino acid deamination
Amino acid deamination Oxidative deamination of amino acids
Oxidative deamination of amino acids Non essential amino acids mnemonics
Non essential amino acids mnemonics How many amino acids are there
How many amino acids are there Quantitative estimation of amino acids by ninhydrin
Quantitative estimation of amino acids by ninhydrin Protein structure
Protein structure Protein metabolism
Protein metabolism Transdeamination of amino acids
Transdeamination of amino acids 17/35
17/35 Strecker synthesis
Strecker synthesis Salt bridge amino acids
Salt bridge amino acids Ketogenic amino acids
Ketogenic amino acids Upon hydrolysis of fibron which amino acids are produced
Upon hydrolysis of fibron which amino acids are produced Alpha carbon
Alpha carbon Acid base properties of amino acids
Acid base properties of amino acids Transdeamination of amino acids
Transdeamination of amino acids Ketogenic amino acids
Ketogenic amino acids Titration curve of valine
Titration curve of valine What is made of amino acids
What is made of amino acids Wikipedia amino acids
Wikipedia amino acids Meister cycle
Meister cycle What are enzymes made of
What are enzymes made of Importance of sulphur containing amino acids
Importance of sulphur containing amino acids Charged amino acids
Charged amino acids