ANALYTICAL CHEMISTRY prof Viktor Kanick Analytick chemie I






![Requirements for analytical reactions n n Activity a. A = [A] y. A [A] Requirements for analytical reactions n n Activity a. A = [A] y. A [A]](https://slidetodoc.com/presentation_image/f004f447878f1363bcc44dfbf43e8f92/image-55.jpg)


![Complex (forming) equilibria c. M = [M] + [ML] +…+ [MLn] = [M] + Complex (forming) equilibria c. M = [M] + [ML] +…+ [MLn] = [M] +](https://slidetodoc.com/presentation_image/f004f447878f1363bcc44dfbf43e8f92/image-66.jpg)





- Slides: 87

ANALYTICAL CHEMISTRY prof. Viktor Kanický, Analytická chemie I 1

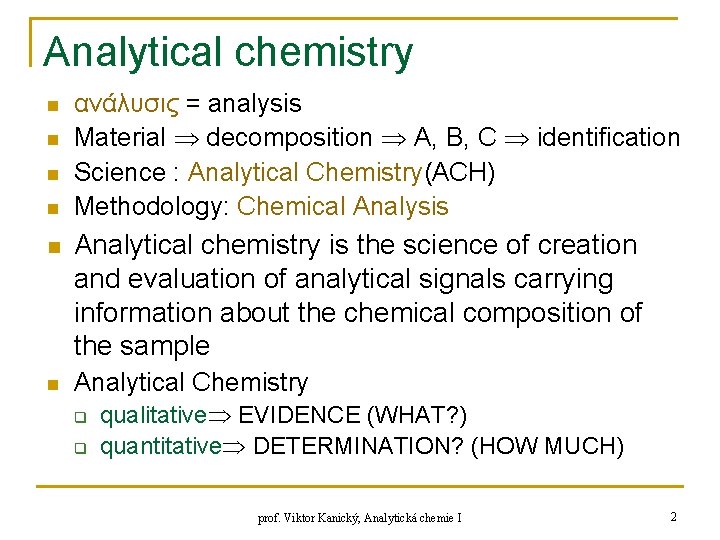
Analytical chemistry n n ανάλυσις = analysis Material decomposition A, B, C identification Science : Analytical Chemistry(ACH) Methodology: Chemical Analysis n Analytical chemistry is the science of creation and evaluation of analytical signals carrying information about the chemical composition of the sample n Analytical Chemistry q q qualitative EVIDENCE (WHAT? ) quantitative DETERMINATION? (HOW MUCH) prof. Viktor Kanický, Analytická chemie I 2

History of Analytical Chemistry n n Egypt, China, Indie, Greece (Demokritos, Platon, Aristoteles, (5. -4. st. BC), Middle Ages– alchemists analysis q q n „dry“ - reaction in solid phase (heating of solids – metallurgy) „wet“ – in solution fundamentals q q q R. Boyle 17. century J. Dalton 18. -19. century A. L. Lavoisier 18. century Fresenius (19. st. ): separation and identification of cations using hydrogen sulphide Instrumental methods: spectral analysis, Bunsen a Kirchhoff (19. cent. ) J. Heyrovský: polarography, Nobel Prize 1959 prof. Viktor Kanický, Analytická chemie I 3

Methodology of analytical chemistry n Analytical approach in Science and Humanities q Partition of problem into particular simpler parts, solution of these partial tasks and combining of individual information for understanding of the whole n n Decomposition of material into chemical species molecules, atoms, ions X Analysis by physical methods– study of matter in solid state, without decomposition, dissolution n Computer Based Analytical Chemistry (COBAC) n ACH is a scientific discipline that develops and applies methods, instruments and strategies to gather information on the composition and nature of matter in space and time prof. Viktor Kanický, Analytická chemie I 4

Chemistry Theory Explanation Analyses Synthesis Analytical chemist Instrument Characterisation prof. Viktor Kanický, Analytická chemie I 5

Classification of analytical methods according to principle n Chemical methods (chemical reactions) q q n Physico-chemical methods q q q n Gravimetry Volumetry (titrimetry) Spectroscopic (radiations, particles - electrons, ions) Separation (separation of comonents in time and space between two phases) Electrochemical (electrode processes) Biochemical methods (enzymes, microorganisms) prof. Viktor Kanický, Analytická chemie I 6

Classification of analytical methods according to object of analysis n Material (examples) q n Determined component type - analyte (examples): q q q n Elemental analysis of samples with inorganic and organic matrix Analysis of organic compounds Determination of radioactive isotopes Analyte content q q n water, geological, metallurgical, ceramics, building, environmental, pharmaceutical, foodstuff, clinical Total analysis (main constituents, =100%) trace( g/g) and ultratrace(ng/g, pg/g) analysis Sample size (g, mg, g, ng, l, nl) prof. Viktor Kanický, Analytická chemie I 7

Elemental analysis n Elemental analysis enables: q q n WHOWHO analysis q q n verification of the presence of the element (qualitative analysis) determination of elemental concentration/content (quantitative analysis) identification of structure, in which the element is present (structural analysis) identification of compound, in which the element is bound (speciation) what (qualitative) how much (quantitative) where (structure) how bound (speciation) The aim is to find structure vs properties relation prof. Viktor Kanický, Analytická chemie I 8

General procedure for analysis n Sampling q q n Sample transfer to a form suitable for analysis q q n mass, volume, flow of electromagnetic radiation or ions, electrochemical potential, current, charge, conductivity Data evaluation q n Decomposition, dissolution, pressing of powdered samples Separation of components (from matrix, separation of individual analytes), concentration of constituents Analytical signal measurement q n Representative sample Homogeneous sample Mean value, standard deviation, error, uncertainties, validation Conclusions, analytical report prof. Viktor Kanický, Analytická chemie I 9

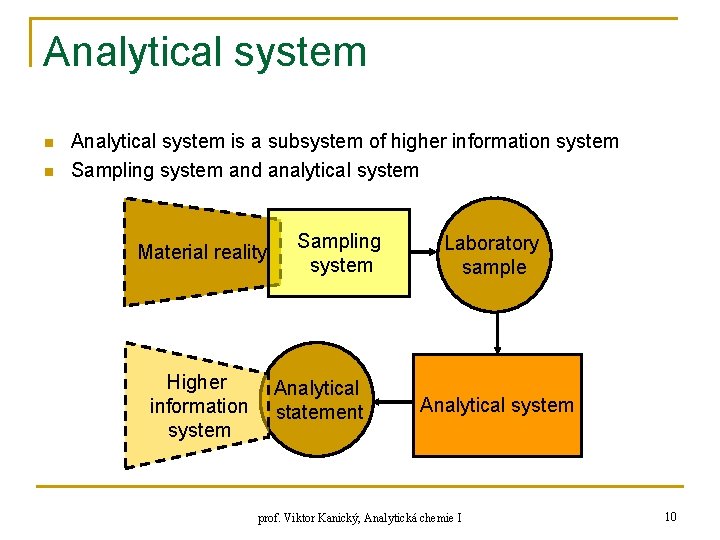
Analytical system n n Analytical system is a subsystem of higher information system Sampling system and analytical system Material reality Higher information system Sampling system Analytical statement Laboratory sample Analytical system prof. Viktor Kanický, Analytická chemie I 10

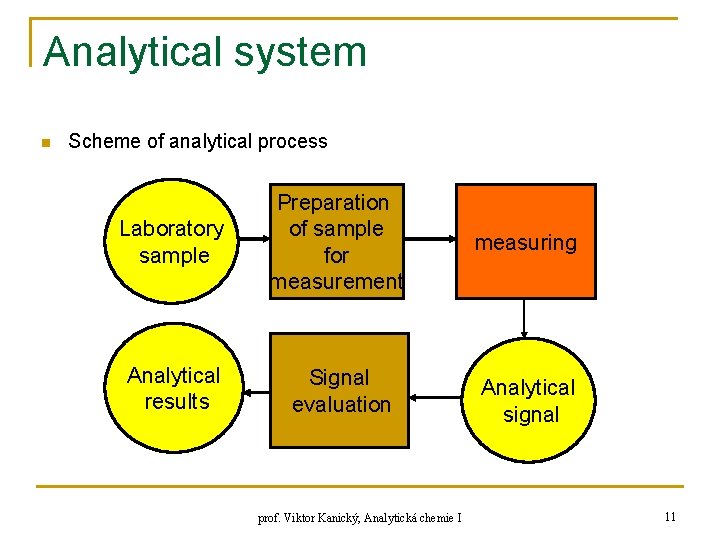
Analytical system n Scheme of analytical process Laboratory sample Preparation of sample for measurement measuring Analytical results Signal evaluation Analytical signal prof. Viktor Kanický, Analytická chemie I 11

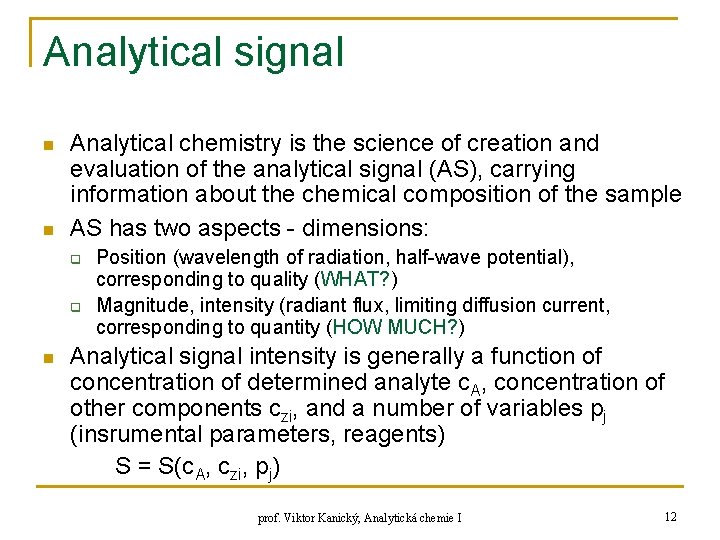
Analytical signal n n Analytical chemistry is the science of creation and evaluation of the analytical signal (AS), carrying information about the chemical composition of the sample AS has two aspects - dimensions: q q n Position (wavelength of radiation, half-wave potential), corresponding to quality (WHAT? ) Magnitude, intensity (radiant flux, limiting diffusion current, corresponding to quantity (HOW MUCH? ) Analytical signal intensity is generally a function of concentration of determined analyte c. A, concentration of other components czi, and a number of variables pj (insrumental parameters, reagents) S = S(c. A, czi, pj) prof. Viktor Kanický, Analytická chemie I 12

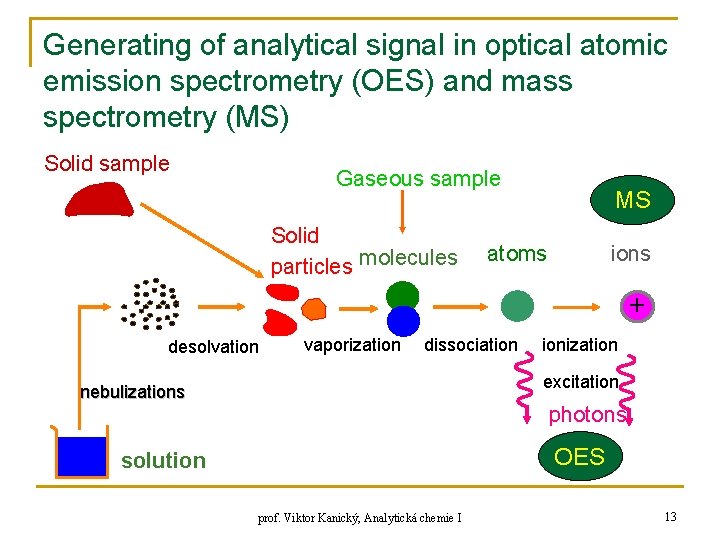
Generating of analytical signal in optical atomic emission spectrometry (OES) and mass spectrometry (MS) Solid sample Gaseous sample Solid particles molecules MS ions atoms + desolvation vaporization dissociation ionization excitation nebulizations photons OES solution prof. Viktor Kanický, Analytická chemie I 13

Atomic (optical) emission spectromety in inductively coupled plasma ICP-AES, ICP-OES n Atomic (line) emission spectrum of chlorine and oxygen in UV region prof. Viktor Kanický, Analytická chemie I 14

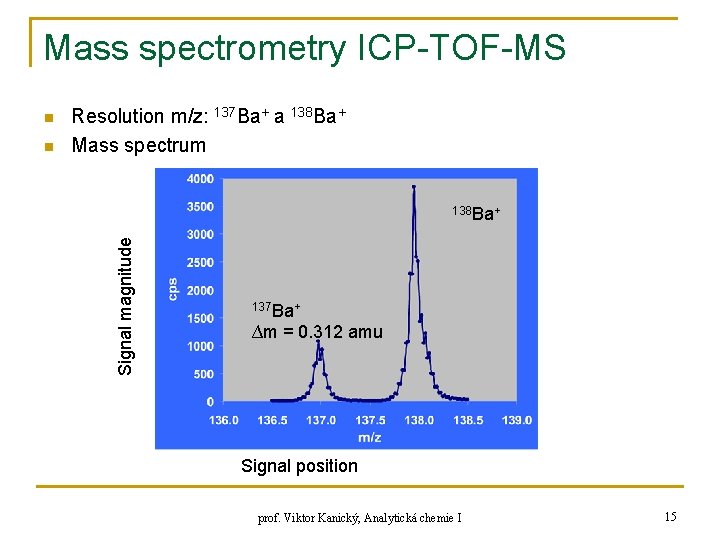
Mass spectrometry ICP-TOF-MS n Resolution m/z: 137 Ba+ a 138 Ba+ Mass spectrum 138 Ba+ Signal magnitude n 137 Ba+ ∆m = 0. 312 amu Signal position prof. Viktor Kanický, Analytická chemie I 15

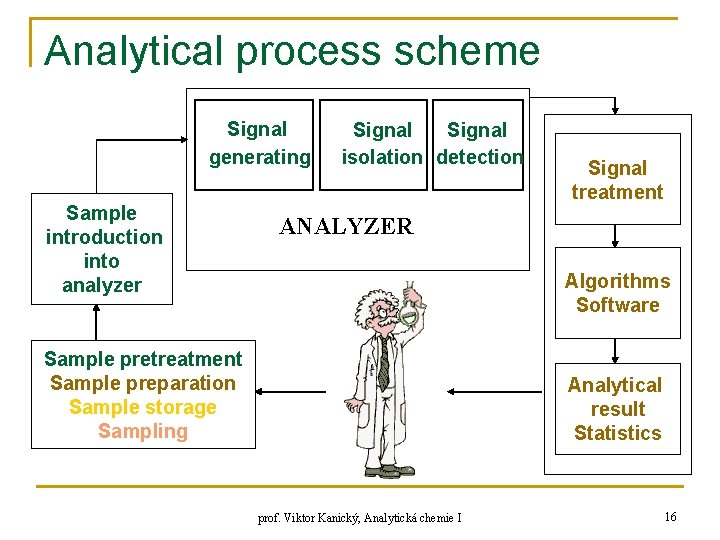
Analytical process scheme Signal generating Sample introduction into analyzer Signal isolation detection Signal treatment ANALYZER Algorithms Software Sample pretreatment Sample preparation Sample storage Sampling Analytical result Statistics prof. Viktor Kanický, Analytická chemie I 16

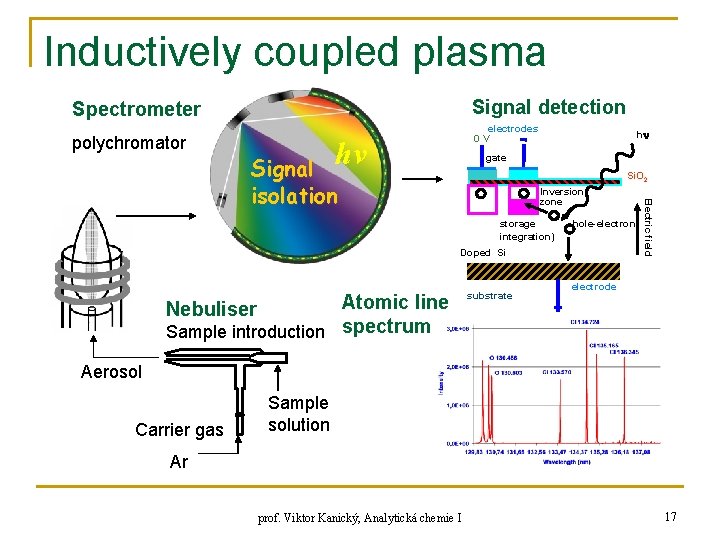
Inductively coupled plasma Signal detection Spectrometer polychromator electrodes 0 V hν h gate Signal isolation Si. O 2 storage integration) hole-electron Doped Si Atomic line Sample introduction spectrum Nebuliser substrate Electric field Inversion zone electrode Aerosol Carrier gas Sample solution Ar prof. Viktor Kanický, Analytická chemie I 17

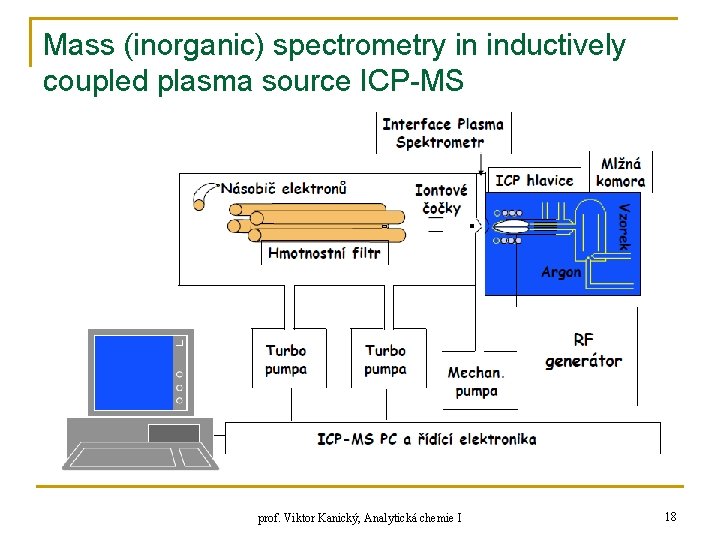
Mass (inorganic) spectrometry in inductively coupled plasma source ICP-MS prof. Viktor Kanický, Analytická chemie I 18

Analytical method n n Sampling and sample storage, preservation of representative material Processing of the part of the sample for quantitative analysis Determination Calculation of results DEFINITION (ISO 3534) q q random error = component of measurement error, which changes in the course of repeated measurements in an unpredictable way systematic error, bias = component of measurement error, which does not change in the course of repeated measurements or changes in a predictable way prof. Viktor Kanický, Analytická chemie I 19

Analytická metoda DEFINICE (ISO 3534) n precision = tightness of agreement between the results obtained with the repeated use of the same experimental procedure under defined conditions (random error) q q n n Repeatability Reproducibility trueness = tightness of agreement between the „true (actual) value“ and the mean value of measured results (systematic error, bias) accuracy = method is accurate if it is satisfied at the same time precision and trueness of the results prof. Viktor Kanický, Analytická chemie I 20

Repeatability n Repeatability represents random fluctuations of the measured values of the analytical signal (or results) around the mean value within one experiment (a series of replicates) n Cause of fluctuations is noise: in case of emission spectrometry – example: q q q n shot noise (photons) flicker noise (sample introduction noise) detector noise Repeatability is usually expressed as standard deviation (SD) or relative standard deviation (RSD) prof. Viktor Kanický, Analytická chemie I 21

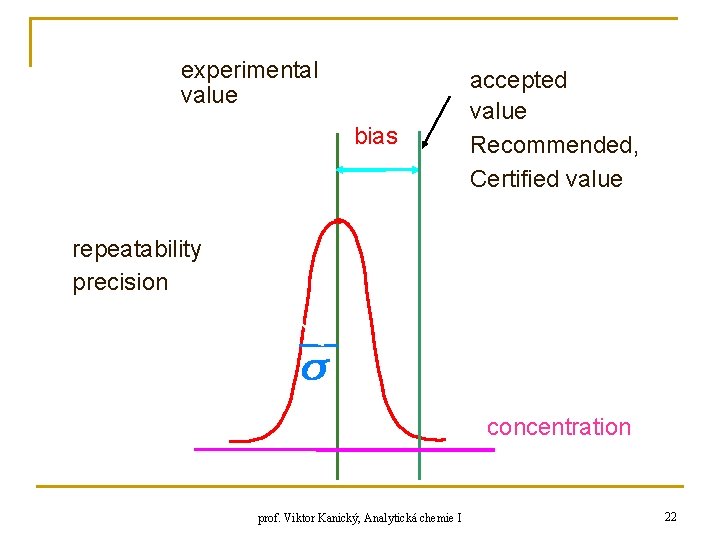
experimental value bias accepted value Recommended, Certified value repeatability precision concentration prof. Viktor Kanický, Analytická chemie I 22

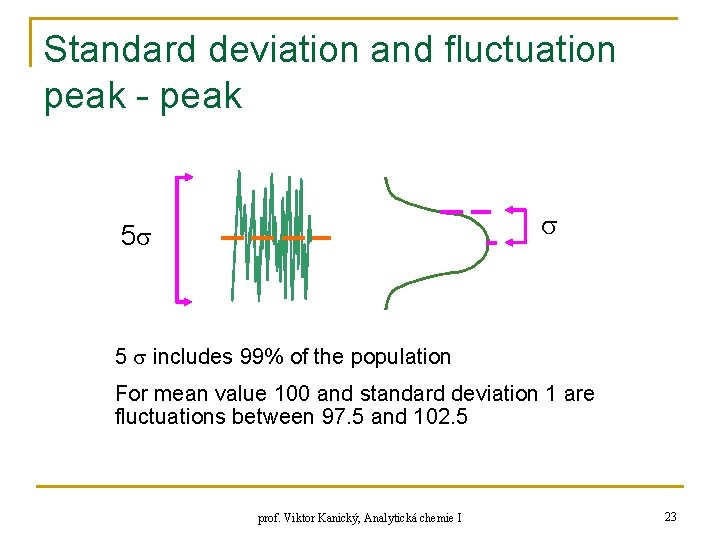
Standard deviation and fluctuation peak - peak 5 5 includes 99% of the population For mean value 100 and standard deviation 1 are fluctuations between 97. 5 and 102. 5 prof. Viktor Kanický, Analytická chemie I 23

Reproducibility n Principle as in the case of repeatability, in addition, one other parameter changes n Reproducibility may be: q q q Between laboratories Between operators in one laboratory Between analyzers In different days etc… prof. Viktor Kanický, Analytická chemie I 24

Repeatability x Trueness repeatability good poor good trueness good poor prof. Viktor Kanický, Analytická chemie I 25

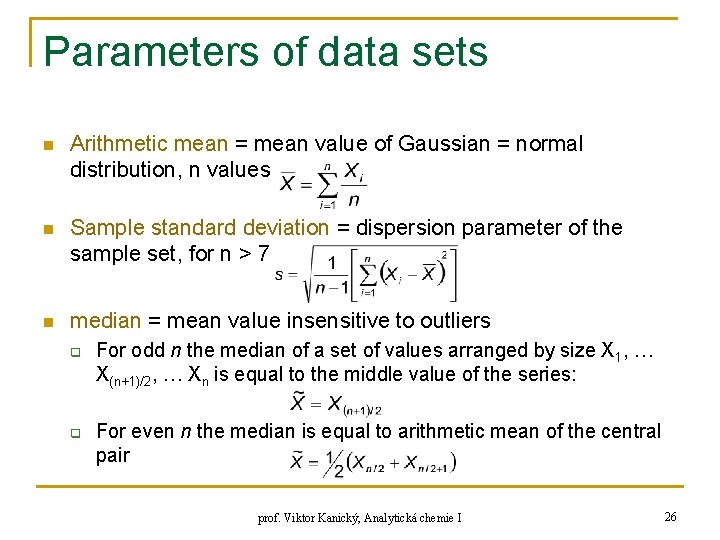
Parameters of data sets n Arithmetic mean = mean value of Gaussian = normal distribution, n values n Sample standard deviation = dispersion parameter of the sample set, for n > 7 n median = mean value insensitive to outliers q q For odd n the median of a set of values arranged by size X 1, … X(n+1)/2, … Xn is equal to the middle value of the series: For even n the median is equal to arithmetic mean of the central pair prof. Viktor Kanický, Analytická chemie I 26

Parameters of data sets - Span n Standard deviation s. R of data set for is calculated from the span of values: prof. Viktor Kanický, Analytická chemie I 27

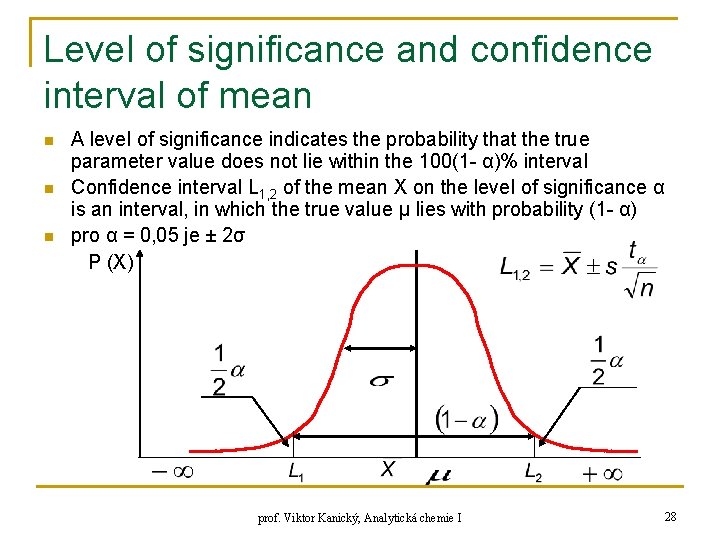
Level of significance and confidence interval of mean n A level of significance indicates the probability that the true parameter value does not lie within the 100(1 - α)% interval Confidence interval L 1, 2 of the mean X on the level of significance α is an interval, in which the true value μ lies with probability (1 - α) pro α = 0, 05 je ± 2σ P (X) prof. Viktor Kanický, Analytická chemie I 28

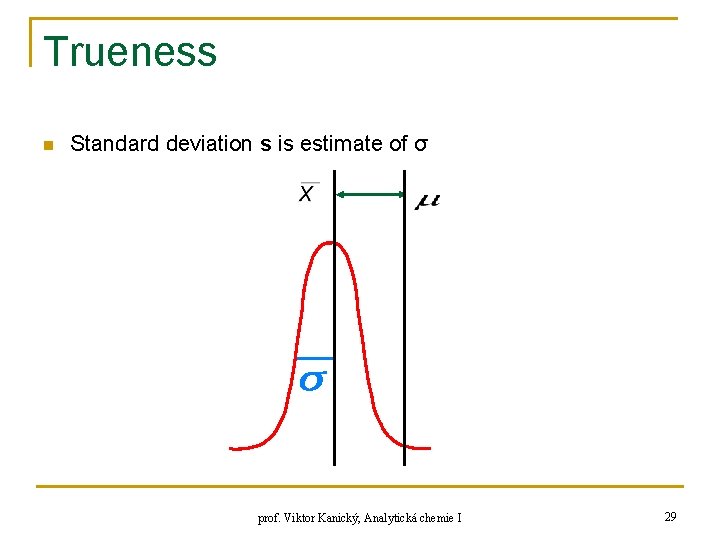
Trueness n Standard deviation s is estimate of σ prof. Viktor Kanický, Analytická chemie I 29

Statistical testing n n Comparison of results of analyses null hypothesis: the assumption that between the compared values is no other difference than that which can be explained by the presence of random errors null hypothesis H 0 is rejected if the actual difference exceeds a critical value that corresponds to the pre-selected significance level α risk that we reject the correct null hypothesis is called the error of the 1 st kind and is given by the significance level α q PI = 1 – α is the probability that we accept the correct null hypothesis prof. Viktor Kanický, Analytická chemie I 30

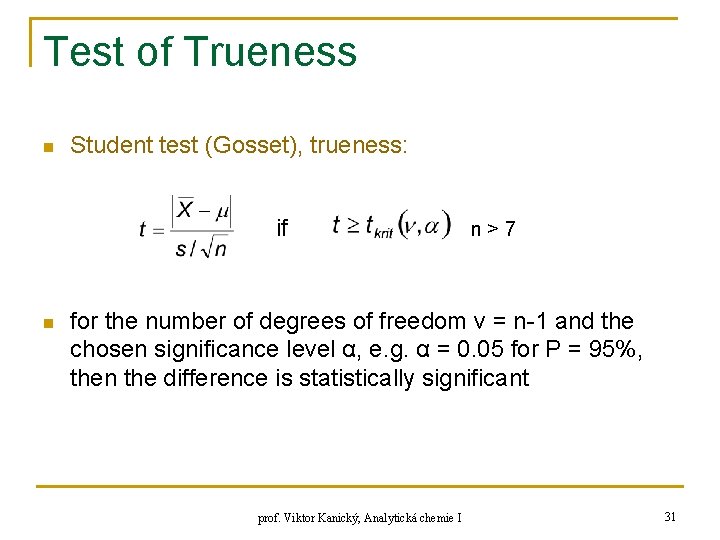
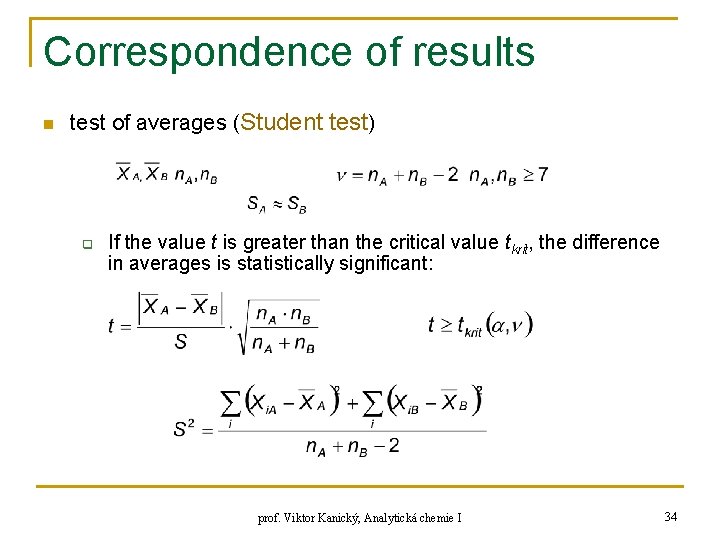
Test of Trueness n Student test (Gosset), trueness: if n n>7 for the number of degrees of freedom ν = n-1 and the chosen significance level α, e. g. α = 0. 05 for P = 95%, then the difference is statistically significant prof. Viktor Kanický, Analytická chemie I 31

Test of Trueness using span n Lord test → statistically significant difference prof. Viktor Kanický, Analytická chemie I 32

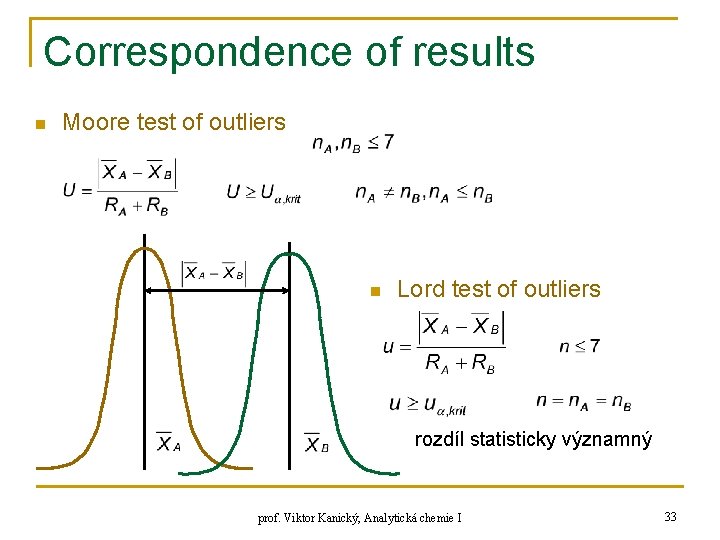
Correspondence of results n Moore test of outliers n Lord test of outliers rozdíl statisticky významný prof. Viktor Kanický, Analytická chemie I 33

Correspondence of results n test of averages (Student test) q If the value t is greater than the critical value tkrit, the difference in averages is statistically significant: prof. Viktor Kanický, Analytická chemie I 34

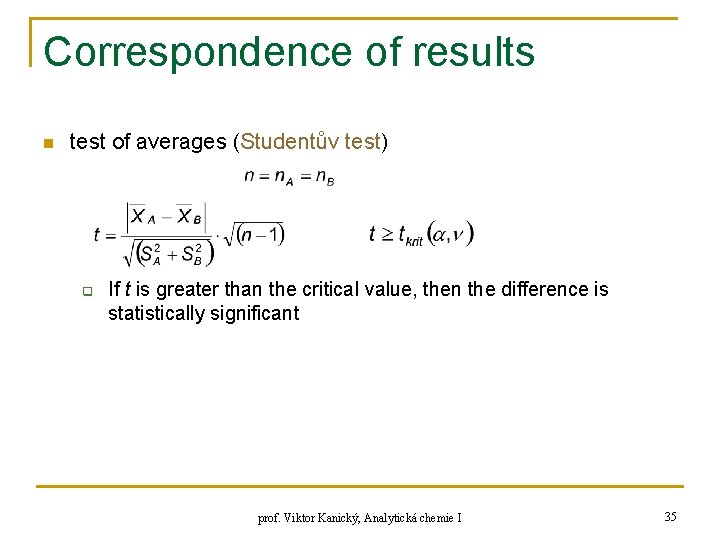
Correspondence of results n test of averages (Studentův test) q If t is greater than the critical value, then the difference is statistically significant prof. Viktor Kanický, Analytická chemie I 35

Exclusion of outliers n T-test; Grubbs test for n > 7 extreme values are outliers prof. Viktor Kanický, Analytická chemie I 36

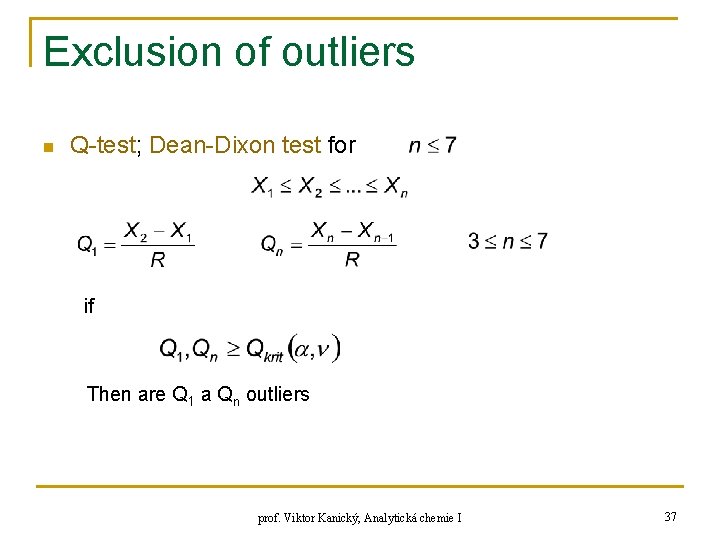
Exclusion of outliers n Q-test; Dean-Dixon test for if Then are Q 1 a Qn outliers prof. Viktor Kanický, Analytická chemie I 37

Types of analytical methods n ISO Guide 32 proposal classifies methods of chemical analysis according to calibration procedure: q q Absolute methods (calculable methods) – result can be calculated on the basis of relations resulting from chemical and physical laws, using measured values (titrimetry, gravimetry, coulometry) Relative methods – analyzed sample is compared with a set of calibration samples with known contents using the detection system, which has a linear response to the concentration of fixed components n differences between calibration and analyzed samples do not affect the signal in comparison with the magnitude of uncertainty n samples before measurement can be adjusted (adjustment of the matrix of calibration samples to the matrix of analyzed samples, elimination of interferences) prof. Viktor Kanický, Analytická chemie I 38

Types of analytical methods q Comparative methods - analyzed sample is compared with a set of calibration samples with known contents using the detection system, which responds not only to the fixed component, but also to change the composition of the matrix n n calibration of such a method requires use of certified reference materials (CRM) it is a quick method to control technological processes (wavedispersive X-ray fluorescence spectrometry in the production of steel, alloys, oxide powder, ceramic materials, etc. ) prof. Viktor Kanický, Analytická chemie I 39

Analytical chemist n n n 80% in industrial laboratories, the analytical chemist is a solver of problems and issues good theoretical knowledge of the methods used and the ability to develop experimental techniques and to select relevant, the optimal method development of specialized analytical procedures for analysis of routine and unique, unusual samples, communication with experts from other disciplines to obtain information about analyzed materials, the ability to choose a compromise between the cost analysis and its accuracy prof. Viktor Kanický, Analytická chemie I 40

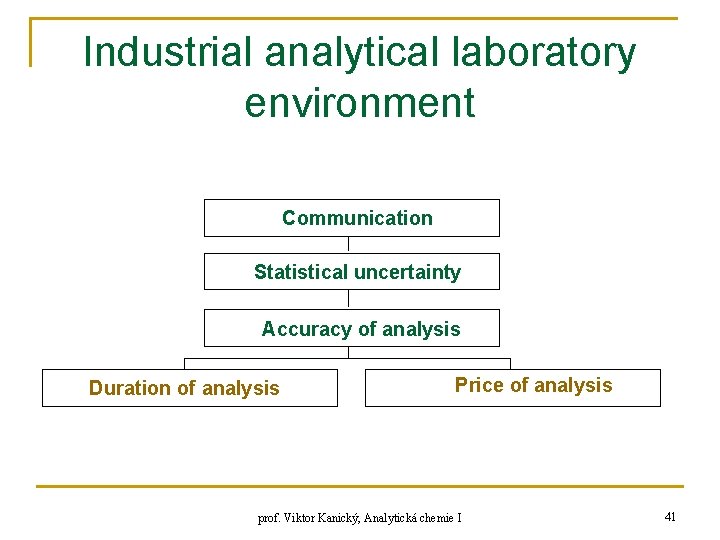
Industrial analytical laboratory environment Communication Statistical uncertainty Accuracy of analysis Duration of analysis Price of analysis prof. Viktor Kanický, Analytická chemie I 41

Method of analytical problem solving n n n knowledge of chemistry of the problem knowledge of sampling and sample processing use of suitable separation methods use of proper calibration and standards selection of the best methods for measuring the analytical signal prof. Viktor Kanický, Analytická chemie I 42

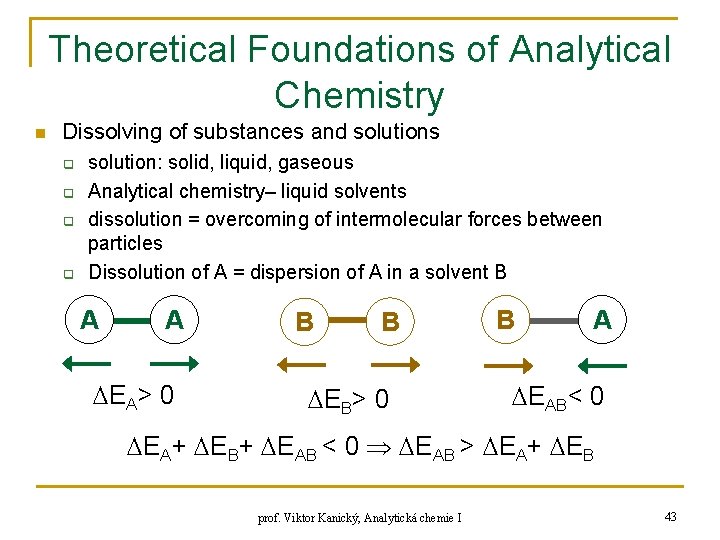
Theoretical Foundations of Analytical Chemistry n Dissolving of substances and solutions q q solution: solid, liquid, gaseous Analytical chemistry– liquid solvents dissolution = overcoming of intermolecular forces between particles Dissolution of A = dispersion of A in a solvent B A A EA> 0 B B EB> 0 B A EAB< 0 EA+ EB+ EAB < 0 EAB > EA+ EB prof. Viktor Kanický, Analytická chemie I 43

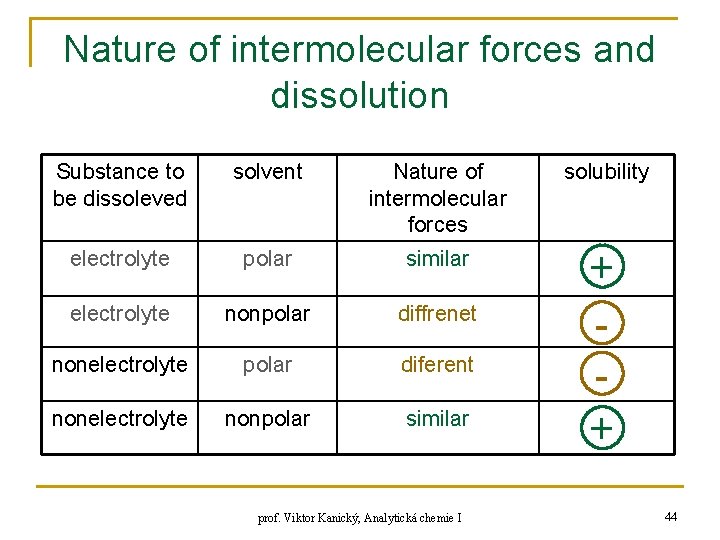
Nature of intermolecular forces and dissolution Substance to be dissoleved solvent Nature of intermolecular forces solubility electrolyte polar similar electrolyte nonpolar diffrenet nonelectrolyte polar diferent nonelectrolyte nonpolar similar + + prof. Viktor Kanický, Analytická chemie I 44

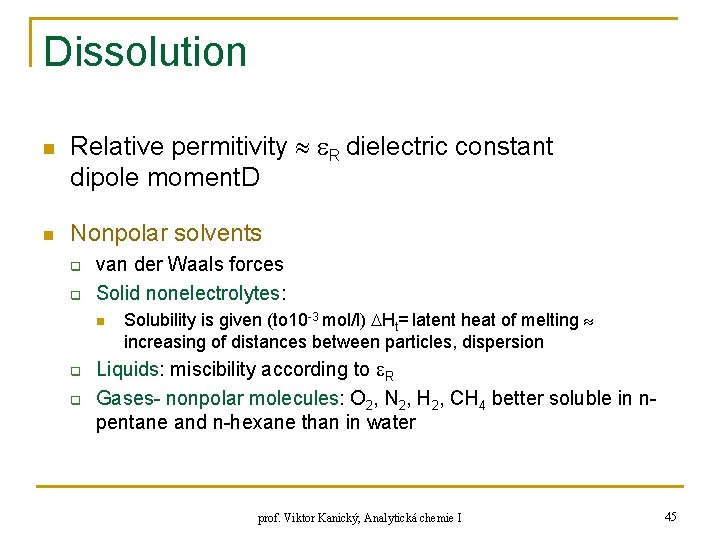
Dissolution n n Relative permitivity R dielectric constant dipole moment. D Nonpolar solvents q q van der Waals forces Solid nonelectrolytes: n q q Solubility is given (to 10 -3 mol/l) Ht= latent heat of melting increasing of distances between particles, dispersion Liquids: miscibility according to R Gases- nonpolar molecules: O 2, N 2, H 2, CH 4 better soluble in npentane and n-hexane than in water prof. Viktor Kanický, Analytická chemie I 45

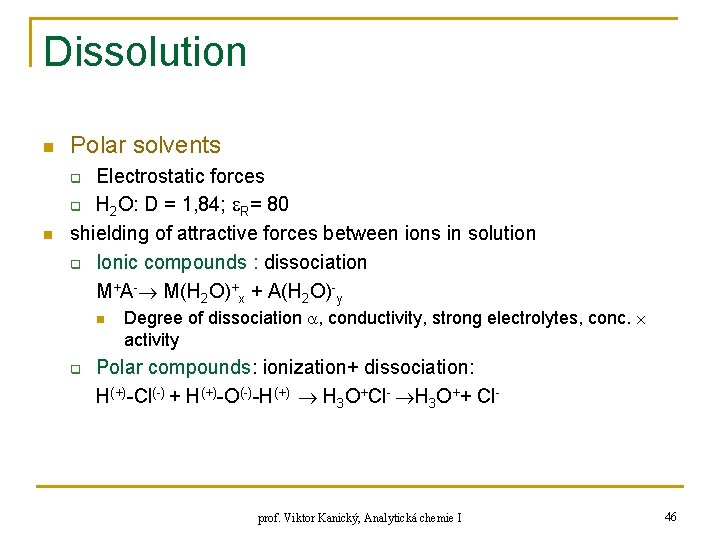
Dissolution n Polar solvents n Electrostatic forces q H 2 O: D = 1, 84; R= 80 shielding of attractive forces between ions in solution q Ionic compounds : dissociation M+A- M(H 2 O)+x + A(H 2 O)-y q n q Degree of dissociation , conductivity, strong electrolytes, conc. activity Polar compounds: ionization+ dissociation: H(+)-Cl(-) + H(+)-O(-)-H(+) H 3 O+Cl- H 3 O++ Cl- prof. Viktor Kanický, Analytická chemie I 46

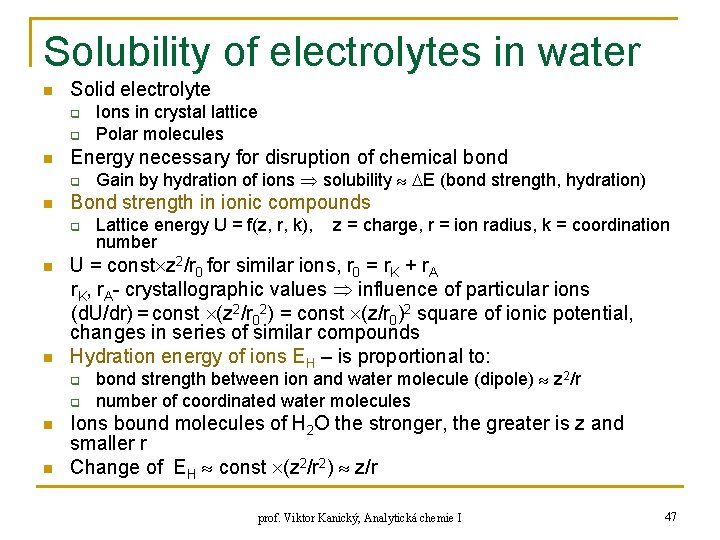
Solubility of electrolytes in water n Solid electrolyte q q n Energy necessary for disruption of chemical bond q n n q n Lattice energy U = f(z, r, k), number z = charge, r = ion radius, k = coordination U = const z 2/r 0 for similar ions, r 0 = r. K + r. A r. K, r. A- crystallographic values influence of particular ions (d. U/dr) = const (z 2/r 02) = const (z/r 0)2 square of ionic potential, changes in series of similar compounds Hydration energy of ions EH – is proportional to: q n Gain by hydration of ions solubility E (bond strength, hydration) Bond strength in ionic compounds q n Ions in crystal lattice Polar molecules bond strength between ion and water molecule (dipole) z 2/r number of coordinated water molecules Ions bound molecules of H 2 O the stronger, the greater is z and smaller r Change of EH const (z 2/r 2) z/r prof. Viktor Kanický, Analytická chemie I 47

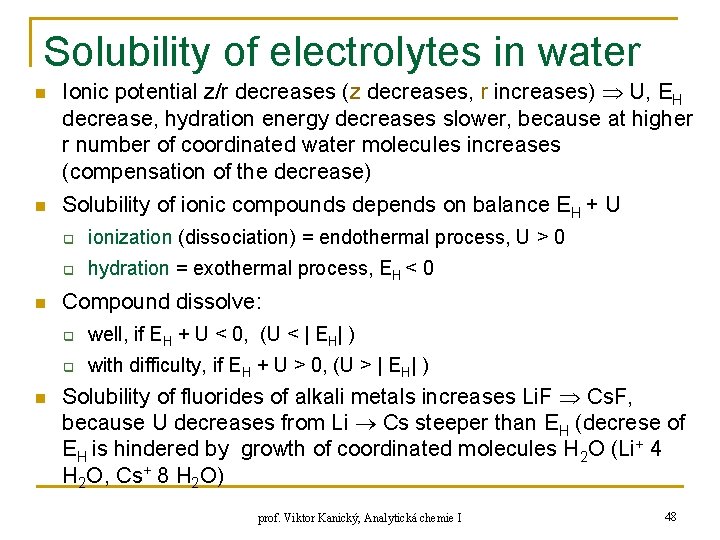
Solubility of electrolytes in water n n Ionic potential z/r decreases (z decreases, r increases) U, EH decrease, hydration energy decreases slower, because at higher r number of coordinated water molecules increases (compensation of the decrease) Solubility of ionic compounds depends on balance EH + U q ionization (dissociation) = endothermal process, U > 0 q hydration = exothermal process, EH < 0 Compound dissolve: q well, if EH + U < 0, (U < | EH| ) q with difficulty, if EH + U > 0, (U > | EH| ) Solubility of fluorides of alkali metals increases Li. F Cs. F, because U decreases from Li Cs steeper than EH (decrese of EH is hindered by growth of coordinated molecules H 2 O (Li+ 4 H 2 O, Cs+ 8 H 2 O) prof. Viktor Kanický, Analytická chemie I 48

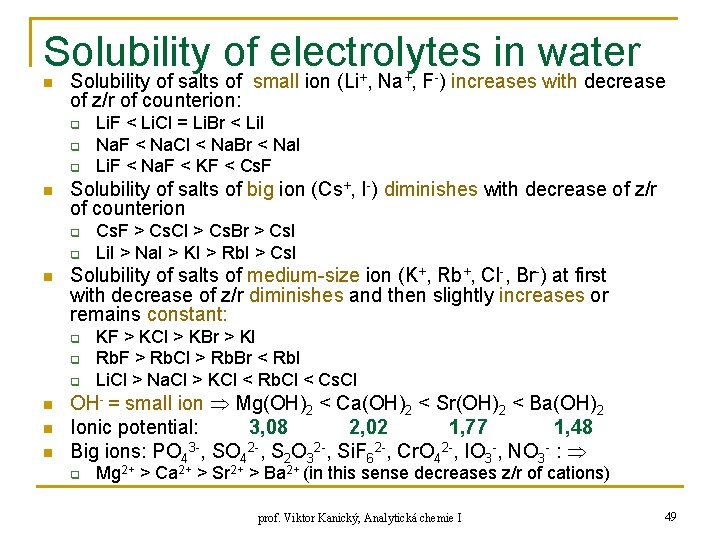
Solubility of electrolytes in water n Solubility of salts of small ion (Li+, Na+, F-) increases with decrease of z/r of counterion: q q q n Solubility of salts of big ion (Cs+, I-) diminishes with decrease of z/r of counterion q q n n Cs. F > Cs. Cl > Cs. Br > Cs. I Li. I > Na. I > KI > Rb. I > Cs. I Solubility of salts of medium-size ion (K+, Rb+, Cl-, Br-) at first with decrease of z/r diminishes and then slightly increases or remains constant: q n Li. F < Li. Cl = Li. Br < Li. I Na. F < Na. Cl < Na. Br < Na. I Li. F < Na. F < KF < Cs. F KF > KCl > KBr > KI Rb. F > Rb. Cl > Rb. Br < Rb. I Li. Cl > Na. Cl > KCl < Rb. Cl < Cs. Cl OH- = small ion Mg(OH)2 < Ca(OH)2 < Sr(OH)2 < Ba(OH)2 Ionic potential: 3, 08 2, 02 1, 77 1, 48 Big ions: PO 43 -, SO 42 -, S 2 O 32 -, Si. F 62 -, Cr. O 42 -, IO 3 -, NO 3 - : q Mg 2+ > Ca 2+ > Sr 2+ > Ba 2+ (in this sense decreases z/r of cations) prof. Viktor Kanický, Analytická chemie I 49

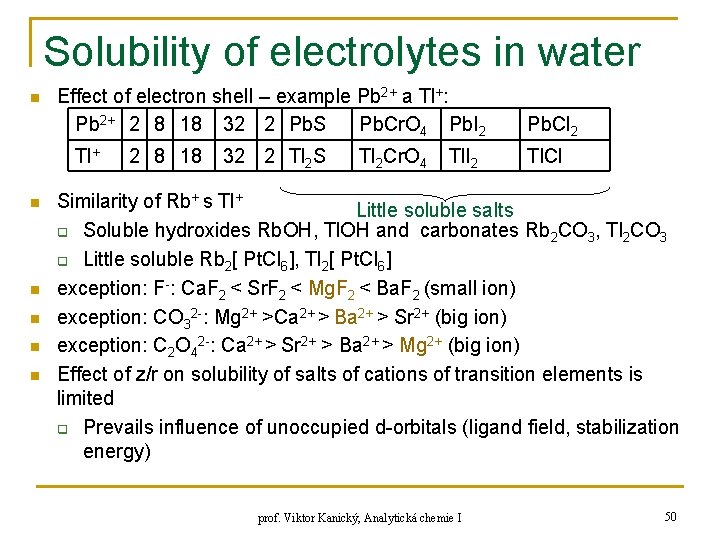
Solubility of electrolytes in water n Effect of electron shell – example Pb 2+ a Tl+: Pb 2+ 2 8 18 32 2 Pb. S Pb. Cr. O 4 Pb. I 2 Tl+ n n n 2 8 18 32 2 Tl 2 S Tl 2 Cr. O 4 Tl. I 2 Pb. Cl 2 Tl. Cl Similarity of Rb+ s Tl+ Little soluble salts q Soluble hydroxides Rb. OH, Tl. OH and carbonates Rb 2 CO 3, Tl 2 CO 3 q Little soluble Rb 2[ Pt. Cl 6], Tl 2[ Pt. Cl 6] exception: F-: Ca. F 2 < Sr. F 2 < Mg. F 2 < Ba. F 2 (small ion) exception: CO 32 -: Mg 2+ >Ca 2+ > Ba 2+ > Sr 2+ (big ion) exception: C 2 O 42 -: Ca 2+ > Sr 2+ > Ba 2+ > Mg 2+ (big ion) Effect of z/r on solubility of salts of cations of transition elements is limited q Prevails influence of unoccupied d-orbitals (ligand field, stabilization energy) prof. Viktor Kanický, Analytická chemie I 50

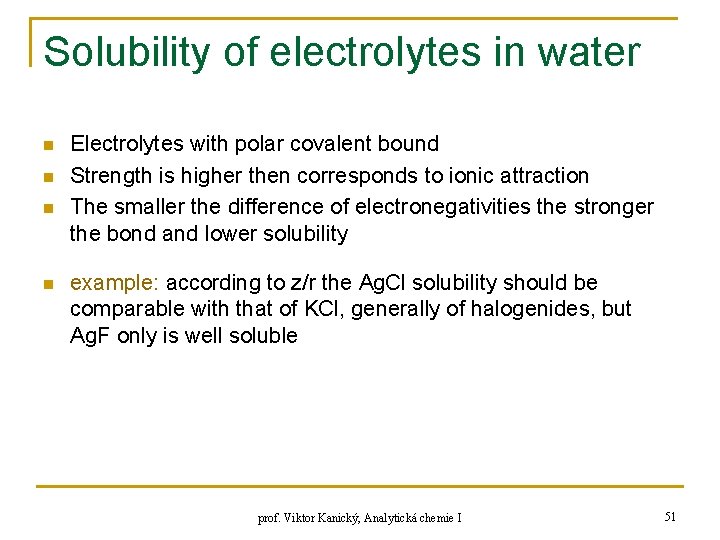
Solubility of electrolytes in water n n Electrolytes with polar covalent bound Strength is higher then corresponds to ionic attraction The smaller the difference of electronegativities the stronger the bond and lower solubility example: according to z/r the Ag. Cl solubility should be comparable with that of KCl, generally of halogenides, but Ag. F only is well soluble prof. Viktor Kanický, Analytická chemie I 51

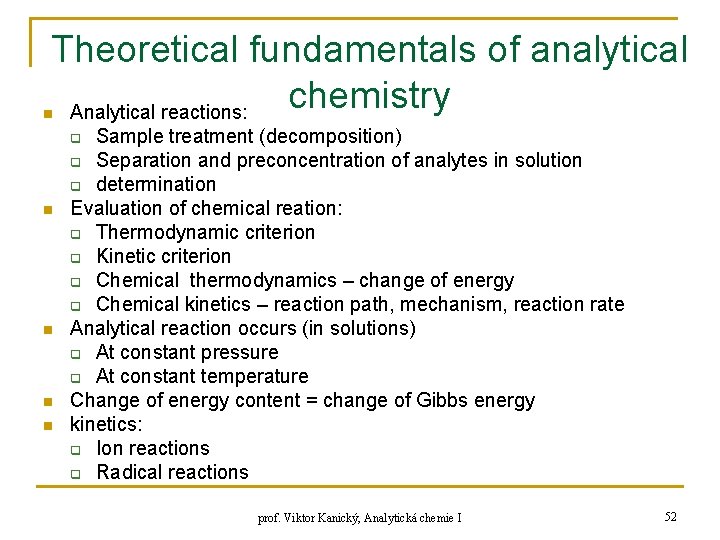
Theoretical fundamentals of analytical chemistry Analytical reactions: n Sample treatment (decomposition) q Separation and preconcentration of analytes in solution q determination Evaluation of chemical reation: q Thermodynamic criterion q Kinetic criterion q Chemical thermodynamics – change of energy q Chemical kinetics – reaction path, mechanism, reaction rate Analytical reaction occurs (in solutions) q At constant pressure q At constant temperature Change of energy content = change of Gibbs energy kinetics: q Ion reactions q Radical reactions q n n prof. Viktor Kanický, Analytická chemie I 52

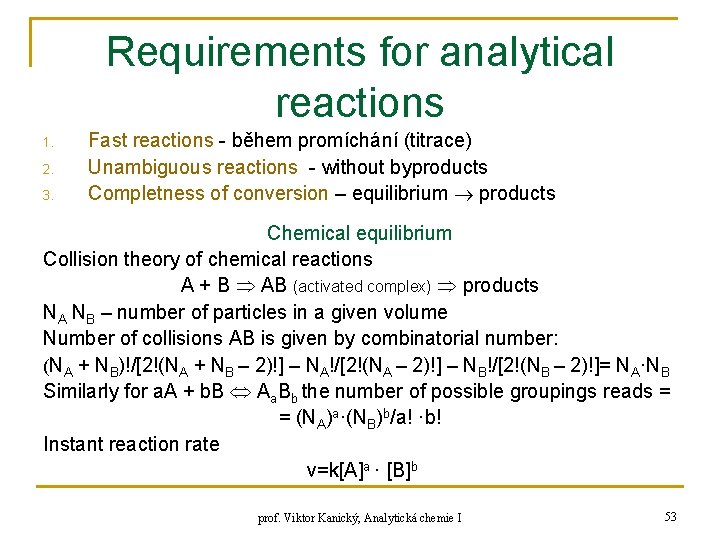
Requirements for analytical reactions 1. 2. 3. Fast reactions - během promíchání (titrace) Unambiguous reactions - without byproducts Completness of conversion – equilibrium products Chemical equilibrium Collision theory of chemical reactions A + B AB (activated complex) products NA NB – number of particles in a given volume Number of collisions AB is given by combinatorial number: (NA + NB)!/[2!(NA + NB – 2)!] – NA!/[2!(NA – 2)!] – NB!/[2!(NB – 2)!]= NA·NB Similarly for a. A + b. B Aa. Bb the number of possible groupings reads = = (NA)a·(NB)b/a! ·b! Instant reaction rate v=k[A]a · [B]b prof. Viktor Kanický, Analytická chemie I 53

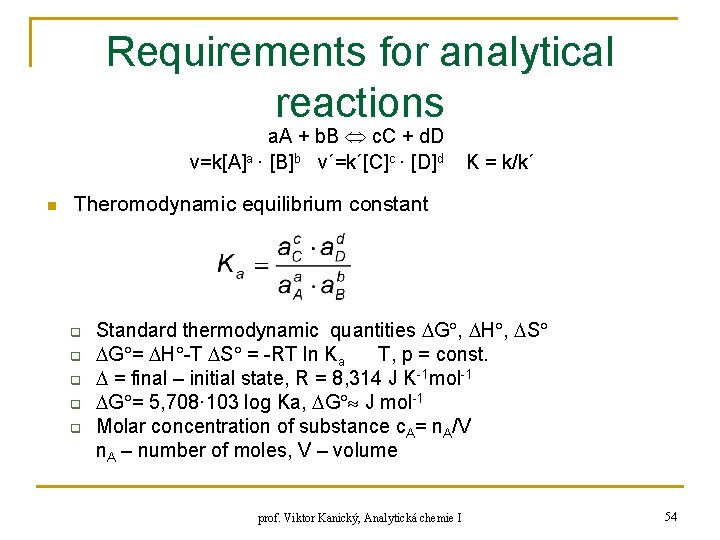
Requirements for analytical reactions a. A + b. B c. C + d. D v=k[A]a · [B]b v´=k´[C]c · [D]d n K = k/k´ Theromodynamic equilibrium constant q q q Standard thermodynamic quantities G , H , S G = H -T S = -RT ln Ka T, p = const. = final – initial state, R = 8, 314 J K-1 mol-1 G = 5, 708· 103 log Ka, G J mol-1 Molar concentration of substance c. A= n. A/V n. A – number of moles, V – volume prof. Viktor Kanický, Analytická chemie I 54
![Requirements for analytical reactions n n Activity a A A y A A Requirements for analytical reactions n n Activity a. A = [A] y. A [A]](https://slidetodoc.com/presentation_image/f004f447878f1363bcc44dfbf43e8f92/image-55.jpg)
Requirements for analytical reactions n n Activity a. A = [A] y. A [A] – equilibrium concentration y. A – activity coefficient, expresses diffrences in behaviour: q solvation, electrostatic effects between ions n Concentration thermodynamic constant n Activity coefficients, theory Debye-Hückel: q q q Molal activity coefficients Molar activity coefficients y Molar fraction, activity coefficients f prof. Viktor Kanický, Analytická chemie I 55

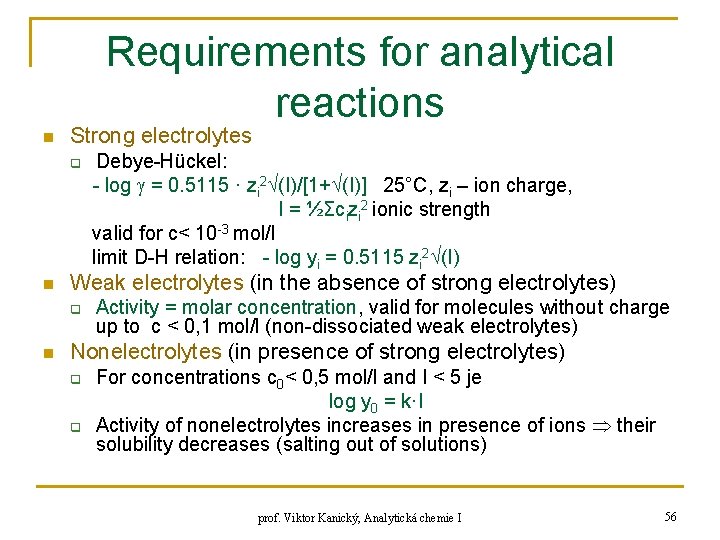
Requirements for analytical reactions n Strong electrolytes q n Weak electrolytes (in the absence of strong electrolytes) q n Debye-Hückel: - log = 0. 5115 · zi 2 (I)/[1+ (I)] 25°C, zi – ion charge, I = ½Σcizi 2 ionic strength valid for c< 10 -3 mol/l limit D-H relation: - log yi = 0. 5115 zi 2 (I) Activity = molar concentration, valid for molecules without charge up to c < 0, 1 mol/l (non-dissociated weak electrolytes) Nonelectrolytes (in presence of strong electrolytes) q q For concentrations c 0< 0, 5 mol/l and I < 5 je log y 0 = k·I Activity of nonelectrolytes increases in presence of ions their solubility decreases (salting out of solutions) prof. Viktor Kanický, Analytická chemie I 56

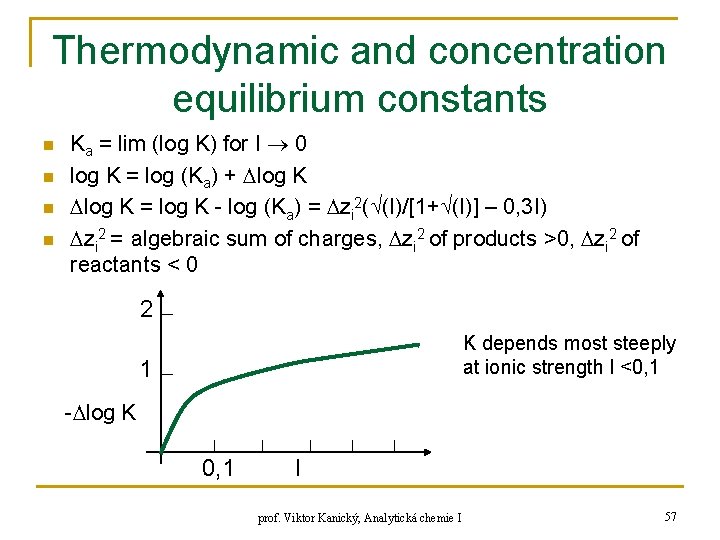
Thermodynamic and concentration equilibrium constants n n Ka = lim (log K) for I 0 log K = log (Ka) + log K = log K - log (Ka) = zi 2( (I)/[1+ (I)] – 0, 3 I) zi 2 = algebraic sum of charges, zi 2 of products >0, zi 2 of reactants < 0 2 K depends most steeply at ionic strength I <0, 1 1 - log K 0, 1 I prof. Viktor Kanický, Analytická chemie I 57

Completness of reaction from equilibrium constant a. A + b. B c. C + d. D c. A, c. B initial concentrations of reactants, conversion to 99, 90 % at equilibrium [A] = [B] = 0, 001 c. A , [C] = [D] = 0, 999 c. A je-li K = 106 99, 9% conversion to products K= x 2/(1 -x)2 n posun rovnováhy nadbytkem činidla (fotometrie, gravimetrie, extrakce) rušení, vedlejší reakce prof. Viktor Kanický, Analytická chemie I 58

Effect of reaction kinetics n Halftime of reaction < 10 s, titration, redox processes at n 1 n 2 are slow n Exploitation in kinetic methods – determination of concentrations from time dependences n Increase in the reaction rate: heating, transfer to reaction complex using catalyst prof. Viktor Kanický, Analytická chemie I 59

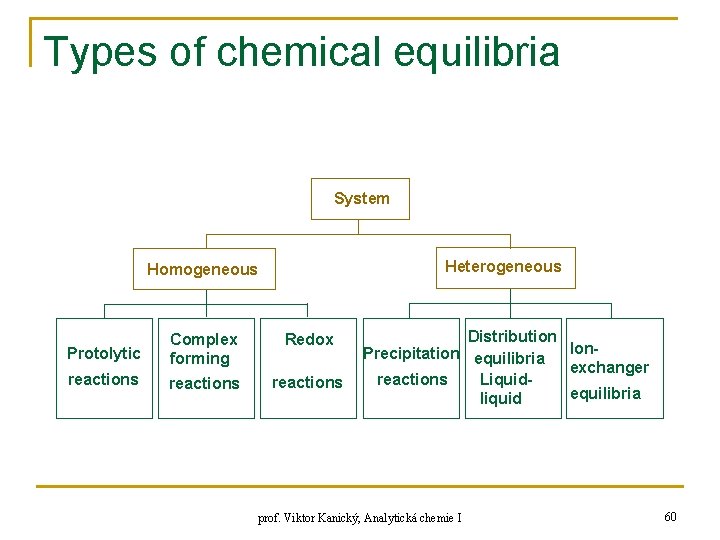
Types of chemical equilibria System Heterogeneous Homogeneous Protolytic Complex forming Redox reactions Distribution Ion. Precipitation equilibria exchanger reactions Liquidequilibria liquid prof. Viktor Kanický, Analytická chemie I 60

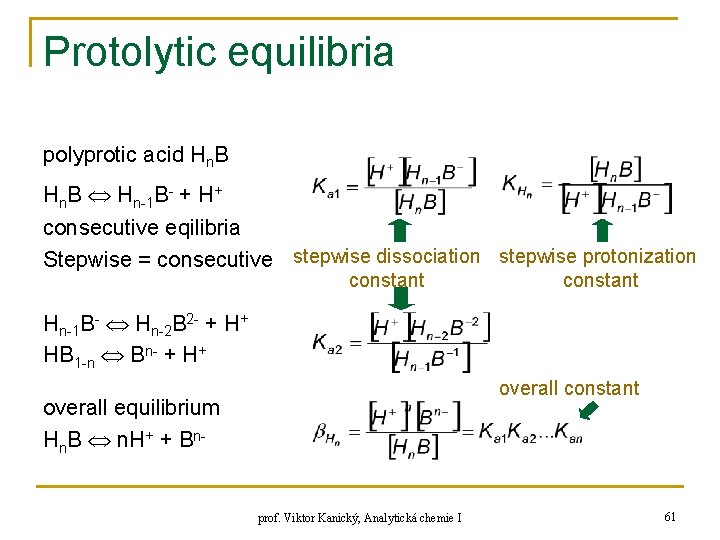
Protolytic equilibria polyprotic acid Hn. B Hn-1 B- + H+ consecutive eqilibria Stepwise = consecutive stepwise dissociation stepwise protonization constant Hn-1 B- Hn-2 B 2 - + H+ HB 1 -n Bn- + H+ overall constant overall equilibrium Hn. B n. H+ + Bn- prof. Viktor Kanický, Analytická chemie I 61

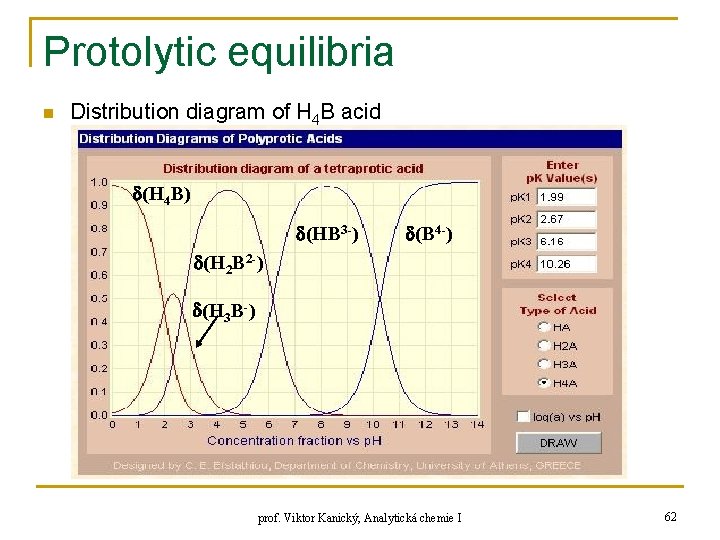
Protolytic equilibria n Distribution diagram of H 4 B acid (H 4 B) (HB 3 -) (B 4 -) (H 2 B 2 -) (H 3 B-) prof. Viktor Kanický, Analytická chemie I 62

Protolytic equilibria n n 2 conjugated acid-base pairs Acid-base equilibrium of amphiprotic solvent= autoprotolysis q n Protolytic equilibrium of acid q q n NH 4 OH NH 4+ + OH- Acidic dissociation constant of base q n HB + SH 2+ + B[SH] >> [HB], [B-], [SH 2+] Dissociation constant of base q n 2 SH 2++ S- KSH= [SH 2+][S-] NH 4+ NH 3 + H+ Ka. Kb=Kw= [H+][OH-] - ion product of water (self-ionization) prof. Viktor Kanický, Analytická chemie I 63

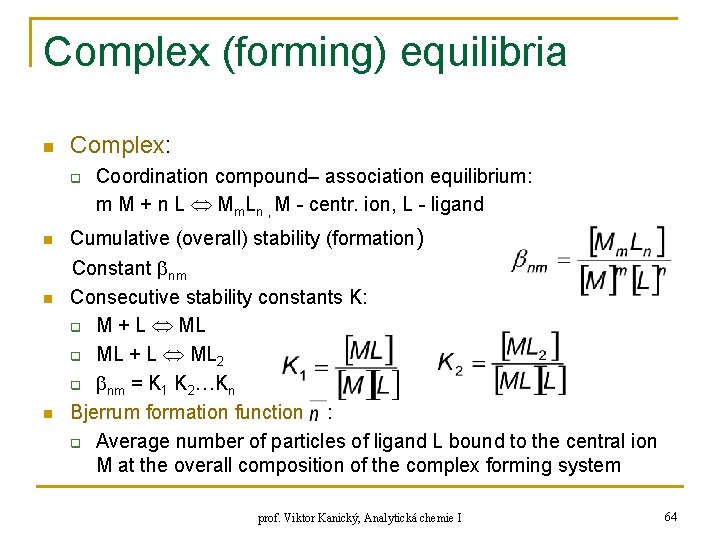
Complex (forming) equilibria n Complex: q n n n Coordination compound– association equilibrium: m M + n L Mm. Ln , M - centr. ion, L - ligand Cumulative (overall) stability (formation) Constant nm Consecutive stability constants K: q M + L ML q ML + L ML 2 q nm = K 1 K 2…Kn Bjerrum formation function : q Average number of particles of ligand L bound to the central ion M at the overall composition of the complex forming system prof. Viktor Kanický, Analytická chemie I 64

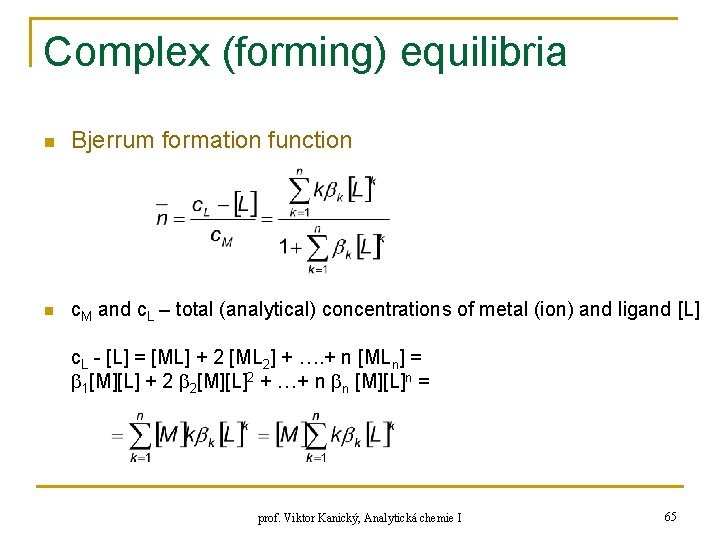
Complex (forming) equilibria n Bjerrum formation function n c. M and c. L – total (analytical) concentrations of metal (ion) and ligand [L] c. L - [L] = [ML] + 2 [ML 2] + …. + n [MLn] = 1[M][L] + 2 2[M][L]2 + …+ n n [M][L]n = prof. Viktor Kanický, Analytická chemie I 65
![Complex forming equilibria c M M ML MLn M Complex (forming) equilibria c. M = [M] + [ML] +…+ [MLn] = [M] +](https://slidetodoc.com/presentation_image/f004f447878f1363bcc44dfbf43e8f92/image-66.jpg)
Complex (forming) equilibria c. M = [M] + [ML] +…+ [MLn] = [M] + [M] 1 [L] + …+ [M] n [L]n = = [M] {1 + 1 [L] +…+ n [L]n } = = [M] {1 + }, [M] in the nominator and denominator reduces 2, 0 relation for Formation f. = f { log [L] } 1 2 3 1, 0 1) K 1= K 2=104 2) K 1= 105 K 2 = 103 3) K 1= 106 K 2 = 102 -6 prof. Viktor Kanický, Analytická chemie I -4 -2 log [L] 66

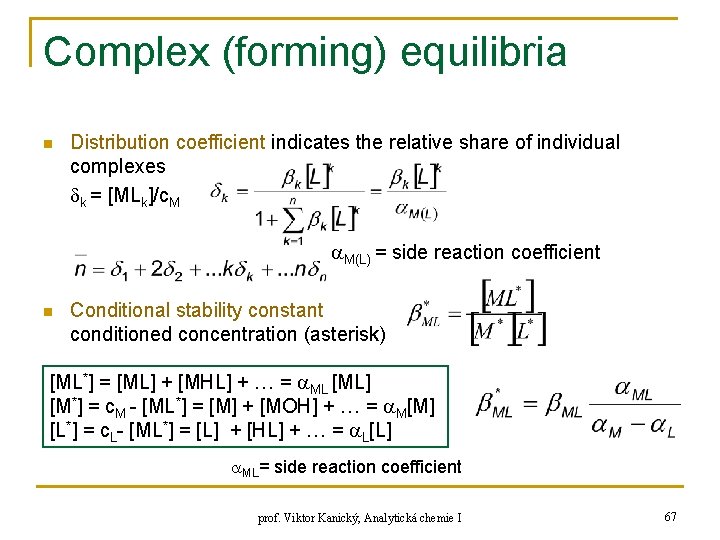
Complex (forming) equilibria n Distribution coefficient indicates the relative share of individual complexes k = [MLk]/c. M M(L) = side reaction coefficient n Conditional stability constant conditioned concentration (asterisk) [ML*] = [ML] + [MHL] + … = ML [ML] [M*] = c. M - [ML*] = [M] + [MOH] + … = M[M] [L*] = c. L- [ML*] = [L] + [HL] + … = L[L] ML= side reaction coefficient prof. Viktor Kanický, Analytická chemie I 67

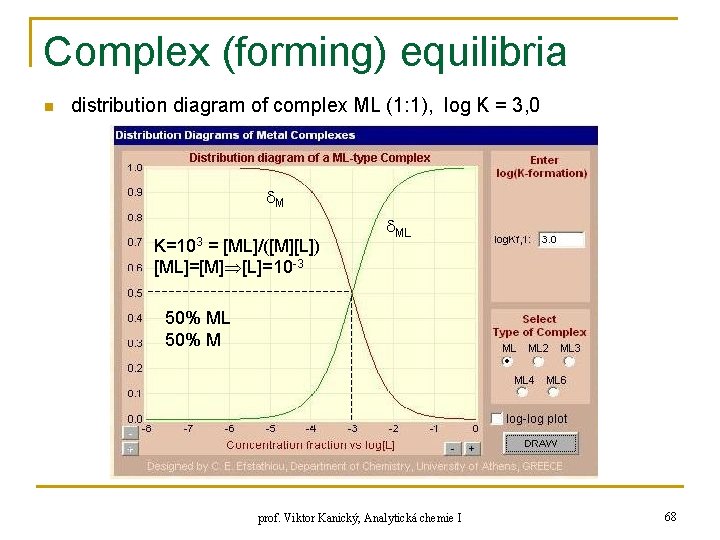
Complex (forming) equilibria n distribution diagram of complex ML (1: 1), log K = 3, 0 M K=103 = [ML]/([M][L]) [ML]=[M] [L]=10 -3 ML 50% M prof. Viktor Kanický, Analytická chemie I 68

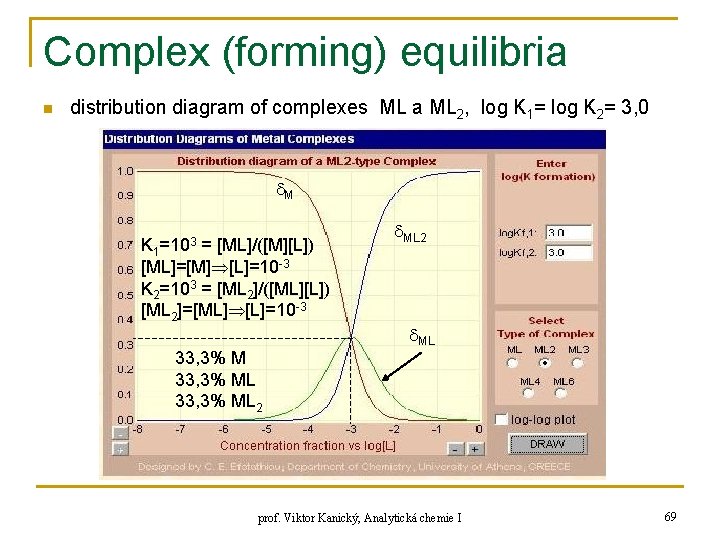
Complex (forming) equilibria n distribution diagram of complexes ML a ML 2, log K 1= log K 2= 3, 0 M K 1=103 = [ML]/([M][L]) [ML]=[M] [L]=10 -3 K 2=103 = [ML 2]/([ML][L]) [ML 2]=[ML] [L]=10 -3 33, 3% ML 2 ML prof. Viktor Kanický, Analytická chemie I 69

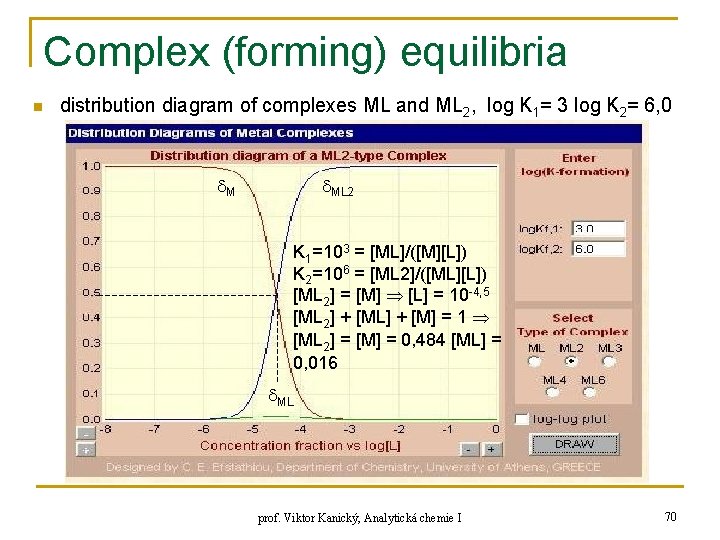
Complex (forming) equilibria n distribution diagram of complexes ML and ML 2, log K 1= 3 log K 2= 6, 0 M ML 2 K 1=103 = [ML]/([M][L]) K 2=106 = [ML 2]/([ML][L]) [ML 2] = [M] [L] = 10 -4, 5 [ML 2] + [ML] + [M] = 1 [ML 2] = [M] = 0, 484 [ML] = 0, 016 ML prof. Viktor Kanický, Analytická chemie I 70

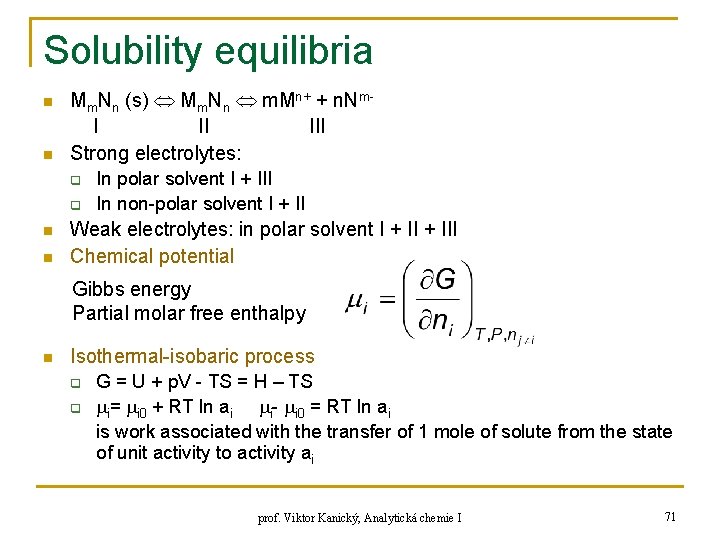
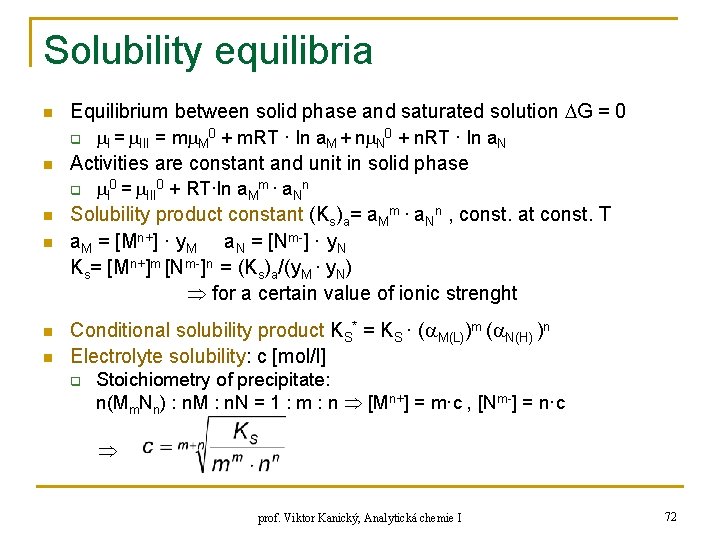
Solubility equilibria n n Mm. Nn (s) Mm. Nn m. Mn+ + n. Nm. I II III Strong electrolytes: q q n n In polar solvent I + III In non-polar solvent I + II Weak electrolytes: in polar solvent I + III Chemical potential Gibbs energy Partial molar free enthalpy n Isothermal-isobaric process q q G = U + p. V - TS = H – TS i= i 0 + RT ln ai i- i 0 = RT ln ai is work associated with the transfer of 1 mole of solute from the state of unit activity to activity ai prof. Viktor Kanický, Analytická chemie I 71

Solubility equilibria n Equilibrium between solid phase and saturated solution G = 0 q n Activities are constant and unit in solid phase q n n I = III = m M 0 + m. RT · ln a. M + n N 0 + n. RT · ln a. N I 0 = III 0 + RT·ln a. Mm · a. Nn Solubility product constant (Ks)a= a. Mm · a. Nn , const. at const. T a. M = [Mn+] · y. M a. N = [Nm-] · y. N Ks= [Mn+]m [Nm-]n = (Ks)a/(y. M · y. N) for a certain value of ionic strenght Conditional solubility product KS* = KS · ( M(L))m ( N(H) )n Electrolyte solubility: c [mol/l] q Stoichiometry of precipitate: n(Mm. Nn) : n. M : n. N = 1 : m : n [Mn+] = m·c , [Nm-] = n·c prof. Viktor Kanický, Analytická chemie I 72

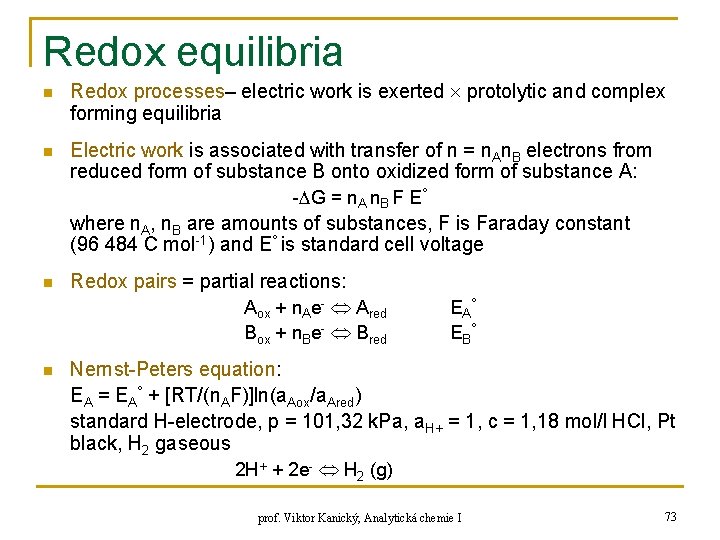
Redox equilibria n n Redox processes– electric work is exerted protolytic and complex forming equilibria Electric work is associated with transfer of n = n. An. B electrons from reduced form of substance B onto oxidized form of substance A: - G = n. A n. B F E° where n. A, n. B are amounts of substances, F is Faraday constant (96 484 C mol-1) and E° is standard cell voltage n Redox pairs = partial reactions: Aox + n. Ae- Ared Box + n. Be- Bred n EA° EB° Nernst-Peters equation: EA = EA° + [RT/(n. AF)]ln(a. Aox/a. Ared) standard H-electrode, p = 101, 32 k. Pa, a. H+ = 1, c = 1, 18 mol/l HCl, Pt black, H 2 gaseous 2 H+ + 2 e- H 2 (g) prof. Viktor Kanický, Analytická chemie I 73

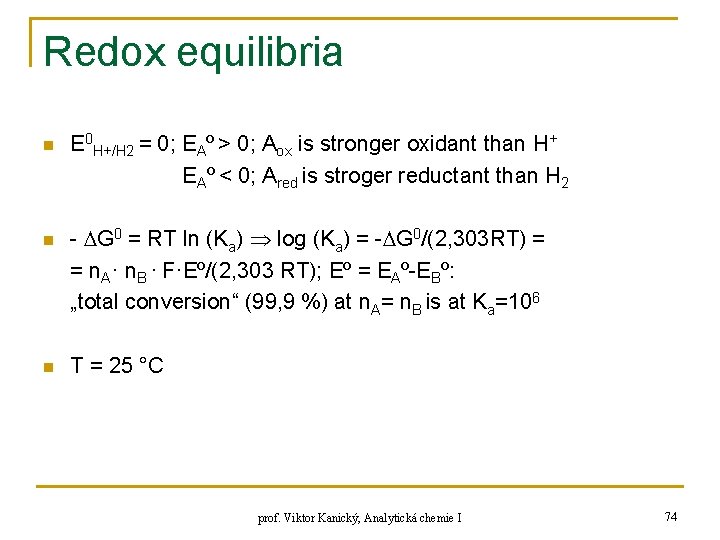
Redox equilibria n n n E 0 H+/H 2 = 0; EAº > 0; Aox is stronger oxidant than H+ EAº < 0; Ared is stroger reductant than H 2 - G 0 = RT ln (Ka) log (Ka) = - G 0/(2, 303 RT) = = n. A· n. B · F·Eº/(2, 303 RT); Eº = EAº-EBº: „total conversion“ (99, 9 %) at n. A= n. B is at Ka=106 T = 25 °C prof. Viktor Kanický, Analytická chemie I 74

Sampling and sample preparation for analysis n composition of the analyzed sample must correspond to the composition of the examined substances q n Sampling of solids ČSN 650611, liquids ČSN 650512 Sampling involves two operations q gross sample collection from analyzed substance n q gross sample – sample portion taken from analyzed substance; from solid material – it is processed by mechanical mixing, quartering, splitting, grinding, sieving and gradual sample reduction analytical sample collection from gross sample n analytický vzorek – must have identical composition with analyzed material prof. Viktor Kanický, Analytická chemie I 75

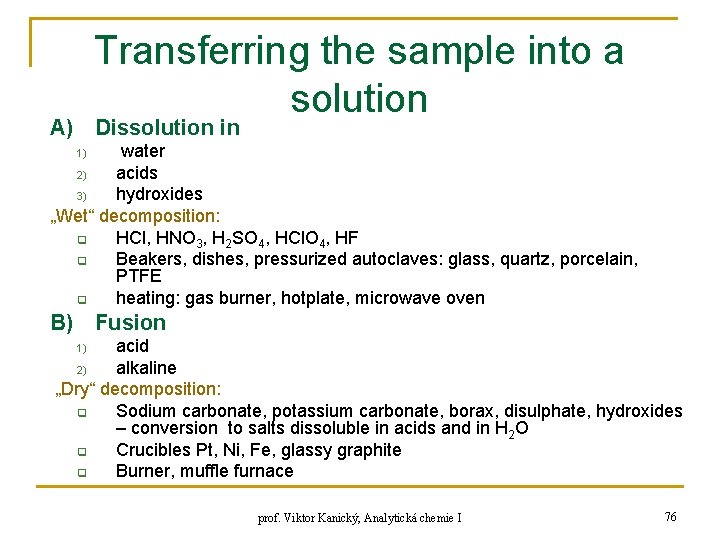
Transferring the sample into a solution A) Dissolution in water 2) acids 3) hydroxides „Wet“ decomposition: q HCl, HNO 3, H 2 SO 4, HCl. O 4, HF q Beakers, dishes, pressurized autoclaves: glass, quartz, porcelain, PTFE q heating: gas burner, hotplate, microwave oven 1) B) Fusion acid 2) alkaline „Dry“ decomposition: q Sodium carbonate, potassium carbonate, borax, disulphate, hydroxides – conversion to salts dissoluble in acids and in H 2 O q Crucibles Pt, Ni, Fe, glassy graphite q Burner, muffle furnace 1) prof. Viktor Kanický, Analytická chemie I 76

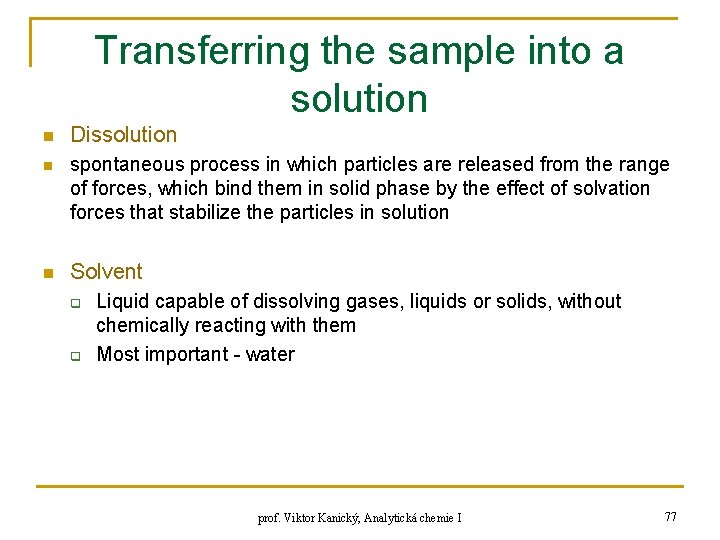
Transferring the sample into a solution n Dissolution n spontaneous process in which particles are released from the range of forces, which bind them in solid phase by the effect of solvation forces that stabilize the particles in solution n Solvent q q Liquid capable of dissolving gases, liquids or solids, without chemically reacting with them Most important - water prof. Viktor Kanický, Analytická chemie I 77

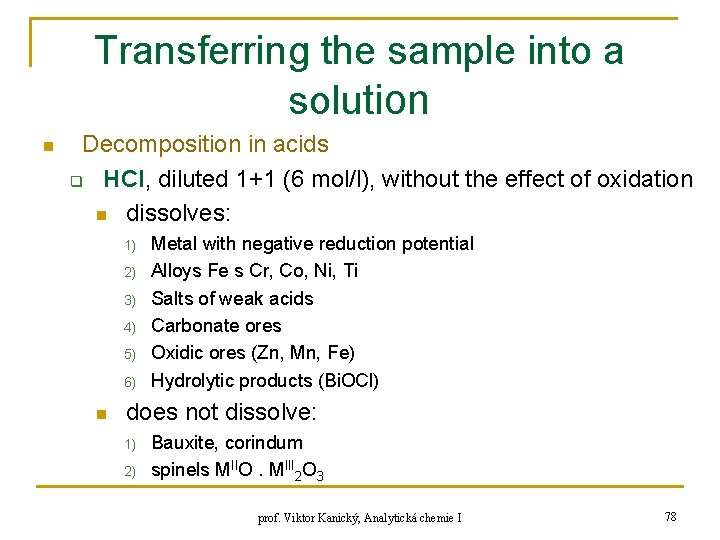
Transferring the sample into a solution n Decomposition in acids q HCl, diluted 1+1 (6 mol/l), without the effect of oxidation n dissolves: 1) 2) 3) 4) 5) 6) n Metal with negative reduction potential Alloys Fe s Cr, Co, Ni, Ti Salts of weak acids Carbonate ores Oxidic ores (Zn, Mn, Fe) Hydrolytic products (Bi. OCl) does not dissolve: 1) 2) Bauxite, corindum spinels MIIO. MIII 2 O 3 prof. Viktor Kanický, Analytická chemie I 78

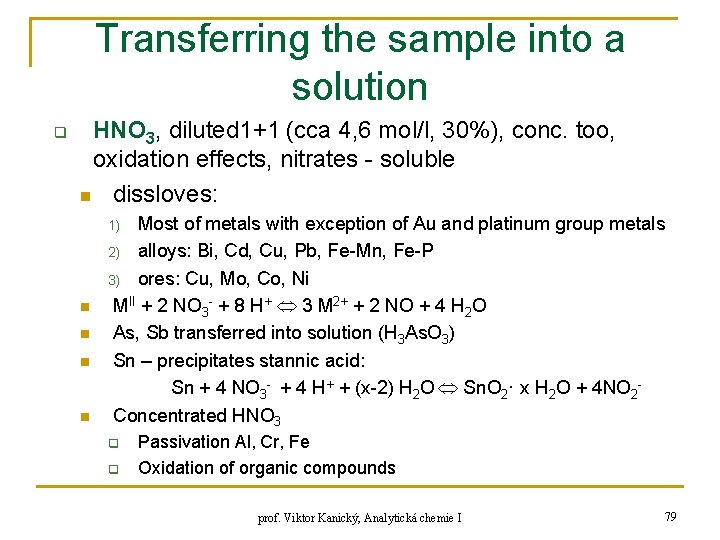
Transferring the sample into a solution q HNO 3, diluted 1+1 (cca 4, 6 mol/l, 30%), conc. too, oxidation effects, nitrates - soluble n dissloves: Most of metals with exception of Au and platinum group metals 2) alloys: Bi, Cd, Cu, Pb, Fe-Mn, Fe-P 3) ores: Cu, Mo, Co, Ni MII + 2 NO 3 - + 8 H+ 3 M 2+ + 2 NO + 4 H 2 O As, Sb transferred into solution (H 3 As. O 3) Sn – precipitates stannic acid: Sn + 4 NO 3 - + 4 H+ + (x-2) H 2 O Sn. O 2· x H 2 O + 4 NO 2 Concentrated HNO 3 1) n n q q Passivation Al, Cr, Fe Oxidation of organic compounds prof. Viktor Kanický, Analytická chemie I 79

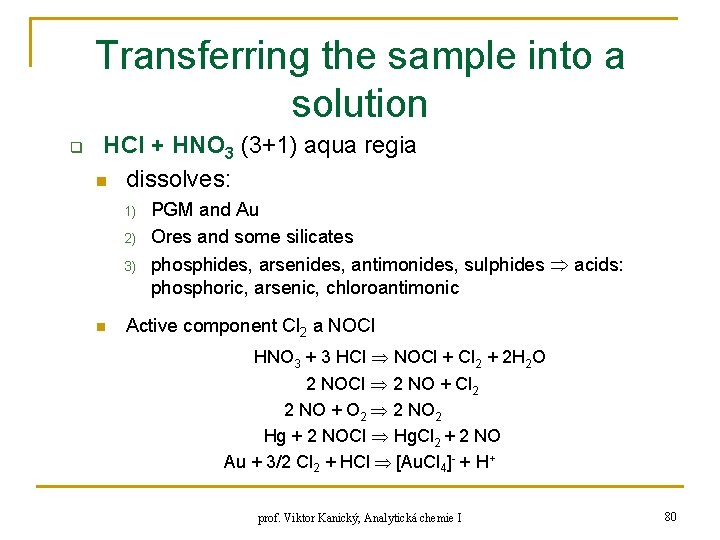
Transferring the sample into a solution q HCl + HNO 3 (3+1) aqua regia n dissolves: 1) 2) 3) n PGM and Au Ores and some silicates phosphides, arsenides, antimonides, sulphides acids: phosphoric, arsenic, chloroantimonic Active component Cl 2 a NOCl HNO 3 + 3 HCl NOCl + Cl 2 + 2 H 2 O 2 NOCl 2 NO + Cl 2 2 NO + O 2 2 NO 2 Hg + 2 NOCl Hg. Cl 2 + 2 NO Au + 3/2 Cl 2 + HCl [Au. Cl 4]- + H+ prof. Viktor Kanický, Analytická chemie I 80

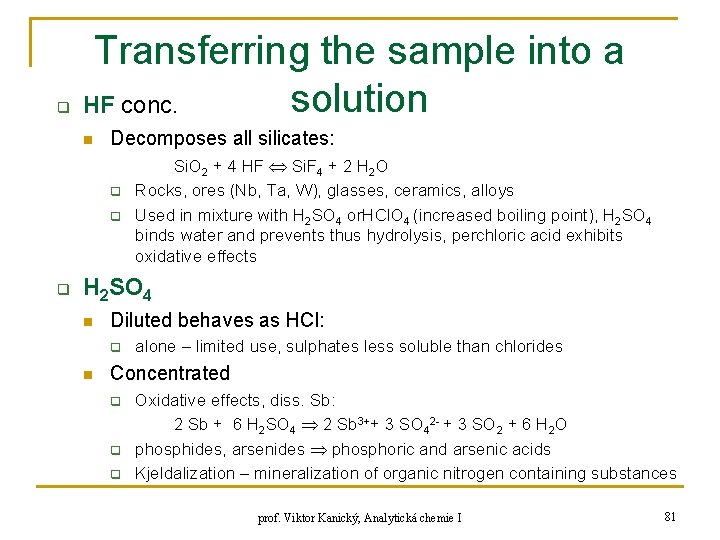
q Transferring the sample into a solution HF conc. n Decomposes all silicates: q q q Si. O 2 + 4 HF Si. F 4 + 2 H 2 O Rocks, ores (Nb, Ta, W), glasses, ceramics, alloys Used in mixture with H 2 SO 4 or. HCl. O 4 (increased boiling point), H 2 SO 4 binds water and prevents thus hydrolysis, perchloric acid exhibits oxidative effects H 2 SO 4 n Diluted behaves as HCl: q n alone – limited use, sulphates less soluble than chlorides Concentrated q q q Oxidative effects, diss. Sb: 2 Sb + 6 H 2 SO 4 2 Sb 3++ 3 SO 42 - + 3 SO 2 + 6 H 2 O phosphides, arsenides phosphoric and arsenic acids Kjeldalization – mineralization of organic nitrogen containing substances prof. Viktor Kanický, Analytická chemie I 81

Transferring the sample into a solution q HCl. O 4 conc. (72%) exhibits oxidative effects at elevated temperature n dissolves: 1) 2) 3) n n q steels(Cr, Si, V, P) carbides In mixture with HF for decomposition of silicates advantage: soluble salts disadvantage: explosive with organic compunds H 3 PO 4 1) 2) alloys ferrovanadium, ferrosilicium, ferrochromium, ferroboron prof. Viktor Kanický, Analytická chemie I 82

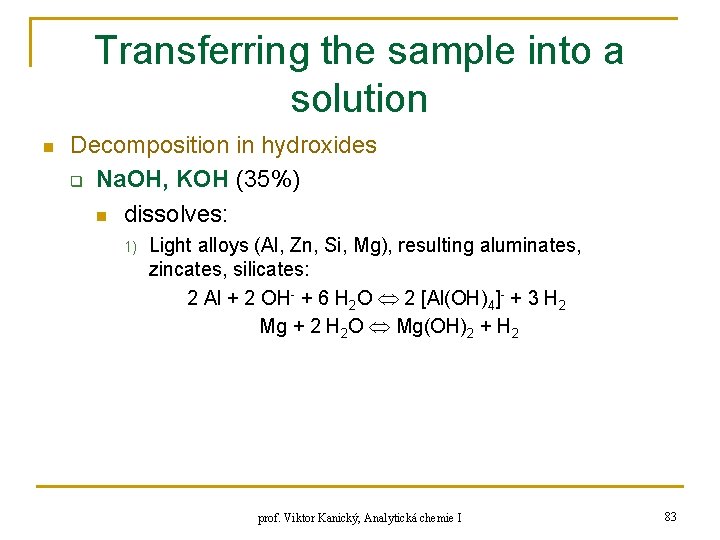
Transferring the sample into a solution n Decomposition in hydroxides q Na. OH, KOH (35%) n dissolves: 1) Light alloys (Al, Zn, Si, Mg), resulting aluminates, zincates, silicates: 2 Al + 2 OH- + 6 H 2 O 2 [Al(OH)4]- + 3 H 2 Mg + 2 H 2 O Mg(OH)2 + H 2 prof. Viktor Kanický, Analytická chemie I 83

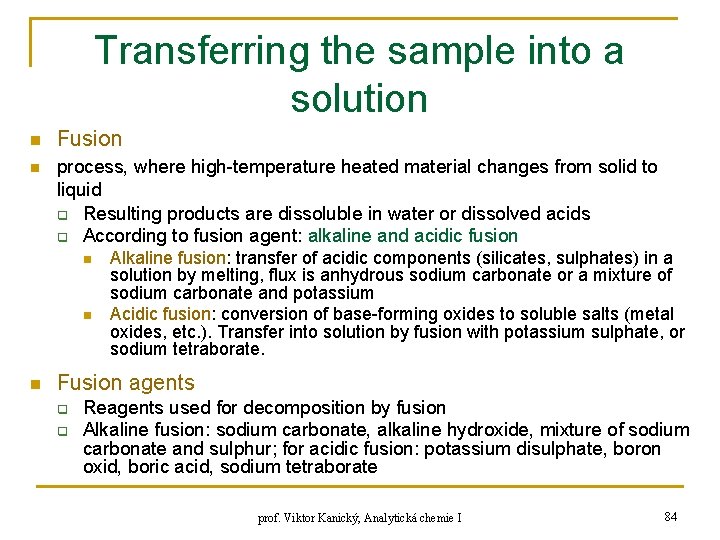
Transferring the sample into a solution n n Fusion process, where high-temperature heated material changes from solid to liquid q Resulting products are dissoluble in water or dissolved acids q According to fusion agent: alkaline and acidic fusion n Alkaline fusion: transfer of acidic components (silicates, sulphates) in a solution by melting, flux is anhydrous sodium carbonate or a mixture of sodium carbonate and potassium Acidic fusion: conversion of base-forming oxides to soluble salts (metal oxides, etc. ). Transfer into solution by fusion with potassium sulphate, or sodium tetraborate. Fusion agents q q Reagents used for decomposition by fusion Alkaline fusion: sodium carbonate, alkaline hydroxide, mixture of sodium carbonate and sulphur; for acidic fusion: potassium disulphate, boron oxid, boric acid, sodium tetraborate prof. Viktor Kanický, Analytická chemie I 84

Převádění vzorku do roztoku n Alkaline fusion n q decomposed: quartz, glass, porcelain, enamels, cement, aluminosilicates Na 2 CO 3 1) 2) q Aluminosilicates are converted into soluble alkaline aluminates and silicates Other oxides are converted to carbonates or depolymerize and in HCl are converted into soluble chlorides Na. OH, KOH fusion in crucibles Ag, Ni or Fe n q decomposed: ores W, Sn, Cr, Ti, Sb, Zr, corindum, bauxit, partially silicates Li 2 B 4 O 7, Li. BO 2 1) Formation of borate glasses soluble in diluted acids –Si retained in solution prof. Viktor Kanický, Analytická chemie I 85

Převádění vzorku do roztoku n Sintration q q Reaction i solid phase at increased temperature but below the melting point of sintration reagent (Na 2 O 2) Pt crucibles, sintered material soluble in water prof. Viktor Kanický, Analytická chemie I 86

Převádění vzorku do roztoku n Acidic fusion q KHSO 4, K 2 S 2 O 7 n n n 2 KHSO 4 K 2 S 2 O 7 + H 2 O Ti. O 2 + 2 K 2 S 2 O 7 Ti(SO 4)2 + 2 K 2 SO 4 decomposed: aluminates, spinels, ores Cu, Sb, Ni, Ti Leaching of sulphates Zr a Ti at cold by addition of H 2 SO 4 Active constituent is SO 3 S 2 O 72 - SO 42 - + SO 3 Al 2 O 3 + 3 SO 3 2 Al 3+ + 3 SO 42 Ti. O 2 + 2 SO 3 Ti 4+ + 2 SO 42 - prof. Viktor Kanický, Analytická chemie I 87
 Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Calibration verification material
Calibration verification material Coprecipitation errors
Coprecipitation errors Round off rule of 5
Round off rule of 5 Kesalahan kuantitatif adalah
Kesalahan kuantitatif adalah Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Normal error curve in analytical chemistry
Normal error curve in analytical chemistry Q test in analytical chemistry
Q test in analytical chemistry Jelaskan kegunaan statistika dalam analisis kimia
Jelaskan kegunaan statistika dalam analisis kimia Special purpose reagent chemicals
Special purpose reagent chemicals What is equivalent weight in chemistry
What is equivalent weight in chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Analytical operations
Analytical operations Excellence in analytical chemistry
Excellence in analytical chemistry Analytical chemistry
Analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry definition
Analytical chemistry definition Viktor fedun
Viktor fedun Viktor pocajt
Viktor pocajt Viktor hartmann baba yaga
Viktor hartmann baba yaga Opel viktor
Opel viktor Viktor mizo wikipedia
Viktor mizo wikipedia Viktor bergen
Viktor bergen Viktor binzberger
Viktor binzberger Dr victor christiansen
Dr victor christiansen Viktor dyk literární směr
Viktor dyk literární směr Cofactor matrix matlab
Cofactor matrix matlab Zsiday viktor
Zsiday viktor Emil slovak md
Emil slovak md Rajzfejlődés szakaszai
Rajzfejlődés szakaszai Careea
Careea Viktor lowenfeld
Viktor lowenfeld Vitalij pecharsky
Vitalij pecharsky Advanced persistent threat assessment
Advanced persistent threat assessment Victor pestov
Victor pestov Viktor haase
Viktor haase Elektro konstruktion
Elektro konstruktion Qp1k84irzpo -site:youtube.com
Qp1k84irzpo -site:youtube.com Viktor frankl gestalt
Viktor frankl gestalt Viktor binzberger
Viktor binzberger Viktor kubal vtipy
Viktor kubal vtipy Viktor mikhaylovich
Viktor mikhaylovich Viktor maier
Viktor maier Dr viktor christiansen
Dr viktor christiansen Viktor staroverov
Viktor staroverov Arany viktor
Arany viktor Viktor arhipov
Viktor arhipov Arany viktor
Arany viktor Viktor marko
Viktor marko Viktor frankl teori
Viktor frankl teori Teori viktor frankl
Teori viktor frankl Viktor dyk současníci
Viktor dyk současníci Humores de hipócrates
Humores de hipócrates Krysař hlavní myšlenka
Krysař hlavní myšlenka Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Soli koncovky
Soli koncovky Chemie
Chemie Jodometrie
Jodometrie Prinzip des kleinsten zwanges
Prinzip des kleinsten zwanges Impuls chemie 4 lösungen pdf
Impuls chemie 4 lösungen pdf Chemie
Chemie Chemie
Chemie Vraag 3
Vraag 3 Podobory chemie
Podobory chemie Impuls chemie lösungen
Impuls chemie lösungen Bahenní plyn
Bahenní plyn Boeken over kwantumchemie en theoretische chemie
Boeken over kwantumchemie en theoretische chemie Concept cartoon chemie
Concept cartoon chemie Ddt chemie
Ddt chemie Chemie
Chemie Mechanismus
Mechanismus Tenside struktur
Tenside struktur Dobrý sluha ale zlý pán chemie
Dobrý sluha ale zlý pán chemie Donauchem pischelsdorf
Donauchem pischelsdorf Schilling chemie
Schilling chemie Soli chemie 9. ročník
Soli chemie 9. ročník Impuls chemie 4
Impuls chemie 4 Chemie k
Chemie k Chemie porn
Chemie porn Chemie 4 klasse
Chemie 4 klasse Van der waals gleichung
Van der waals gleichung Gefahrensymbole physik
Gefahrensymbole physik Woudschoten chemie 2021
Woudschoten chemie 2021 Methan oxidationszahl
Methan oxidationszahl Substrat chemie
Substrat chemie Molecuulorbitaal
Molecuulorbitaal Chemie
Chemie