QUALITATIVE ANALYTICAL CHEMISTRY prof Viktor Kanick Analytick chemie











- Slides: 41

QUALITATIVE ANALYTICAL CHEMISTRY prof. Viktor Kanický, Analytická chemie I 1

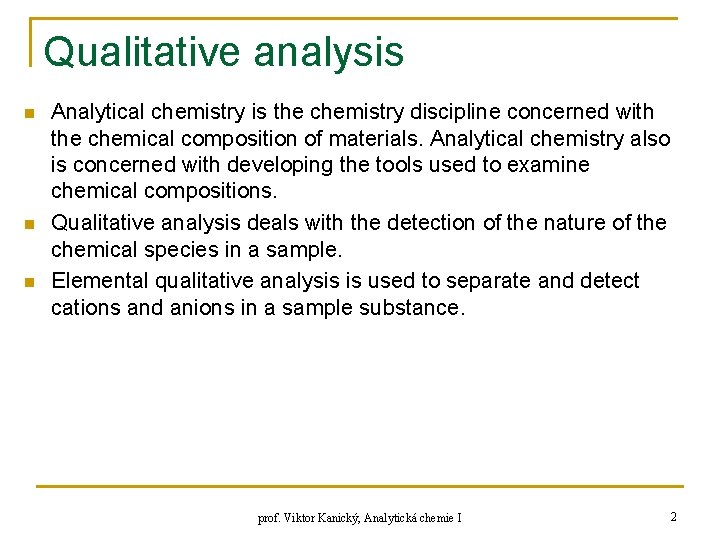
Qualitative analysis n n n Analytical chemistry is the chemistry discipline concerned with the chemical composition of materials. Analytical chemistry also is concerned with developing the tools used to examine chemical compositions. Qualitative analysis deals with the detection of the nature of the chemical species in a sample. Elemental qualitative analysis is used to separate and detect cations and anions in a sample substance. prof. Viktor Kanický, Analytická chemie I 2

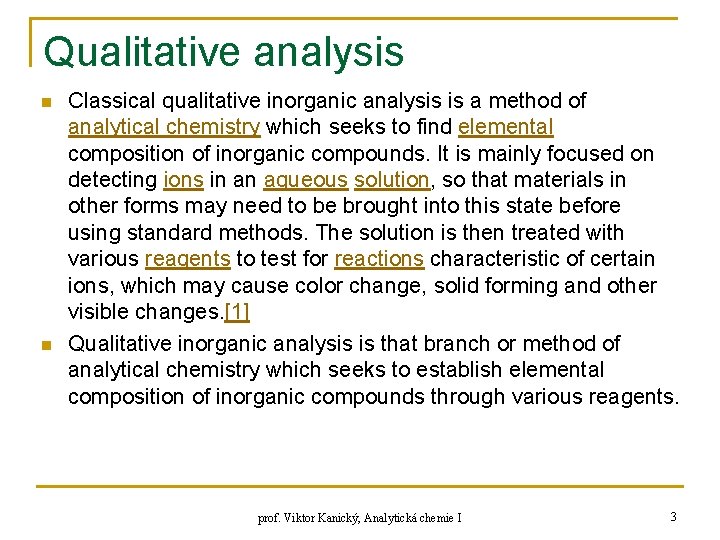
Qualitative analysis n n Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, so that materials in other forms may need to be brought into this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, solid forming and other visible changes. [1] Qualitative inorganic analysis is that branch or method of analytical chemistry which seeks to establish elemental composition of inorganic compounds through various reagents. prof. Viktor Kanický, Analytická chemie I 3

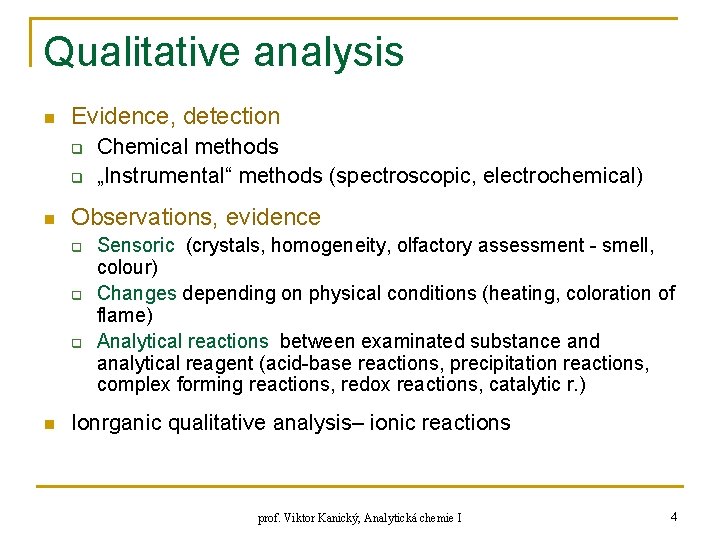
Qualitative analysis n Evidence, detection q q n Observations, evidence q q q n Chemical methods „Instrumental“ methods (spectroscopic, electrochemical) Sensoric (crystals, homogeneity, olfactory assessment - smell, colour) Changes depending on physical conditions (heating, coloration of flame) Analytical reactions between examinated substance and analytical reagent (acid-base reactions, precipitation reactions, complex forming reactions, redox reactions, catalytic r. ) Ionrganic qualitative analysis– ionic reactions prof. Viktor Kanický, Analytická chemie I 4

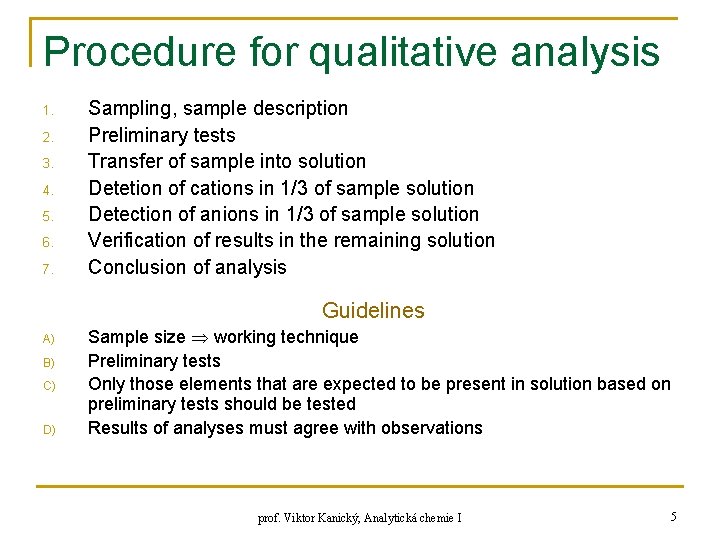
Procedure for qualitative analysis 1. 2. 3. 4. 5. 6. 7. Sampling, sample description Preliminary tests Transfer of sample into solution Detetion of cations in 1/3 of sample solution Detection of anions in 1/3 of sample solution Verification of results in the remaining solution Conclusion of analysis Guidelines A) B) C) D) Sample size working technique Preliminary tests Only those elements that are expected to be present in solution based on preliminary tests should be tested Results of analyses must agree with observations prof. Viktor Kanický, Analytická chemie I 5

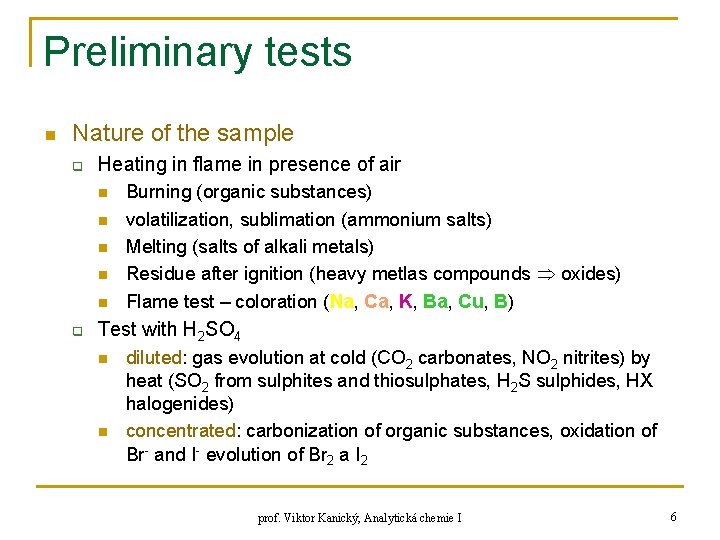
Preliminary tests n Nature of the sample q Heating in flame in presence of air n n n q Burning (organic substances) volatilization, sublimation (ammonium salts) Melting (salts of alkali metals) Residue after ignition (heavy metlas compounds oxides) Flame test – coloration (Na, Ca, K, Ba, Cu, B) Test with H 2 SO 4 n n diluted: gas evolution at cold (CO 2 carbonates, NO 2 nitrites) by heat (SO 2 from sulphites and thiosulphates, H 2 S sulphides, HX halogenides) concentrated: carbonization of organic substances, oxidation of Br- and I- evolution of Br 2 a I 2 prof. Viktor Kanický, Analytická chemie I 6

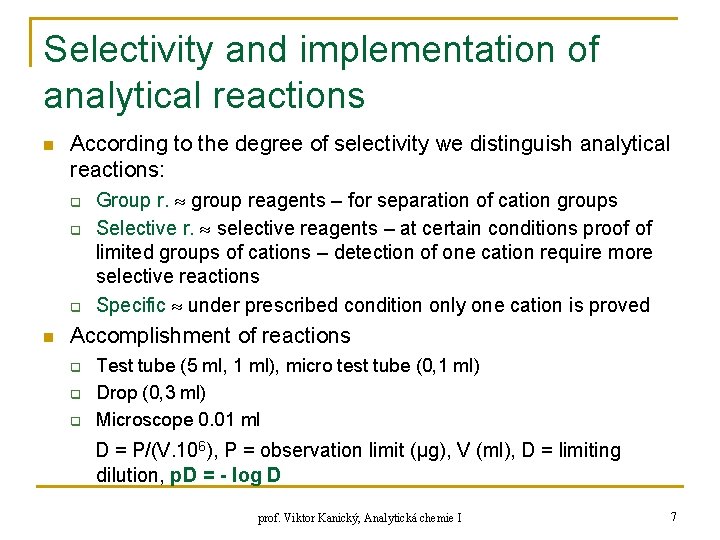
Selectivity and implementation of analytical reactions n According to the degree of selectivity we distinguish analytical reactions: q q q n Group r. group reagents – for separation of cation groups Selective r. selective reagents – at certain conditions proof of limited groups of cations – detection of one cation require more selective reactions Specific under prescribed condition only one cation is proved Accomplishment of reactions q q q Test tube (5 ml, 1 ml), micro test tube (0, 1 ml) Drop (0, 3 ml) Microscope 0. 01 ml D = P/(V. 106), P = observation limit (μg), V (ml), D = limiting dilution, p. D = - log D prof. Viktor Kanický, Analytická chemie I 7

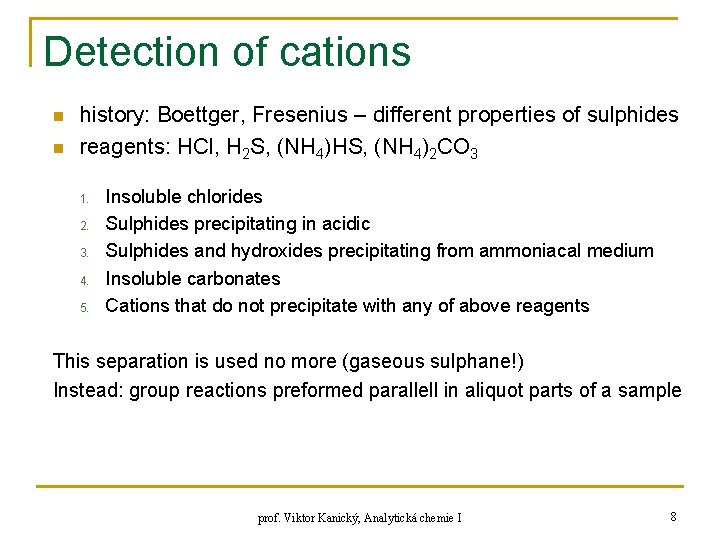
Detection of cations n n history: Boettger, Fresenius – different properties of sulphides reagents: HCl, H 2 S, (NH 4)HS, (NH 4)2 CO 3 1. 2. 3. 4. 5. Insoluble chlorides Sulphides precipitating in acidic Sulphides and hydroxides precipitating from ammoniacal medium Insoluble carbonates Cations that do not precipitate with any of above reagents This separation is used no more (gaseous sulphane!) Instead: group reactions preformed parallell in aliquot parts of a sample prof. Viktor Kanický, Analytická chemie I 8

DETECTION OF CATIONS, GROUP REACTIONS, SELECTIVE REACTIONS prof. Viktor Kanický, Analytická chemie I 9

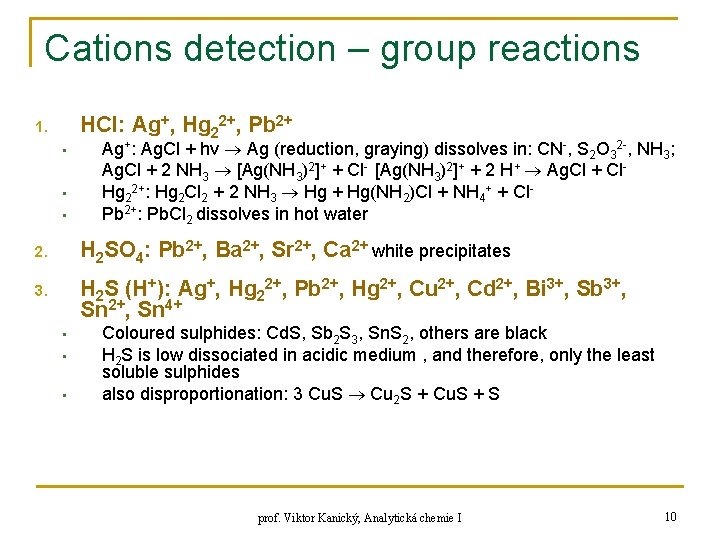
Cations detection – group reactions HCl: Ag+, Hg 22+, Pb 2+ 1. • • • Ag+: Ag. Cl + hν Ag (reduction, graying) dissolves in: CN-, S 2 O 32 -, NH 3; Ag. Cl + 2 NH 3 [Ag(NH 3)2]+ + Cl- [Ag(NH 3)2]+ + 2 H+ Ag. Cl + Cl. Hg 22+: Hg 2 Cl 2 + 2 NH 3 Hg + Hg(NH 2)Cl + NH 4+ + Cl. Pb 2+: Pb. Cl 2 dissolves in hot water 2. H 2 SO 4: Pb 2+, Ba 2+, Sr 2+, Ca 2+ white precipitates 3. H 2 S (H+): Ag+, Hg 22+, Pb 2+, Hg 2+, Cu 2+, Cd 2+, Bi 3+, Sb 3+, Sn 2+, Sn 4+ • • • Coloured sulphides: Cd. S, Sb 2 S 3, Sn. S 2, others are black H 2 S is low dissociated in acidic medium , and therefore, only the least soluble sulphides also disproportionation: 3 Cu. S Cu 2 S + Cu. S + S prof. Viktor Kanický, Analytická chemie I 10

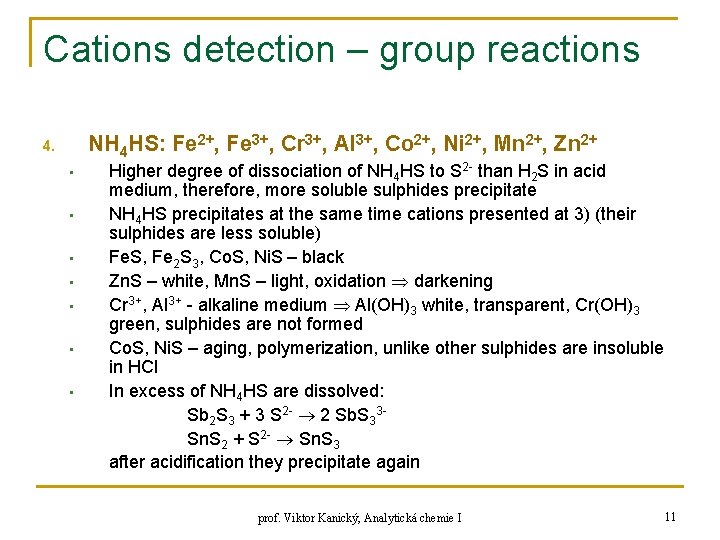
Cations detection – group reactions NH 4 HS: Fe 2+, Fe 3+, Cr 3+, Al 3+, Co 2+, Ni 2+, Mn 2+, Zn 2+ 4. • • Higher degree of dissociation of NH 4 HS to S 2 - than H 2 S in acid medium, therefore, more soluble sulphides precipitate NH 4 HS precipitates at the same time cations presented at 3) (their sulphides are less soluble) Fe. S, Fe 2 S 3, Co. S, Ni. S – black Zn. S – white, Mn. S – light, oxidation darkening Cr 3+, Al 3+ - alkaline medium Al(OH)3 white, transparent, Cr(OH)3 green, sulphides are not formed Co. S, Ni. S – aging, polymerization, unlike other sulphides are insoluble in HCl In excess of NH 4 HS are dissolved: Sb 2 S 3 + 3 S 2 - 2 Sb. S 33 Sn. S 2 + S 2 - Sn. S 3 after acidification they precipitate again prof. Viktor Kanický, Analytická chemie I 11

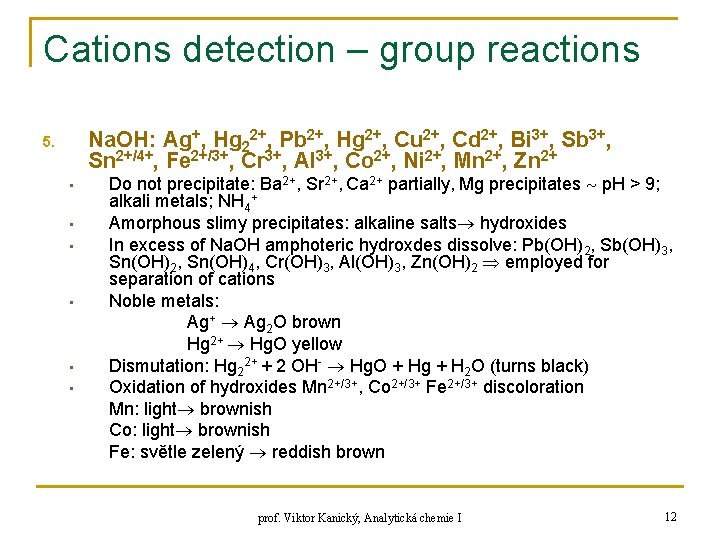
Cations detection – group reactions Na. OH: Ag+, Hg 22+, Pb 2+, Hg 2+, Cu 2+, Cd 2+, Bi 3+, Sb 3+, Sn 2+/4+, Fe 2+/3+, Cr 3+, Al 3+, Co 2+, Ni 2+, Mn 2+, Zn 2+ 5. • • • Do not precipitate: Ba 2+, Sr 2+, Ca 2+ partially, Mg precipitates p. H > 9; alkali metals; NH 4+ Amorphous slimy precipitates: alkaline salts hydroxides In excess of Na. OH amphoteric hydroxdes dissolve: Pb(OH)2, Sb(OH)3, Sn(OH)2, Sn(OH)4, Cr(OH)3, Al(OH)3, Zn(OH)2 employed for separation of cations Noble metals: Ag+ Ag 2 O brown Hg 2+ Hg. O yellow Dismutation: Hg 22+ + 2 OH- Hg. O + Hg + H 2 O (turns black) Oxidation of hydroxides Mn 2+/3+, Co 2+/3+ Fe 2+/3+ discoloration Mn: light brownish Co: light brownish Fe: světle zelený reddish brown prof. Viktor Kanický, Analytická chemie I 12

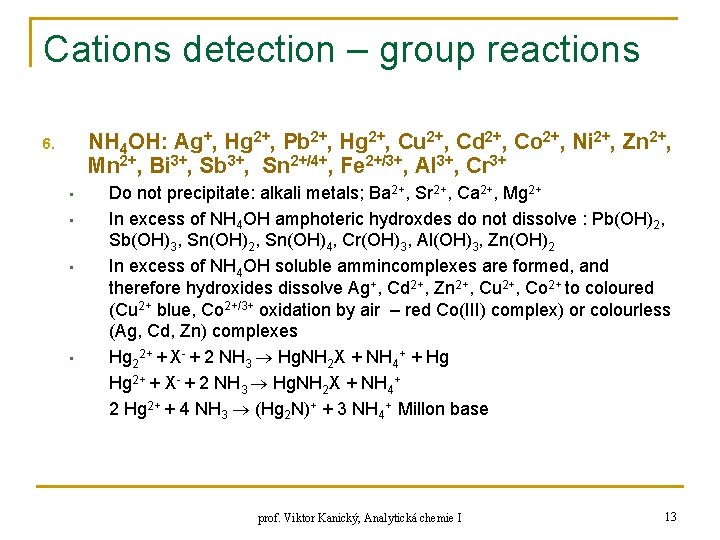
Cations detection – group reactions NH 4 OH: Ag+, Hg 2+, Pb 2+, Hg 2+, Cu 2+, Cd 2+, Co 2+, Ni 2+, Zn 2+, Mn 2+, Bi 3+, Sb 3+, Sn 2+/4+, Fe 2+/3+, Al 3+, Cr 3+ 6. • • Do not precipitate: alkali metals; Ba 2+, Sr 2+, Ca 2+, Mg 2+ In excess of NH 4 OH amphoteric hydroxdes do not dissolve : Pb(OH)2, Sb(OH)3, Sn(OH)2, Sn(OH)4, Cr(OH)3, Al(OH)3, Zn(OH)2 In excess of NH 4 OH soluble ammincomplexes are formed, and therefore hydroxides dissolve Ag+, Cd 2+, Zn 2+, Cu 2+, Co 2+ to coloured (Cu 2+ blue, Co 2+/3+ oxidation by air – red Co(III) complex) or colourless (Ag, Cd, Zn) complexes Hg 22+ + X- + 2 NH 3 Hg. NH 2 X + NH 4+ + Hg Hg 2+ + X- + 2 NH 3 Hg. NH 2 X + NH 4+ 2 Hg 2+ + 4 NH 3 (Hg 2 N)+ + 3 NH 4+ Millon base prof. Viktor Kanický, Analytická chemie I 13

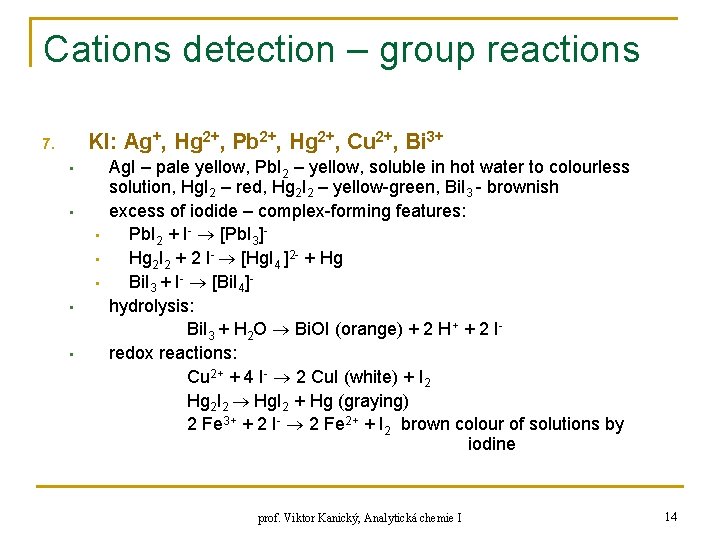
Cations detection – group reactions KI: Ag+, Hg 2+, Pb 2+, Hg 2+, Cu 2+, Bi 3+ 7. • • Ag. I – pale yellow, Pb. I 2 – yellow, soluble in hot water to colourless solution, Hg. I 2 – red, Hg 2 I 2 – yellow-green, Bi. I 3 - brownish excess of iodide – complex-forming features: Pb. I 2 + I- [Pb. I 3]Hg 2 I 2 + 2 I- [Hg. I 4 ]2 - + Hg Bi. I 3 + I- [Bi. I 4]hydrolysis: Bi. I 3 + H 2 O Bi. OI (orange) + 2 H+ + 2 Iredox reactions: Cu 2+ + 4 I- 2 Cu. I (white) + I 2 Hg 2 I 2 Hg. I 2 + Hg (graying) 2 Fe 3+ + 2 I- 2 Fe 2+ + I 2 brown colour of solutions by iodine prof. Viktor Kanický, Analytická chemie I 14

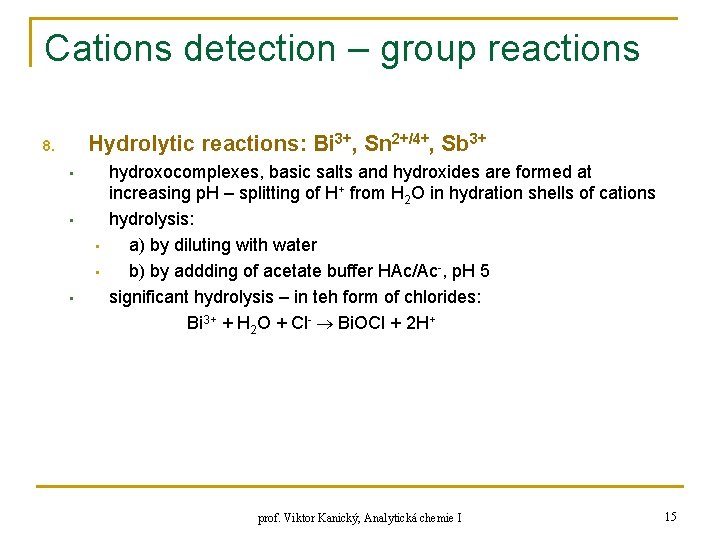
Cations detection – group reactions Hydrolytic reactions: Bi 3+, Sn 2+/4+, Sb 3+ 8. • • • hydroxocomplexes, basic salts and hydroxides are formed at increasing p. H – splitting of H+ from H 2 O in hydration shells of cations hydrolysis: a) by diluting with water b) by addding of acetate buffer HAc/Ac-, p. H 5 significant hydrolysis – in teh form of chlorides: Bi 3+ + H 2 O + Cl- Bi. OCl + 2 H+ prof. Viktor Kanický, Analytická chemie I 15

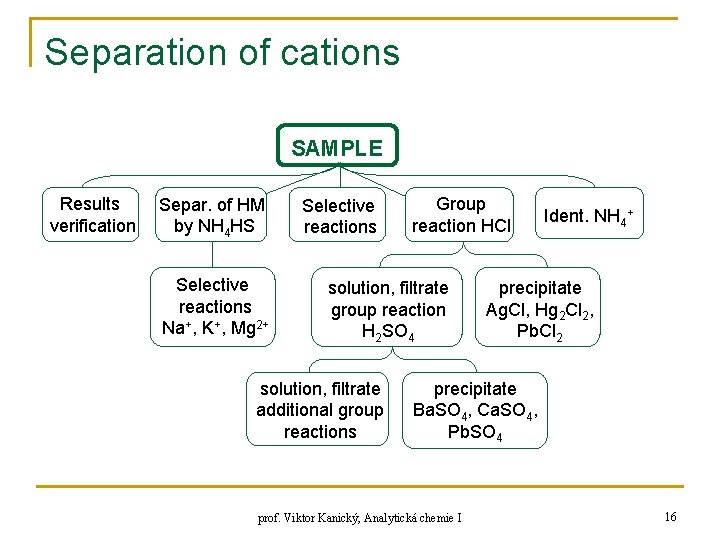
Separation of cations SAMPLE Results verification Separ. of HM by NH 4 HS Selective reactions Na+, K+, Mg 2+ Selective reactions Group reaction HCl solution, filtrate group reaction H 2 SO 4 solution, filtrate additional group reactions Ident. NH 4+ precipitate Ag. Cl, Hg 2 Cl 2, Pb. Cl 2 precipitate Ba. SO 4, Ca. SO 4, Pb. SO 4 prof. Viktor Kanický, Analytická chemie I 16

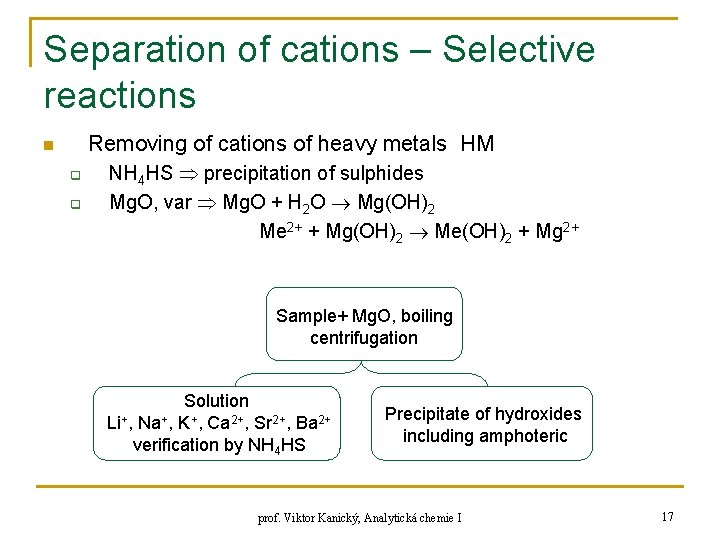
Separation of cations – Selective reactions Removing of cations of heavy metals HM n q q NH 4 HS precipitation of sulphides Mg. O, var Mg. O + H 2 O Mg(OH)2 Me 2+ + Mg(OH)2 Me(OH)2 + Mg 2+ Sample+ Mg. O, boiling centrifugation Solution Li+, Na+, K+, Ca 2+, Sr 2+, Ba 2+ verification by NH 4 HS Precipitate of hydroxides including amphoteric prof. Viktor Kanický, Analytická chemie I 17

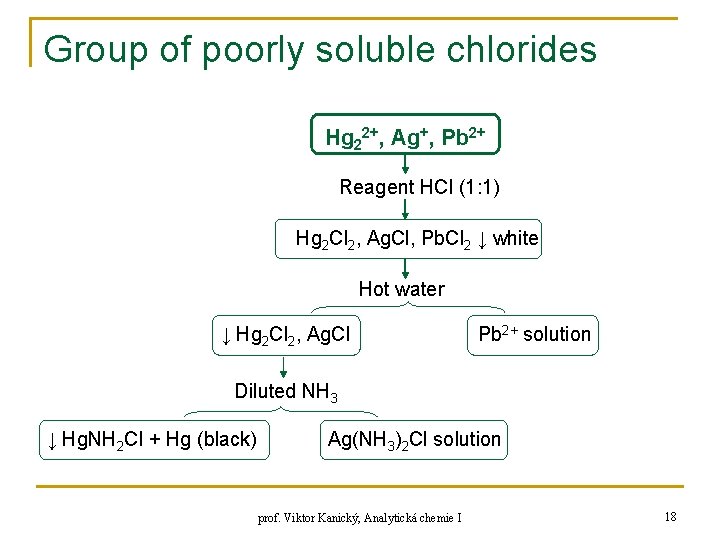
Group of poorly soluble chlorides Hg 22+, Ag+, Pb 2+ Reagent HCl (1: 1) Hg 2 Cl 2, Ag. Cl, Pb. Cl 2 ↓ white Hot water ↓ Hg 2 Cl 2, Ag. Cl Pb 2+ solution Diluted NH 3 ↓ Hg. NH 2 Cl + Hg (black) Ag(NH 3)2 Cl solution prof. Viktor Kanický, Analytická chemie I 18

Selective reactions of cations forming poorly soluble chlorides Sample + 1 mol. l-1 H 2 SO 4 centrifugation solution addition of 2 M HCl and centrifugation + NH 3 1: 1 centrifugation Hg. NH 2 Cl + Hg 0 precipitate addition of H 2 S turns black due to Pb. S crystals Ag. Cl microscope, Tananajev reaction prof. Viktor Kanický, Analytická chemie I 19

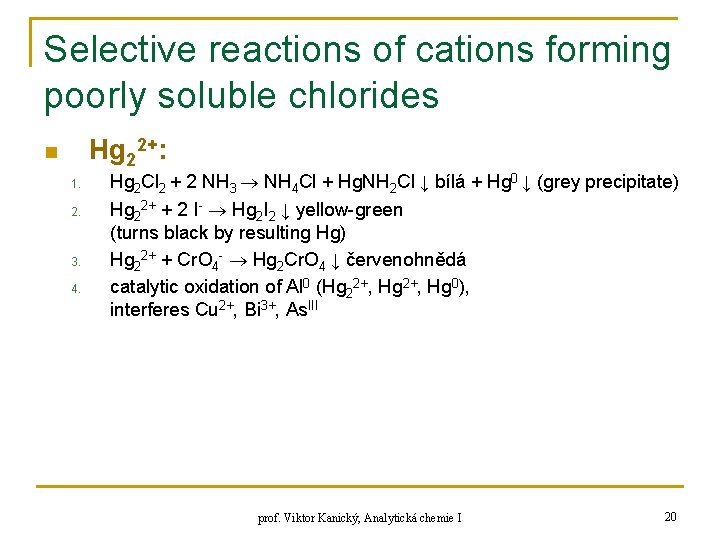
Selective reactions of cations forming poorly soluble chlorides Hg 22+: n 1. 2. 3. 4. Hg 2 Cl 2 + 2 NH 3 NH 4 Cl + Hg. NH 2 Cl ↓ bílá + Hg 0 ↓ (grey precipitate) Hg 22+ + 2 I- Hg 2 I 2 ↓ yellow-green (turns black by resulting Hg) Hg 22+ + Cr. O 4 - Hg 2 Cr. O 4 ↓ červenohnědá catalytic oxidation of Al 0 (Hg 22+, Hg 0), interferes Cu 2+, Bi 3+, As. III prof. Viktor Kanický, Analytická chemie I 20

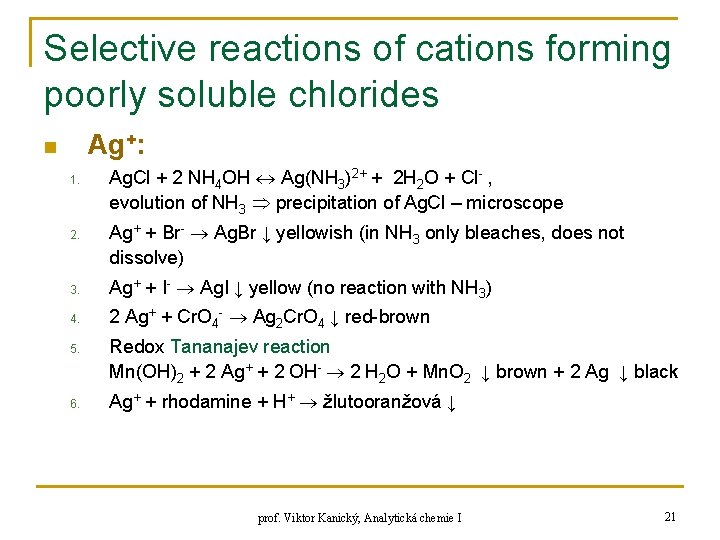
Selective reactions of cations forming poorly soluble chlorides Ag+: n 1. 2. Ag. Cl + 2 NH 4 OH Ag(NH 3)2+ + 2 H 2 O + Cl- , evolution of NH 3 precipitation of Ag. Cl – microscope Ag+ + Br- Ag. Br ↓ yellowish (in NH 3 only bleaches, does not dissolve) 3. Ag+ + I- Ag. I ↓ yellow (no reaction with NH 3) 4. 2 Ag+ + Cr. O 4 - Ag 2 Cr. O 4 ↓ red-brown 5. 6. Redox Tananajev reaction Mn(OH)2 + 2 Ag+ + 2 OH- 2 H 2 O + Mn. O 2 ↓ brown + 2 Ag ↓ black Ag+ + rhodamine + H+ žlutooranžová ↓ prof. Viktor Kanický, Analytická chemie I 21

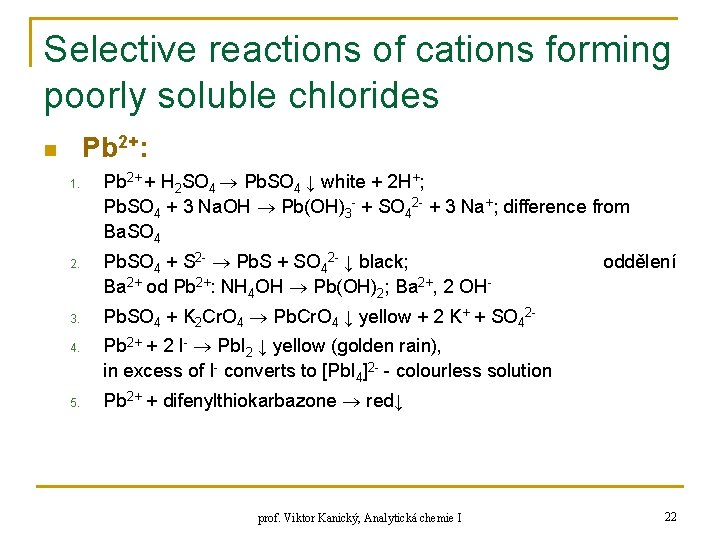
Selective reactions of cations forming poorly soluble chlorides Pb 2+: n 1. 2. 3. 4. 5. Pb 2+ + H 2 SO 4 Pb. SO 4 ↓ white + 2 H+; Pb. SO 4 + 3 Na. OH Pb(OH)3 - + SO 42 - + 3 Na+; difference from Ba. SO 4 Pb. SO 4 + S 2 - Pb. S + SO 42 - ↓ black; Ba 2+ od Pb 2+: NH 4 OH Pb(OH)2; Ba 2+, 2 OH- oddělení Pb. SO 4 + K 2 Cr. O 4 Pb. Cr. O 4 ↓ yellow + 2 K+ + SO 42 Pb 2+ + 2 I- Pb. I 2 ↓ yellow (golden rain), in excess of I- converts to [Pb. I 4]2 - - colourless solution Pb 2+ + difenylthiokarbazone red↓ prof. Viktor Kanický, Analytická chemie I 22

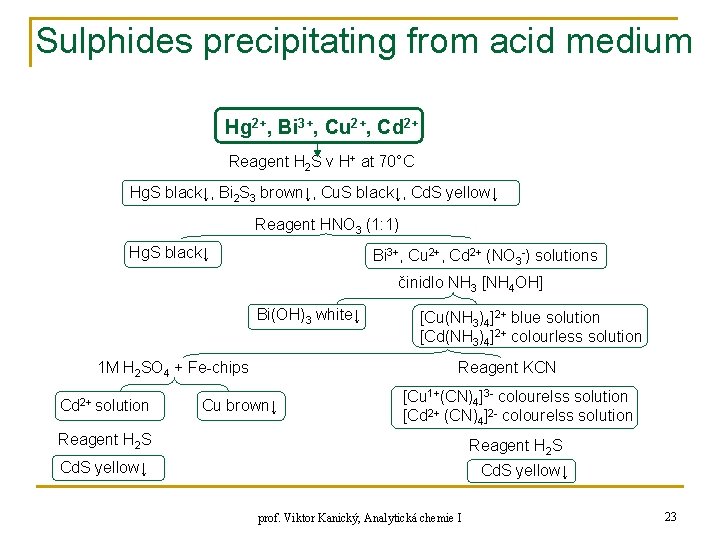
Sulphides precipitating from acid medium Hg 2+, Bi 3+, Cu 2+, Cd 2+ Reagent H 2 S v H+ at 70°C Hg. S black↓, Bi 2 S 3 brown↓, Cu. S black↓, Cd. S yellow↓ Reagent HNO 3 (1: 1) Hg. S black↓ Bi 3+, Cu 2+, Cd 2+ (NO 3 -) solutions činidlo NH 3 [NH 4 OH] Bi(OH)3 white↓ 1 M H 2 SO 4 + Fe-chips Cd 2+ solution [Cu(NH 3)4]2+ blue solution [Cd(NH 3)4]2+ colourless solution Reagent KCN Cu brown↓ [Cu 1+(CN)4]3 - colourelss solution [Cd 2+ (CN)4]2 - colourelss solution Reagent H 2 S Cd. S yellow↓ prof. Viktor Kanický, Analytická chemie I 23

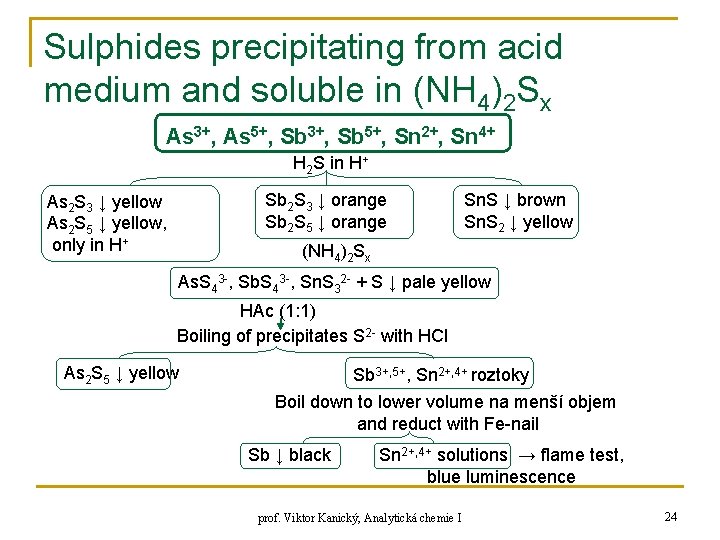
Sulphides precipitating from acid medium and soluble in (NH 4)2 Sx As 3+, As 5+, Sb 3+, Sb 5+, Sn 2+, Sn 4+ H 2 S in H+ Sb 2 S 3 ↓ orange Sb 2 S 5 ↓ orange As 2 S 3 ↓ yellow As 2 S 5 ↓ yellow, only in H+ Sn. S ↓ brown Sn. S 2 ↓ yellow (NH 4)2 Sx As. S 43 -, Sb. S 43 -, Sn. S 32 - + S ↓ pale yellow HAc (1: 1) Boiling of precipitates S 2 - with HCl As 2 S 5 ↓ yellow Sb 3+, 5+, Sn 2+, 4+ roztoky Boil down to lower volume na menší objem and reduct with Fe-nail Sb ↓ black Sn 2+, 4+ solutions → flame test, blue luminescence prof. Viktor Kanický, Analytická chemie I 24

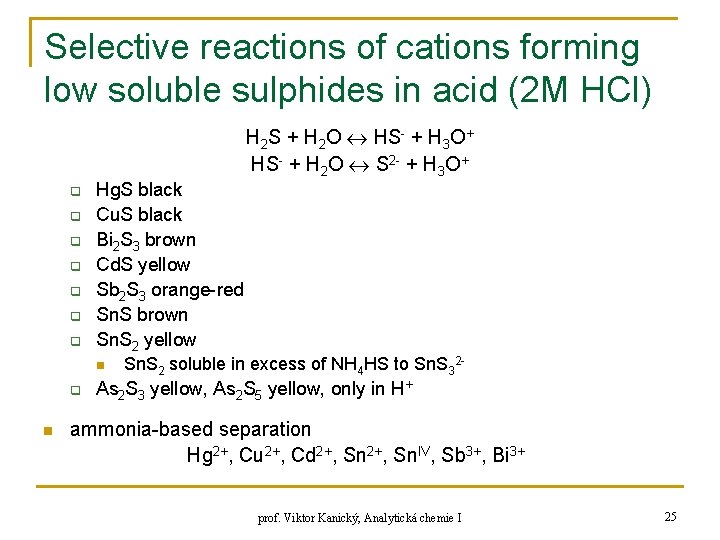
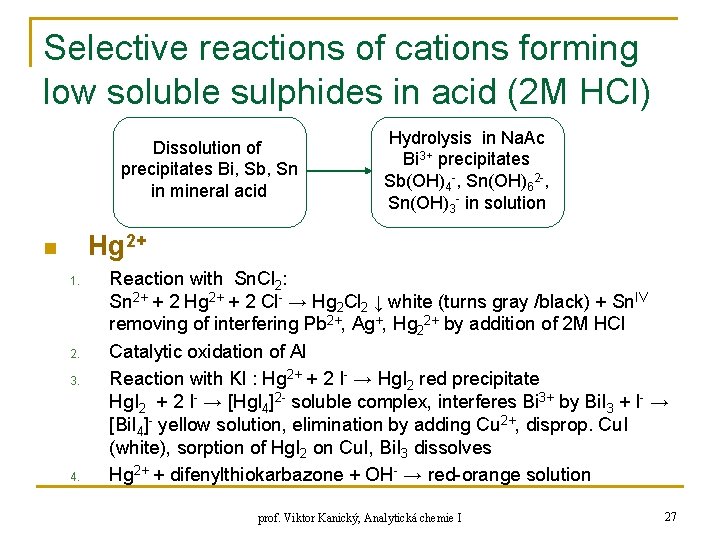
Selective reactions of cations forming low soluble sulphides in acid (2 M HCl) H 2 S + H 2 O HS- + H 3 O+ HS- + H 2 O S 2 - + H 3 O+ q q q q Hg. S black Cu. S black Bi 2 S 3 brown Cd. S yellow Sb 2 S 3 orange-red Sn. S brown Sn. S 2 yellow n q n Sn. S 2 soluble in excess of NH 4 HS to Sn. S 32 - As 2 S 3 yellow, As 2 S 5 yellow, only in H+ ammonia-based separation Hg 2+, Cu 2+, Cd 2+, Sn. IV, Sb 3+, Bi 3+ prof. Viktor Kanický, Analytická chemie I 25

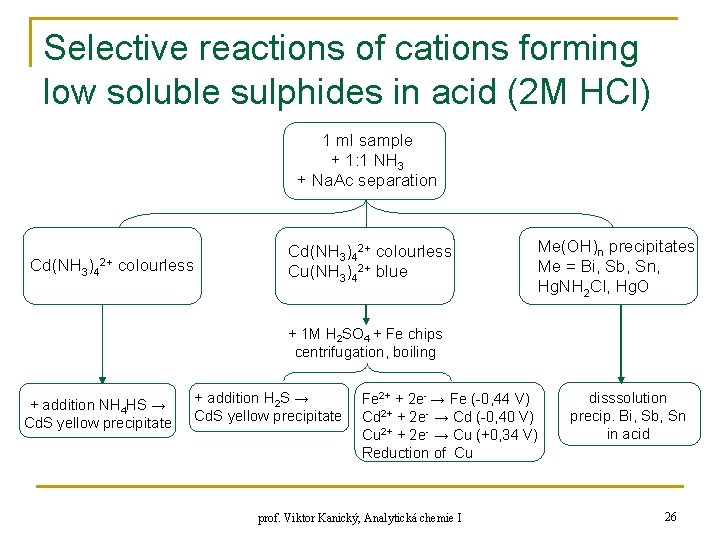
Selective reactions of cations forming low soluble sulphides in acid (2 M HCl) 1 ml sample + 1: 1 NH 3 + Na. Ac separation Cd(NH 3)42+ colourless Cu(NH 3)42+ blue Me(OH)n precipitates Me = Bi, Sb, Sn, Hg. NH 2 Cl, Hg. O + 1 M H 2 SO 4 + Fe chips centrifugation, boiling + addition NH 4 HS → Cd. S yellow precipitate + addition H 2 S → Cd. S yellow precipitate Fe 2+ + 2 e- → Fe (-0, 44 V) Cd 2+ + 2 e- → Cd (-0, 40 V) Cu 2+ + 2 e- → Cu (+0, 34 V) Reduction of Cu prof. Viktor Kanický, Analytická chemie I disssolution precip. Bi, Sb, Sn in acid 26

Selective reactions of cations forming low soluble sulphides in acid (2 M HCl) Dissolution of precipitates Bi, Sb, Sn in mineral acid Hydrolysis in Na. Ac Bi 3+ precipitates Sb(OH)4 -, Sn(OH)62 -, Sn(OH)3 - in solution Hg 2+ n 1. 2. 3. 4. Reaction with Sn. Cl 2: Sn 2+ + 2 Hg 2+ + 2 Cl- → Hg 2 Cl 2 ↓ white (turns gray /black) + Sn. IV removing of interfering Pb 2+, Ag+, Hg 22+ by addition of 2 M HCl Catalytic oxidation of Al Reaction with KI : Hg 2+ + 2 I- → Hg. I 2 red precipitate Hg. I 2 + 2 I- → [Hg. I 4]2 - soluble complex, interferes Bi 3+ by Bi. I 3 + I- → [Bi. I 4]- yellow solution, elimination by adding Cu 2+, disprop. Cu. I (white), sorption of Hg. I 2 on Cu. I, Bi. I 3 dissolves Hg 2+ + difenylthiokarbazone + OH- → red-orange solution prof. Viktor Kanický, Analytická chemie I 27

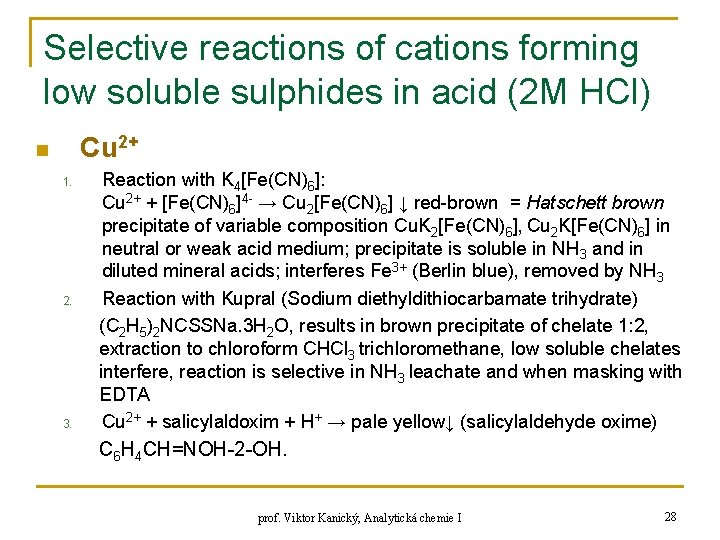
Selective reactions of cations forming low soluble sulphides in acid (2 M HCl) Cu 2+ n 1. 2. 3. Reaction with K 4[Fe(CN)6]: Cu 2+ + [Fe(CN)6]4 - → Cu 2[Fe(CN)6] ↓ red-brown = Hatschett brown precipitate of variable composition Cu. K 2[Fe(CN)6], Cu 2 K[Fe(CN)6] in neutral or weak acid medium; precipitate is soluble in NH 3 and in diluted mineral acids; interferes Fe 3+ (Berlin blue), removed by NH 3 Reaction with Kupral (Sodium diethyldithiocarbamate trihydrate) (C 2 H 5)2 NCSSNa. 3 H 2 O, results in brown precipitate of chelate 1: 2, extraction to chloroform CHCl 3 trichloromethane, low soluble chelates interfere, reaction is selective in NH 3 leachate and when masking with EDTA Cu 2+ + salicylaldoxim + H+ → pale yellow↓ (salicylaldehyde oxime) C 6 H 4 CH=NOH-2 -OH. prof. Viktor Kanický, Analytická chemie I 28

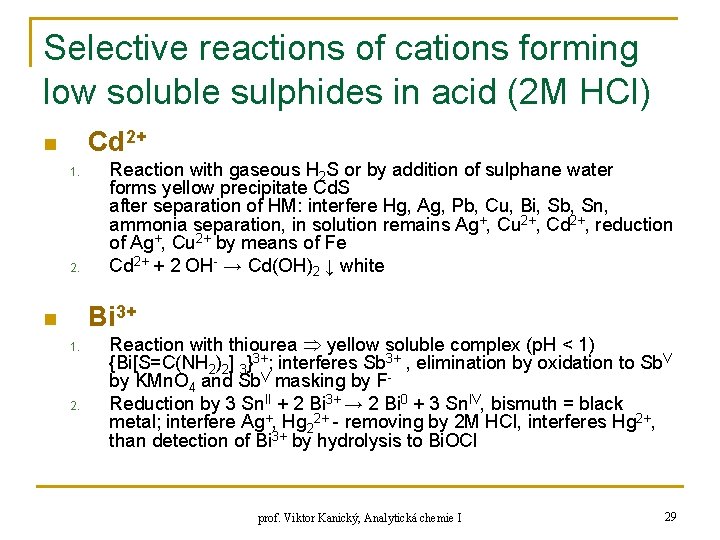
Selective reactions of cations forming low soluble sulphides in acid (2 M HCl) Cd 2+ n 1. 2. Reaction with gaseous H 2 S or by addition of sulphane water forms yellow precipitate Cd. S after separation of HM: interfere Hg, Ag, Pb, Cu, Bi, Sb, Sn, ammonia separation, in solution remains Ag+, Cu 2+, Cd 2+, reduction of Ag+, Cu 2+ by means of Fe Cd 2+ + 2 OH- → Cd(OH)2 ↓ white Bi 3+ n 1. 2. Reaction with thiourea yellow soluble complex (p. H < 1) {Bi[S=C(NH 2)2] 3}3+; interferes Sb 3+ , elimination by oxidation to Sb. V by KMn. O 4 and Sb. V masking by FReduction by 3 Sn. II + 2 Bi 3+ → 2 Bi 0 + 3 Sn. IV, bismuth = black metal; interfere Ag+, Hg 22+ - removing by 2 M HCl, interferes Hg 2+, than detection of Bi 3+ by hydrolysis to Bi. OCl prof. Viktor Kanický, Analytická chemie I 29

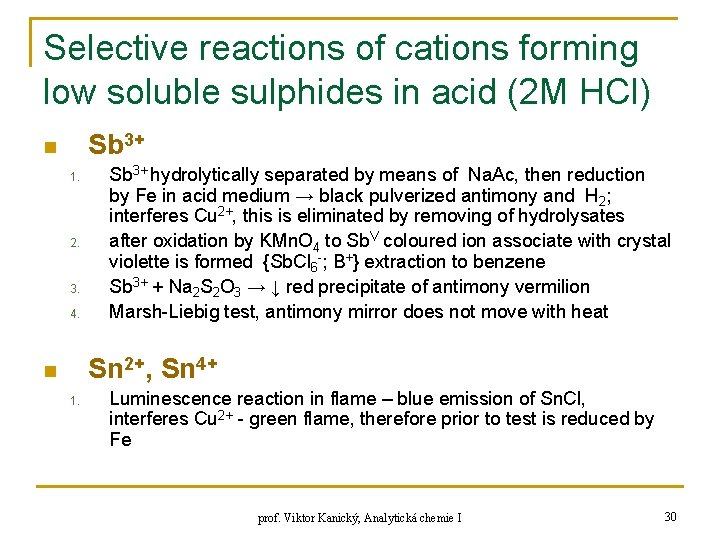
Selective reactions of cations forming low soluble sulphides in acid (2 M HCl) Sb 3+ n 1. 2. 3. 4. Sb 3+ hydrolytically separated by means of Na. Ac, then reduction by Fe in acid medium → black pulverized antimony and H 2; interferes Cu 2+, this is eliminated by removing of hydrolysates after oxidation by KMn. O 4 to Sb. V coloured ion associate with crystal violette is formed {Sb. Cl 6 -; B+} extraction to benzene Sb 3+ + Na 2 S 2 O 3 → ↓ red precipitate of antimony vermilion Marsh-Liebig test, antimony mirror does not move with heat Sn 2+, Sn 4+ n 1. Luminescence reaction in flame – blue emission of Sn. Cl, interferes Cu 2+ - green flame, therefore prior to test is reduced by Fe prof. Viktor Kanický, Analytická chemie I 30

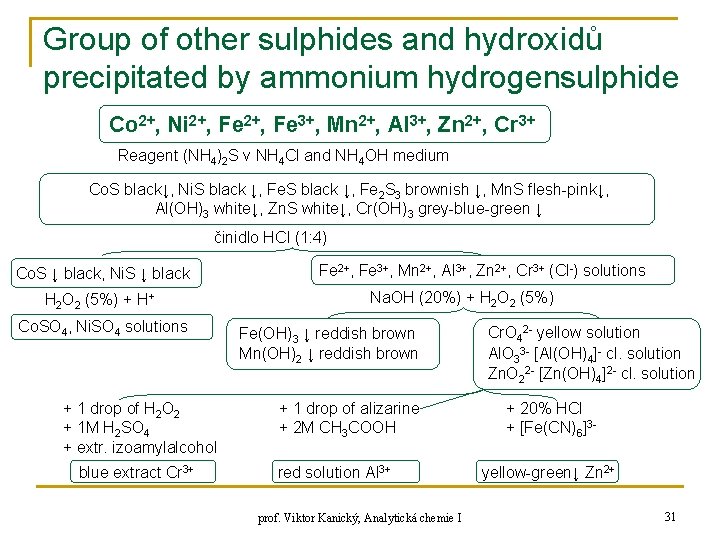
Group of other sulphides and hydroxidů precipitated by ammonium hydrogensulphide Co 2+, Ni 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ Reagent (NH 4)2 S v NH 4 Cl and NH 4 OH medium Co. S black↓, Ni. S black ↓, Fe 2 S 3 brownish ↓, Mn. S flesh-pink↓, Al(OH)3 white↓, Zn. S white↓, Cr(OH)3 grey-blue-green ↓ činidlo HCl (1: 4) Co. S ↓ black, Ni. S ↓ black H 2 O 2 (5%) + H+ Co. SO 4, Ni. SO 4 solutions + 1 drop of H 2 O 2 + 1 M H 2 SO 4 + extr. izoamylalcohol blue extract Cr 3+ Fe 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ (Cl-) solutions Na. OH (20%) + H 2 O 2 (5%) Fe(OH)3 ↓ reddish brown Mn(OH)2 ↓ reddish brown + 1 drop of alizarine + 2 M CH 3 COOH red solution Al 3+ prof. Viktor Kanický, Analytická chemie I Cr. O 42 - yellow solution Al. O 33 - [Al(OH)4]- cl. solution Zn. O 22 - [Zn(OH)4]2 - cl. solution + 20% HCl + [Fe(CN)6]3 yellow-green↓ Zn 2+ 31

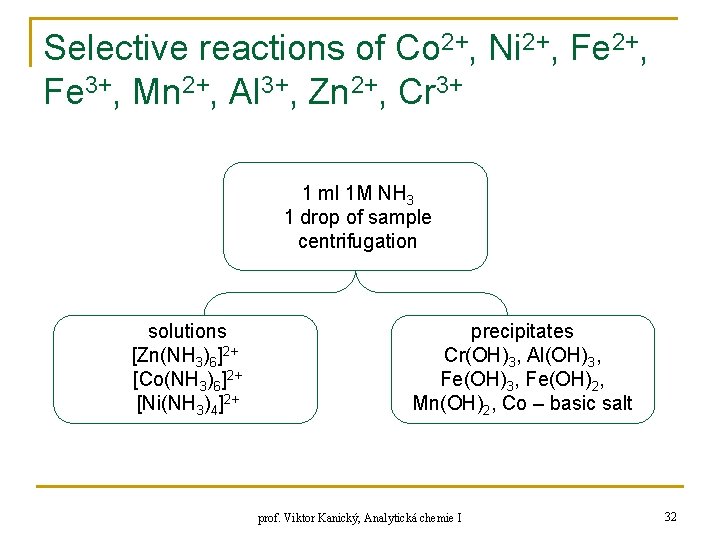
Selective reactions of Co 2+, Ni 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ 1 ml 1 M NH 3 1 drop of sample centrifugation solutions [Zn(NH 3)6]2+ [Co(NH 3)6]2+ [Ni(NH 3)4]2+ precipitates Cr(OH)3, Al(OH)3, Fe(OH)2, Mn(OH)2, Co – basic salt prof. Viktor Kanický, Analytická chemie I 32

Selektivní reakce kationů Co 2+, Ni 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ Fe 3+ n 1. 2. 3. 4. s thiokyanatanem (SCN)- v kyselém prostředí Fe 3+ + SCN- → červené komplexy [Fe(SCN)]2+, [Fe(SCN)2]+… Fe[Fe(SCN)6] typický krvavě červený roztok; ruší fluoridy s K 4[Fe(CN)6] v kys. prostředí vzniká sraženina nebo koloidní roztok Berlínské modři K{Fe. III[Fe. II(CN)6]} respektive Fe. III 4[Fe. II(CN)6]3; ruší Cu 2+ ve velkém nadbytku s kyselinou sulfosalicylovou v kys. prostř. vzniká fialový roztok rozpustného chelátu; ruší fluoridy a dihydrogenfosforečnany Fe 3+ + 3 NH 3 + 3 H 2 O → 3 NH 4+ + Fe(OH)3 ↓ rezavě hnědá, v nadbytku NH 3 nerozpustná Fe 2+ n 1. 2. 3. s 1, 10 -fenanthrolinem při p. H 2 -9 vzniká stabilní červený chelát s kynoželezitanem K 3[Fe(CN)6] vzniká Berlínská modř bezvodé soli Fe 2+ jsou bílé, hydratované světle zelené: Fe. SO 4. 7 H 2 O, (NH 4)Fe(SO 4)2. 6 H 2 O (Mohrova sůl) a Fe. Cl 2. 4 H 2 O prof. Viktor Kanický, Analytická chemie I 33

Selektivní reakce kationů Co 2+, Ni 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ Al 3+ n 1. 2. s alizarinem S (1, 2 -dihydroxyantrachinon-3 -sulfonan) vzniká červený lak – chelát Al. L, povrchově adsorbován na Al(OH)3 v NH 3 prostředí, ruší: Fe 3+, Cu 2+ - oddělení pomocí Na. OH s kvercetinem – vzniká zeleně fluoreskující, ve vodě málo rozpustný Al - chelát při p. H 1 -4, ruší: Zn 2+ a Sb 3+ – nepatrná fluorescence, maskování: F- Cr 3+ n 1. 2. s peroxidem H 2 O 2 v alkalickém prostředí za varu → Cr. O 42 - žlutý roztok, po okyselení přechází s přebytečným peroxidem na nestálý modrý peroxid Cr. VIO 5, ruší: Mn 2+ - alkalické dělení Cr 3+ + 3 NH 3 + 3 H 2 O → 3 NH 4+ + Cr(OH)3 ↓ šedozelená prof. Viktor Kanický, Analytická chemie I 34

Selektivní reakce kationů Co 2+, Ni 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ Zn 2+ n 1. 2. s kyanoželeznatanem K 4[Fe(CN)6] v prostředí 1 M HCl vzniká bílá sraženina K 2 Zn[Fe(CN)6]; ruší Fe 3+, Cu 2+, Mn 2+, Ni 2+ a Co 2+ - dělení v 10% Na. OH s difenylthiokarbazonem v alkalickém prostředí vzniká zelený roztok, vytřepáním v roztoku CCl 4 přechází na purpurovou červeň Co 2+ n 1. 2. 3. 4. s thiokyanatanem vzniká modrý rozpustný komplex [Co(SCN)4]2 -, extrakce např. do izoamylalkoholu, ruší Fe 3+, Bi 3+ - maskování s F-, Cu 2+ tvoří hnědou sraženinu bezvodé soli jsou modré, hydratované růžové např. : Co. Cl 2. 6 H 2 O, Co(NO 3)2. 6 H 2 O, Co. SO 4. 7 H 2 O Co 2+ + 2 NH 3 + 2 H 2 O → ↓ světle modrá zásaditých solí, rozpouští se v nadbytku NH 3 na [Co(NH 3)4]2+, který oxiduje na [Co(NH 3)6]3+ (hnědý roztok) s 1 -nitroso-2 -naftolem vzniká červenohnědá sraženina chelátu Co. L 3, ruší Cu 2+, Fe 3+, Hg 2+, Ni 2+ - jejich cheláty se v 10% HCl rozloží prof. Viktor Kanický, Analytická chemie I 35

Selektivní reakce kationů Co 2+, Ni 2+, Fe 3+, Mn 2+, Al 3+, Zn 2+, Cr 3+ Mn 2+ n 1. 2. 3. oxidací v kyselých roztocích jodistanem za horka vzniká fialový Mn. O 4 Mn 2+ + 2 NH 3 + 3 H 2 O → bílá ↓ zásaditých solí, v důsledku oxidace hnědne bezvodé soli jsou bezbarvé hydratované růžové: Mn. SO 4. 7 H 2 O, Mn(NO 3)2. 6 H 2 O, Mn. Cl 2. 4 H 2 O Ni 2+ n 1. 2. 3. s diacetylaldioximem (Čugajevovo činidlo) v amoniakálním prostředí vzniká růžově červená sraženina chelátu Ni(DH)2 Ni 2+ + 2 NH 3 + 2 H 2 O → ↓ zásadité soli, v nadbytku NH 3 vznik [Ni(NH 3)4]2+ modrý roztok hydratované soli Ni 2+ jsou zelené, bezvodé jsou bezbarvé prof. Viktor Kanický, Analytická chemie I 36

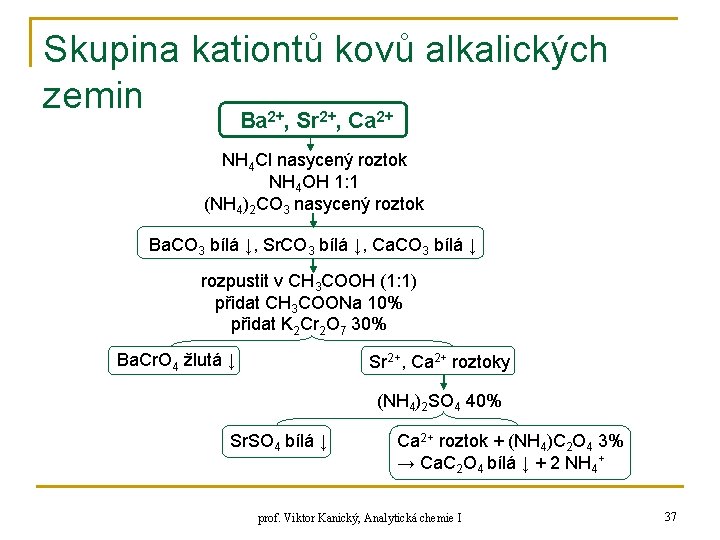
Skupina kationtů kovů alkalických zemin Ba 2+, Sr 2+, Ca 2+ NH 4 Cl nasycený roztok NH 4 OH 1: 1 (NH 4)2 CO 3 nasycený roztok Ba. CO 3 bílá ↓, Sr. CO 3 bílá ↓, Ca. CO 3 bílá ↓ rozpustit v CH 3 COOH (1: 1) přidat CH 3 COONa 10% přidat K 2 Cr 2 O 7 30% Ba. Cr. O 4 žlutá ↓ Sr 2+, Ca 2+ roztoky (NH 4)2 SO 4 40% Sr. SO 4 bílá ↓ Ca 2+ roztok + (NH 4)C 2 O 4 3% → Ca. C 2 O 4 bílá ↓ + 2 NH 4+ prof. Viktor Kanický, Analytická chemie I 37

Selektivní reakce alkalických kovů alkalických zemin n Li+, Na+, K+, NH 4+ q bezbarvé, dobře rozpustné soli; netvoří stabilní komplexy plamenové zkoušky (ne NH 4+) - zbarvení emisí alkal. kovů q reakce s organickými činidly q n Ca 2+, Sr 2+, Ba 2+ q sraženiny: SO 42 -, Cr. O 42 -, OH-, F-, C 2 O 42 -, CO 32 - rozpustnost: n n n SO 42 -, Cr. O 42 - : OH-, F-, C 2 O 42 - : CO 32 - : Ca 2+ > Sr 2+ > Ba 2+ Ca 2+ < Sr 2+ < Ba 2+ Ca 2+ ≈ Ba 2+ < Sr 2+ prof. Viktor Kanický, Analytická chemie I 38

Selektivní reakce alkalických kovů alkalických zemin n plamenové zkoušky – těkavé chloridy, Pt drát q q q Li 2+ karmínově červená Na+ žlutá K+ fialová + červená Ca 2+ cihlově červená Sr 2+ červená + oranž. Ba 2+ zelená 670, 0 nm 589, 6 a 589, 0 nm 404, 7 a 768, 0 nm 620, 0 nm 674, 7; 662, 8 a 606, 0 nm (oranž. ) 531; 524 a 514 nm prof. Viktor Kanický, Analytická chemie I 39

Selektivní reakce alkalických kovů alkalických zemin n Li+ 1. n Na+ 1. n s octanem uranylo-zinečnatým v kyselině octové vzniká světle žlutá krystalická sraženina Na. Mg(UO 2)3(CH 3 COO)9. 9 H 2 O, ruší TK K+ 1. 2. n Li. Cl je rozpustný v organických rozpouštědlech chloridy Na, K, Ca a Ba, vhodné pro oddělení pro plamenovou zkoušku s dipikrylaminem vzniká oranžovo-červená sraženina draselné soli hexanitrodifenylaminátu, ruší TK, NH 4+ s kyselinou pikrovou vzniká pikran draselný žlutá sraženina NH 4+ 1. s Nesslerovým činidlem v alkalickém prostředí vzniká hnědá až žlutá sraženina; příprava Ness. činidla: Hg. Cl 2 + 2 KI Hg. I 2 … + 2 KI [Hg. I 4]2 -; v Na. OH reakce [Hg. I 4]2 - + NH 4+ Hg 2 I 3 NH 2 ruší všechny kationty, které se srážejí v alkalickém prostředí prof. Viktor Kanický, Analytická chemie I 40

Selektivní reakce alkalických kovů alkalických zemin n Mg 2+ 1. 2. 3. n Ca 2+ 1. n s kyselinou šťavelovou ve slabě kyselém prostředí vzniká bílá krystalická sraženina šťavelanu vápenatého; Neruší Sr 2+, Ba 2+, alkalické kovy, ruší TK odstranění s Mg. O Sr 2+ 1. n s Magnezonem (4 -nitrobenzen azorezorcin nebo 4 -nitrobenzenazo-1 naftol) v Na. OH tvoří modrou sraženinu chelátu; slepý pokus: žlutá fialová v roztoku (acidobazický indikátor); modrý chelát – zbarvení při adsorpci na Mg(OH)2 s thiazolovou (titanovou) žlutí vzniká červená sraženina; slepý pokus dává žlutý až oranžový roztok s 8 -hydroxichinolinem v alkalickém prostředí vzniká žlutá sraženina (jehličky) žlutá sraženina s K 2 Cr. O 4 po oddělení TK, ruší Ca 2+, na rozdíl od Ba 2+ se nesráží Sr 2+ ve 2 mol. l-1 kys. octové Ba 2+ 1. sráží se s K 2 Cr. O 4 ve 2 mol. l-1 HAc, v neutr. / alkal. prostředí, sráží se 1 mol. l-1 H 2 SO 4 prof. Viktor Kanický, Analytická chemie I 41
 Analytic induction and grounded theory
Analytic induction and grounded theory Analytical chemistry definition
Analytical chemistry definition Kesalahan kuantitatif adalah
Kesalahan kuantitatif adalah Jelaskan kegunaan statistika dalam analisis kimia
Jelaskan kegunaan statistika dalam analisis kimia Chemistry apparatus
Chemistry apparatus Calibration verification and linearity
Calibration verification and linearity Gaussian curve
Gaussian curve What is equivalent weight in chemistry
What is equivalent weight in chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Round off rule of 5
Round off rule of 5 Q test in analytical chemistry
Q test in analytical chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Special purpose reagent chemicals
Special purpose reagent chemicals Analytical chemistry
Analytical chemistry Excellence in analytical chemistry
Excellence in analytical chemistry Coprecipitation errors
Coprecipitation errors General chemistry with qualitative analysis
General chemistry with qualitative analysis Qualitative analysis chemistry
Qualitative analysis chemistry Quantitative and qualitative in chemistry
Quantitative and qualitative in chemistry Quantitative chemistry igcse
Quantitative chemistry igcse Viktor frankl teori
Viktor frankl teori Viktor mikhaylovich
Viktor mikhaylovich Teoria de viktor frankl
Teoria de viktor frankl Viktor dyk literární směr
Viktor dyk literární směr Viktor haase
Viktor haase Viktor arhipov
Viktor arhipov Viktor hartmann gnomus
Viktor hartmann gnomus Rhoda kellogg wikipedia
Rhoda kellogg wikipedia Viktor kubal vtipy
Viktor kubal vtipy Viktor dyk současníci
Viktor dyk současníci Viktor binzberger
Viktor binzberger Victor pestov
Victor pestov Arany viktor
Arany viktor Dr victor christiansen
Dr victor christiansen Advanced persistent threat assessment
Advanced persistent threat assessment Viktor staroverov
Viktor staroverov Viktor pocajt
Viktor pocajt Viktor frankl teori
Viktor frankl teori Viktor binzberger
Viktor binzberger Teori viktor frankl
Teori viktor frankl