Analytical Chemistry Definition the science of extraction identification





- Slides: 36

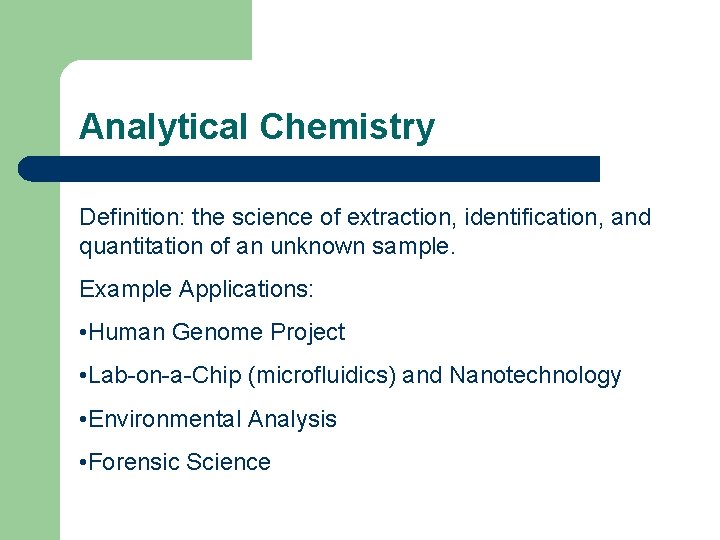
Analytical Chemistry Definition: the science of extraction, identification, and quantitation of an unknown sample. Example Applications: • Human Genome Project • Lab-on-a-Chip (microfluidics) and Nanotechnology • Environmental Analysis • Forensic Science

Course Philosophy l l l develop good lab habits and technique background in classical “wet chemical” methods (titrations, gravimetric analysis, electrochemical techniques) Quantitation using instrumentation (UV-Vis, AAS, GC)

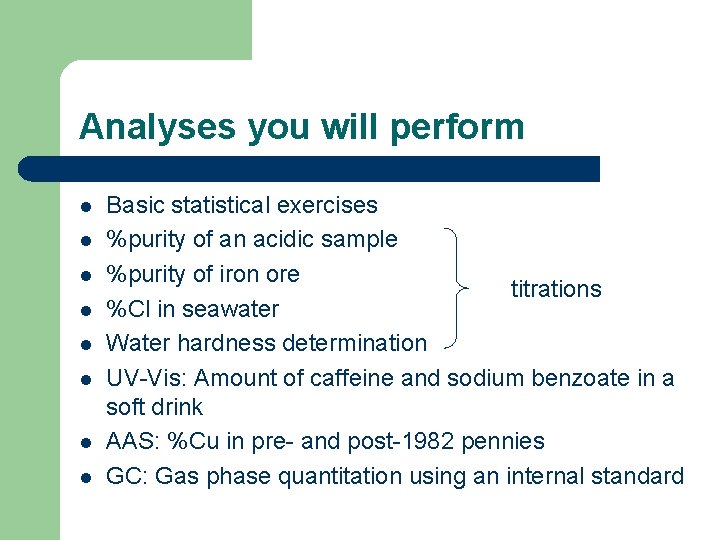
Analyses you will perform l l l l Basic statistical exercises %purity of an acidic sample %purity of iron ore titrations %Cl in seawater Water hardness determination UV-Vis: Amount of caffeine and sodium benzoate in a soft drink AAS: %Cu in pre- and post-1982 pennies GC: Gas phase quantitation using an internal standard

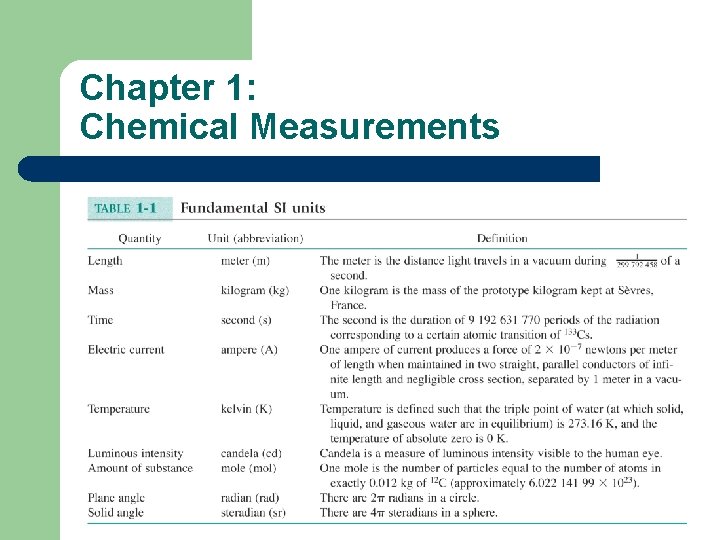
Chapter 1: Chemical Measurements



Example, p. 15: convert 0. 27 p. C to electrons

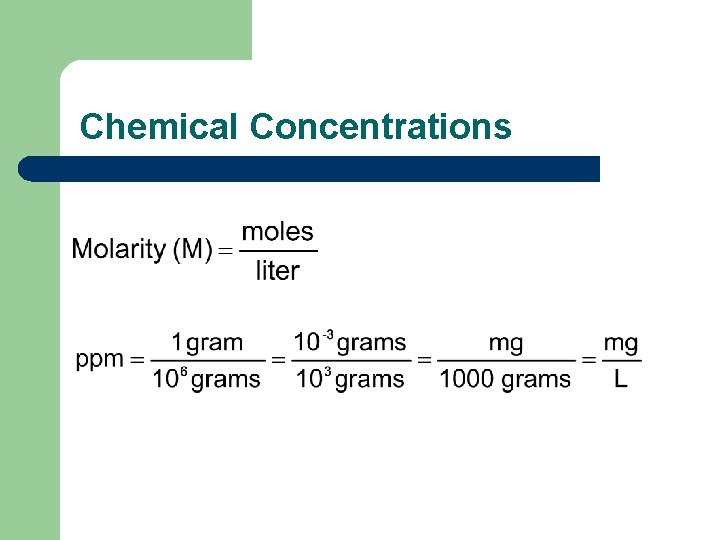
Chemical Concentrations

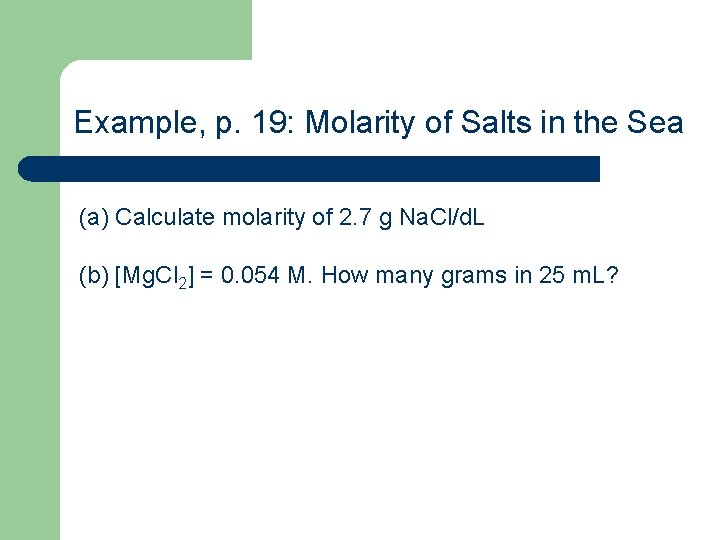
Example, p. 19: Molarity of Salts in the Sea (a) Calculate molarity of 2. 7 g Na. Cl/d. L (b) [Mg. Cl 2] = 0. 054 M. How many grams in 25 m. L?

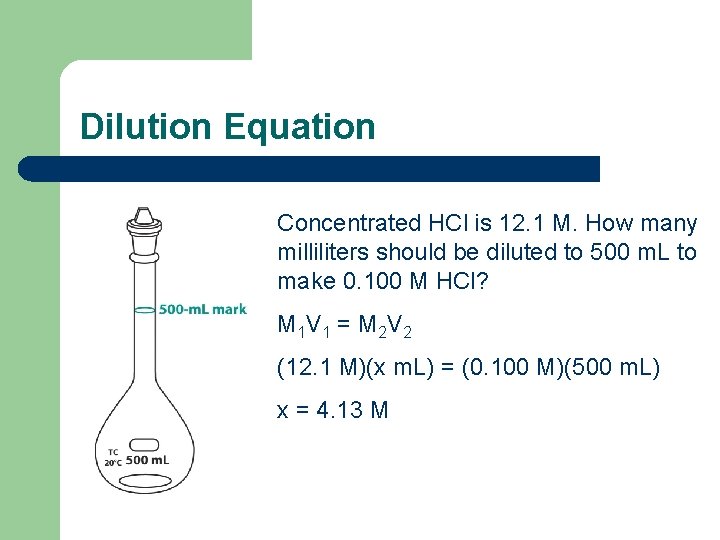
Dilution Equation Concentrated HCl is 12. 1 M. How many milliliters should be diluted to 500 m. L to make 0. 100 M HCl? M 1 V 1 = M 2 V 2 (12. 1 M)(x m. L) = (0. 100 M)(500 m. L) x = 4. 13 M

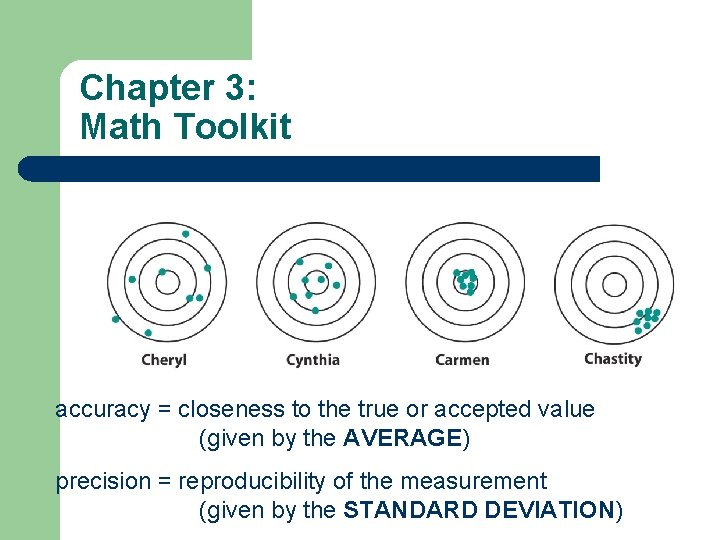
Chapter 3: Math Toolkit accuracy = closeness to the true or accepted value (given by the AVERAGE) precision = reproducibility of the measurement (given by the STANDARD DEVIATION)

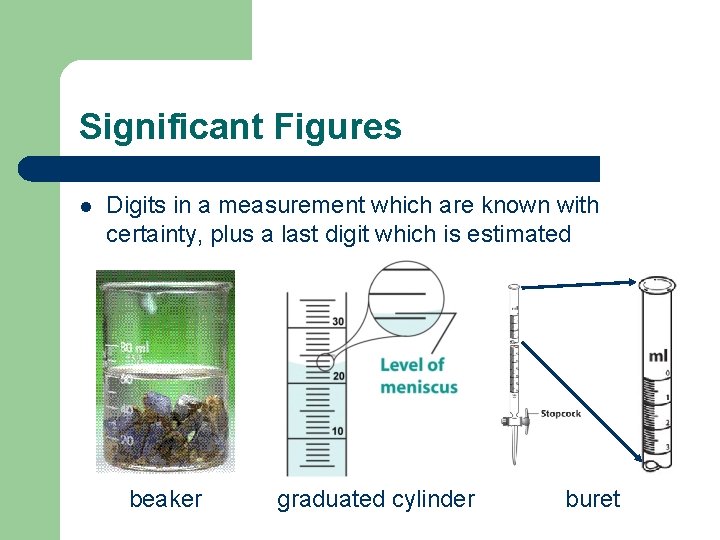
Significant Figures l Digits in a measurement which are known with certainty, plus a last digit which is estimated beaker graduated cylinder buret

Rules for Determining How Many Significant Figures There are in a Number · · · All nonzero digits are significant (4. 006, 12. 012, 10. 070) Interior zeros are significant (4. 006, 12. 012, 10. 070) Trailing zeros FOLLOWING a decimal point are significant (10. 070) Trailing zeros PRECEEDING an assumed decimal point may or may not be significant Leading zeros are not significant. They simply locate the decimal point (0. 00002)

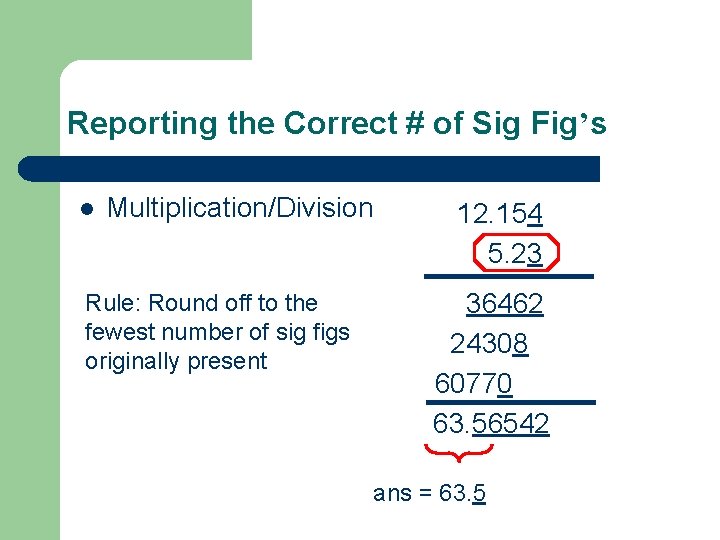
Reporting the Correct # of Sig Fig’s l Multiplication/Division Rule: Round off to the fewest number of sig figs originally present 12. 154 5. 23 36462 24308 60770 63. 56542 ans = 63. 5

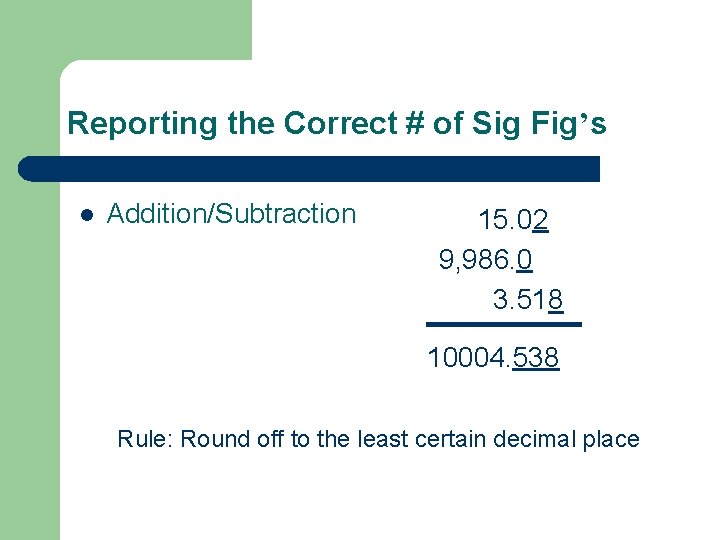
Reporting the Correct # of Sig Fig’s l Addition/Subtraction 15. 02 9, 986. 0 3. 518 10004. 538 Rule: Round off to the least certain decimal place

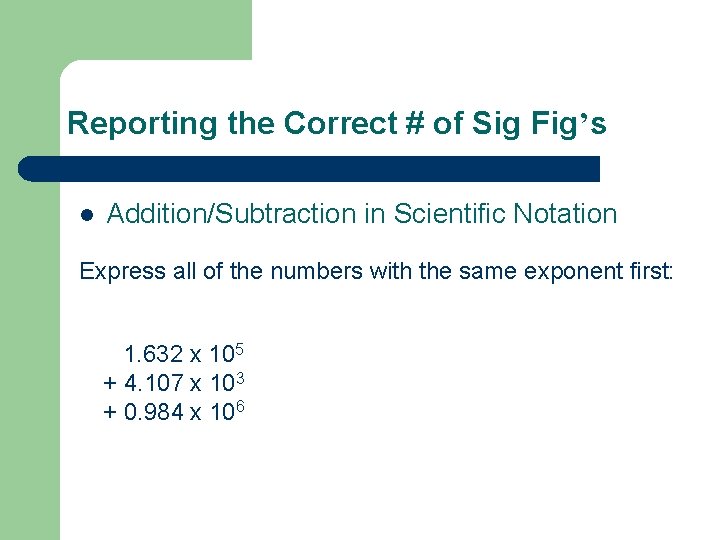
Reporting the Correct # of Sig Fig’s l Addition/Subtraction in Scientific Notation Express all of the numbers with the same exponent first: 1. 632 x 105 + 4. 107 x 103 + 0. 984 x 106

Reporting the Correct # of Sig Fig’s l Logs and anti-logs

Rounding Off Rules l l l digit to be dropped > 5, round UP 158. 7 = 159 digit to be dropped < 5, round DOWN 158. 4 = 158 digit to be dropped = 5, make answer EVEN 158. 5 = 158. 0 l BUT 157. 5 = 158. 0 158. 501 = 159. 000

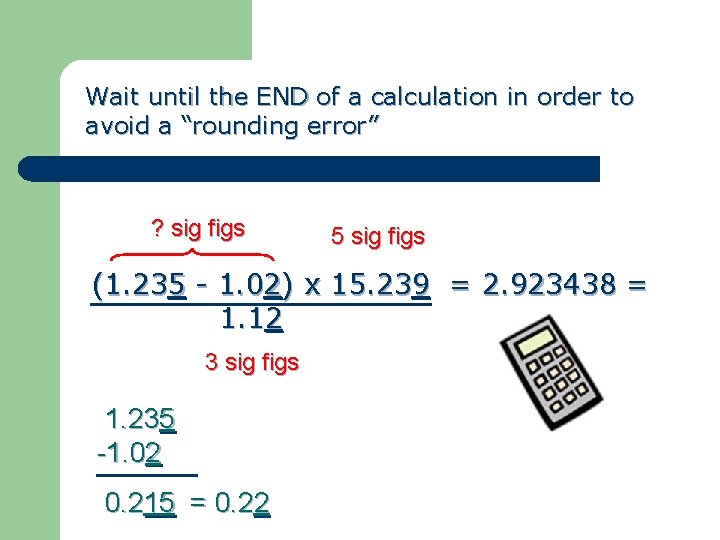
Wait until the END of a calculation in order to avoid a “rounding error” ? sig figs 5 sig figs (1. 235 - 1. 02) x 15. 239 = 2. 923438 = 1. 12 3 sig figs 1. 235 -1. 02 0. 215 = 0. 22

Propagation of Errors A way to keep track of the error in a calculation based on the errors of the variables used in the calculation error in variable x 1 = e 1 = "standard deviation" (see Ch 4) e. g. 43. 27 0. 12 m. L percent relative error = %e 1 = e 1*100 x 1 e. g. 0. 12*100/43. 27 = 0. 28%

Addition & Subtraction Suppose you're adding three volumes together and you want to know what the total error (et) is: 43. 27 0. 12 42. 98 0. 22 43. 06 0. 15 129. 31 et

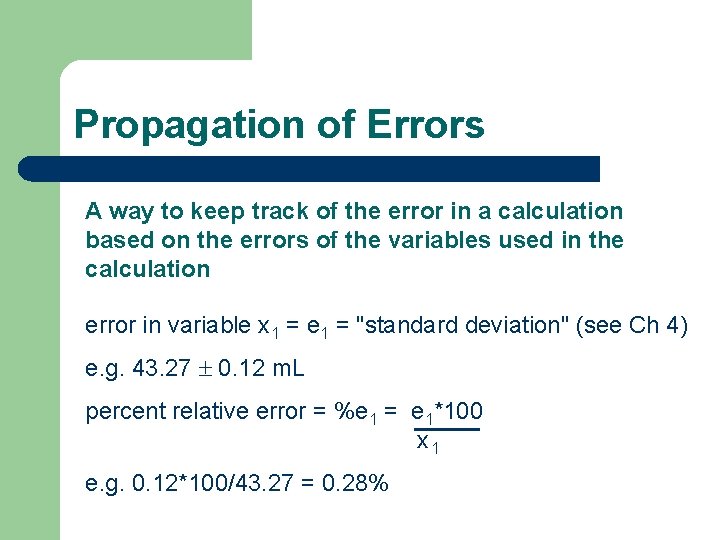
Multplication & Division

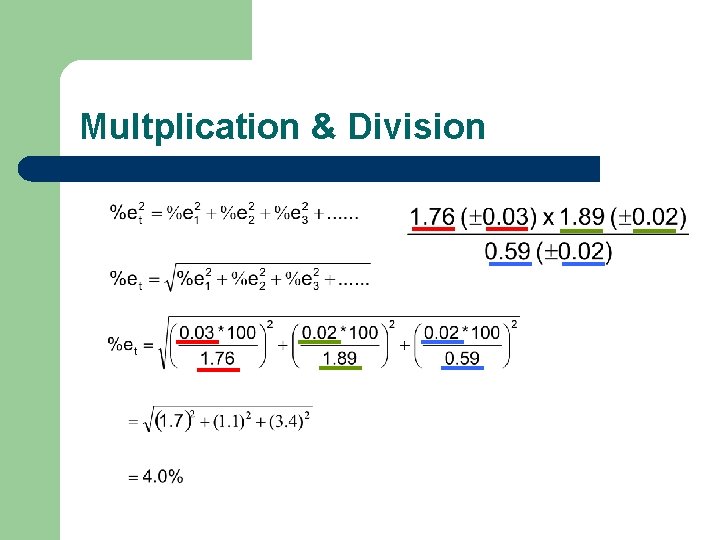
Combined Example

Chapter 4: Statistics

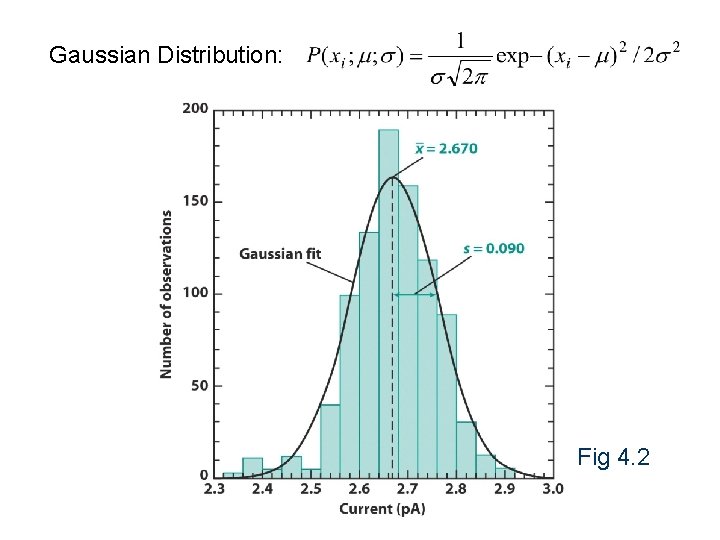
Gaussian Distribution: Fig 4. 2

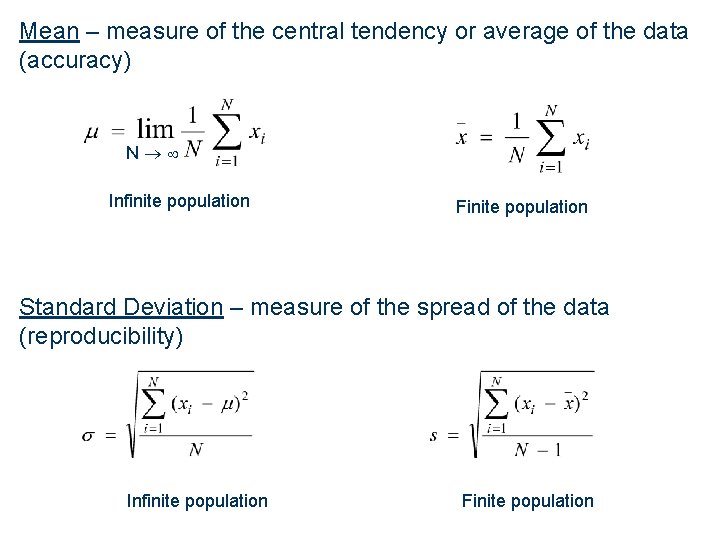
Mean – measure of the central tendency or average of the data (accuracy) N Infinite population Finite population Standard Deviation – measure of the spread of the data (reproducibility) Infinite population Finite population

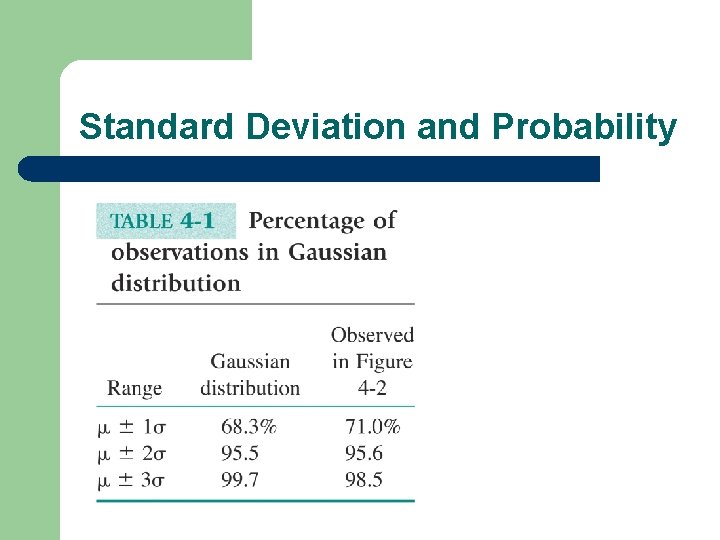
Standard Deviation and Probability

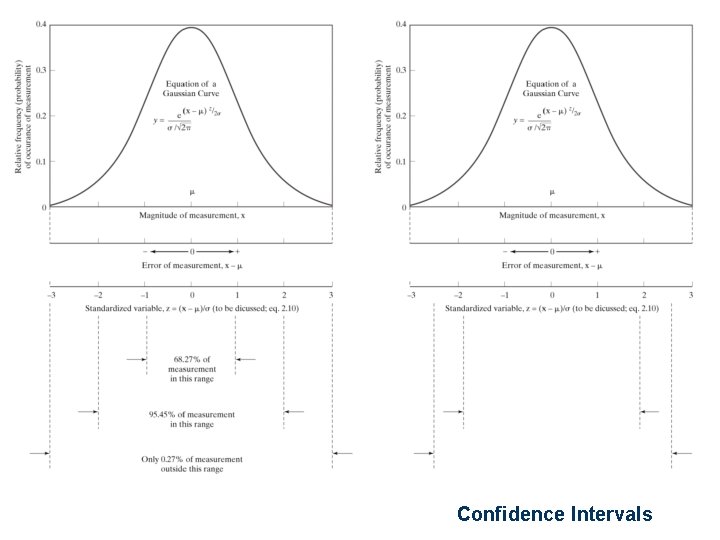
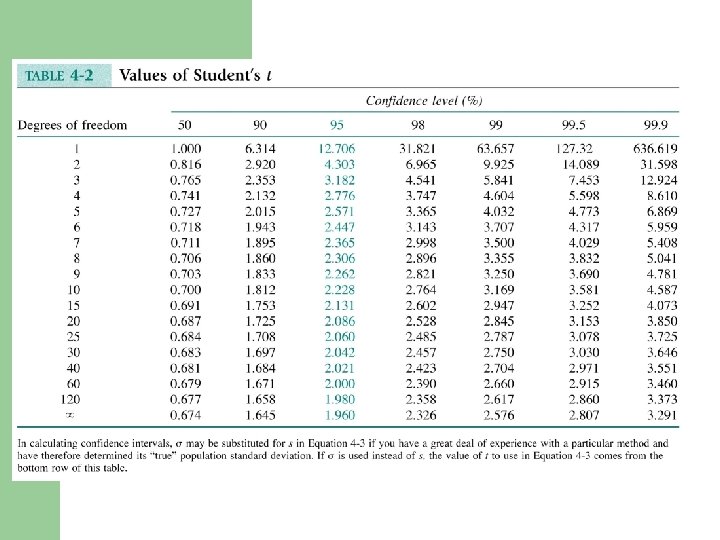
Confidence Intervals

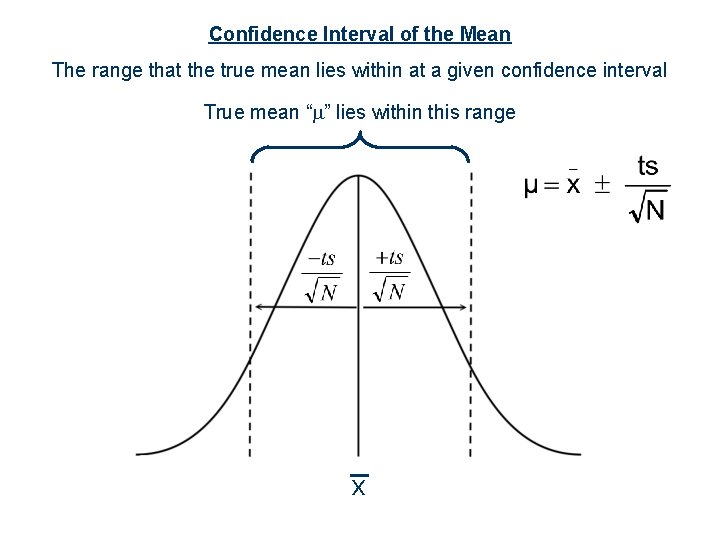
Confidence Interval of the Mean The range that the true mean lies within at a given confidence interval True mean “ ” lies within this range x


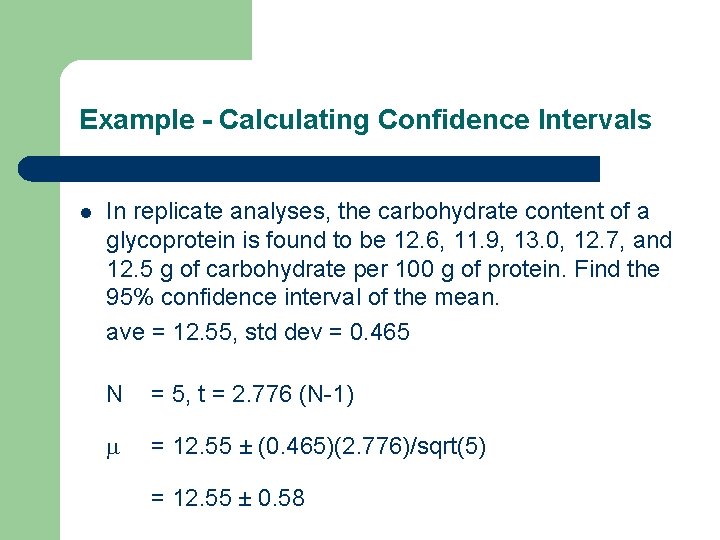
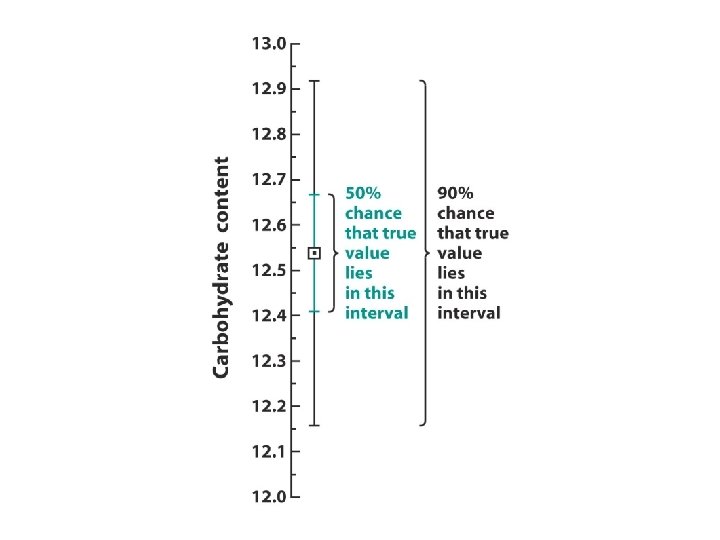
Example - Calculating Confidence Intervals l In replicate analyses, the carbohydrate content of a glycoprotein is found to be 12. 6, 11. 9, 13. 0, 12. 7, and 12. 5 g of carbohydrate per 100 g of protein. Find the 95% confidence interval of the mean. ave = 12. 55, std dev = 0. 465 N = 5, t = 2. 776 (N-1) = 12. 55 ± (0. 465)(2. 776)/sqrt(5) = 12. 55 ± 0. 58


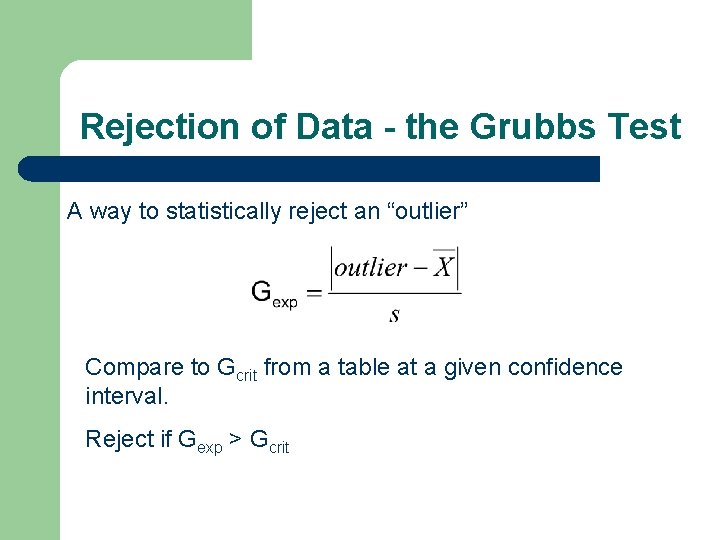
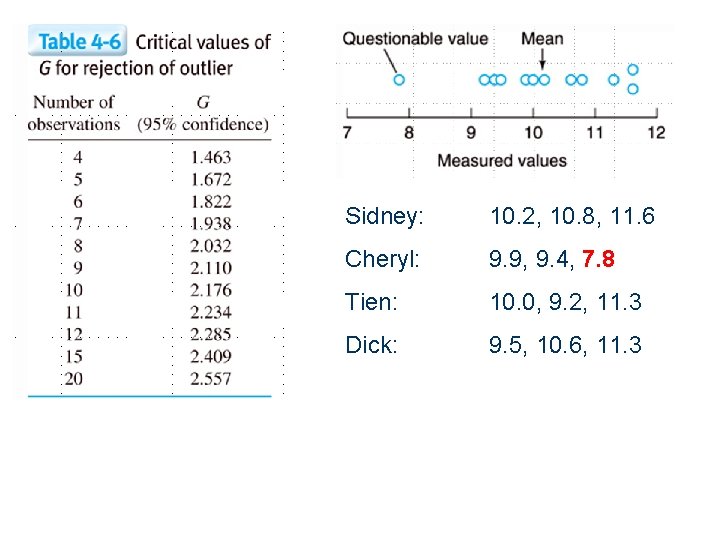
Rejection of Data - the Grubbs Test A way to statistically reject an “outlier” Compare to Gcrit from a table at a given confidence interval. Reject if Gexp > Gcrit

Sidney: 10. 2, 10. 8, 11. 6 Cheryl: 9. 9, 9. 4, 7. 8 Tien: 10. 0, 9. 2, 11. 3 Dick: 9. 5, 10. 6, 11. 3

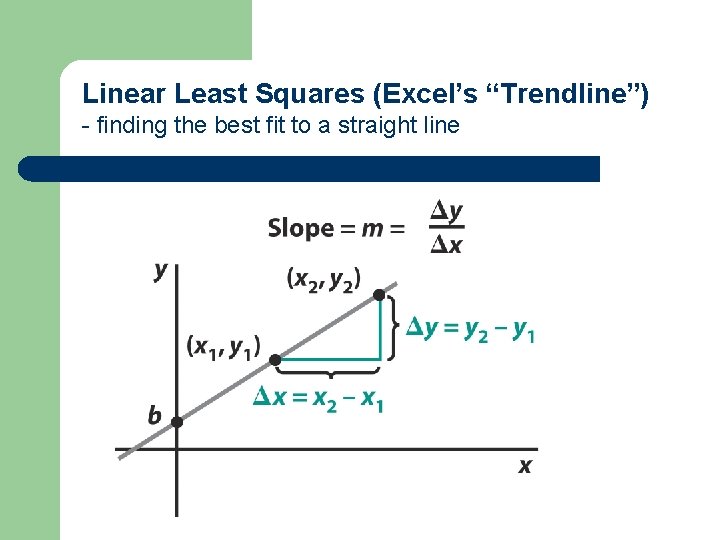
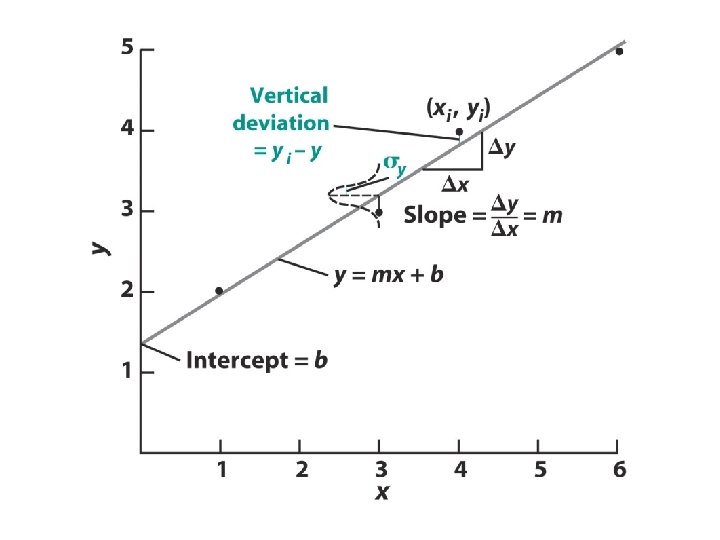
Linear Least Squares (Excel’s “Trendline”) - finding the best fit to a straight line

 Analysis
Analysis Redox reaction in alkaline medium
Redox reaction in alkaline medium What is analytical chemistry definition
What is analytical chemistry definition Presumptive identification vs positive identification
Presumptive identification vs positive identification Error in analytical chemistry
Error in analytical chemistry Analytical chemistry statistics
Analytical chemistry statistics Macrobalance analytical balance
Macrobalance analytical balance Analytical measurement range
Analytical measurement range Normal error curve in analytical chemistry
Normal error curve in analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Q test in analytical chemistry
Q test in analytical chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Introduction to analytical chemistry
Introduction to analytical chemistry Analytical chemistry
Analytical chemistry Excellence in analytical chemistry
Excellence in analytical chemistry What are the applications of gravimetric analysis
What are the applications of gravimetric analysis My favorite subject is science แปลว่า
My favorite subject is science แปลว่า Vet science parasite id
Vet science parasite id Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Difference between infusion and decoction
Difference between infusion and decoction Ventouse extraction
Ventouse extraction Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5