Treatment of acute rejection of the renal allograft


































![ØIn patients with evidence of microvascular inflammation on biopsy (ie, Banff glomerulitis score [g] ØIn patients with evidence of microvascular inflammation on biopsy (ie, Banff glomerulitis score [g]](https://slidetodoc.com/presentation_image_h2/0ea14e873dce0ac514d3949387eae525/image-46.jpg)













- Slides: 65

Treatment of acute rejection of the renal allograft DR. SEYED SADRADDIN RASI HASHEMI

INTRODUCTION

Acute renal allograft rejection is a major cause of allograft dysfunction. Some kidneys do not regain function even with maximal antirejection therapy.

Even among patients who recover, acute rejection episodes can have a negative impact on long-term graft survival. Acute rejection is a major predictor of interstitial fibrosis/tubular atrophy (IF/TA), formerly called chronic allograft nephropathy, which is responsible for most deathcensored graft loss after the first year posttransplant.

There has been a dramatic reduction in the incidence of acute rejection due to the introduction of potent immunosuppressive drugs in the past three decades.

DEFINITIONS

• Acute renal allograft rejection is defined as an acute deterioration in allograft function associated with specific pathologic changes in the graft. • There are two principal histologic forms of acute rejection:

ØThere are two principal histologic forms of acute rejection: • Acute T cell-mediated (cellular) rejection (TCMR), which is characterized by lymphocytic infiltration of the tubules, interstitium, and, in some cases, the arterial intima. • Active (acute) antibody-mediated rejection (ABMR), the diagnosis of which requires morphologic evidence of acute tissue injury, evidence of circulating donor-specific alloantibodies, and evidence of antibody-endothelial cell interaction (such as C 4 d deposition in the allograft).

ABMR and acute TCMR may coexist at the same time in the allograft.

Acute T cellmediated (cellular) rejection (TCMR)

INTRODUCTION

The use of potent immunosuppressive agents as part of induction and maintenance therapy for kidney transplantation has significantly reduced the incidence of acute rejection to approximately 8 percent at most transplant centers

• A renal allograft biopsy is required to establish the diagnosis and determine the severity of rejection in order to determine the most appropriate approach to therapy. • TCMR and ABMR may also coexist at the same time in the renal allograft (ie, mixed acute rejection). The presence of histologic evidence of acute rejection on biopsy without an elevation in the serum creatinine concentration is known as subclinical rejection.

• Acute TCMR occurs most commonly within the first year after transplantation and rarely occurs after five years posttransplan.

TREATMENT

— The treatment approach in patients with histologic evidence of acute TCMR is guided predominantly by the histopathologic severity of rejection (algorithm 1).

Initial treatment of acute T cellmediated rejection of the renal allograft

ØIn patients with borderline TCMR, there is no consensus regarding optimal management. Some transplant centers do not administer specific treatment for rejection but augment maintenance immunosuppression by targeting higher tacrolimus levels (ie, 5 to 7 ng/m. L) in patients whose tacrolimus trough levels are in the lower range (ie, 3 to 5 ng/m. L) and/or by optimizing mycophenolate dosing.

ØIn most patients with Banff grade I rejection, we administer pulse high-dose intravenous( IV) glucocorticoids, followed by an oral glucocorticoid taper. • In addition, we augment maintenance immunosuppression. • In patients with Banff grade IB rejection and few or no chronic histologic lesions who present less than one year posttransplant, we give rabbit antithymocyte globulin (r. ATG) -Thymoglobulin in addition to pulse glucocorticoids.

Banff grade I rejection ØIn patients with biopsy-proven Banff grade IA or IB TCMR (with no evidence of ABMR), we typically advocate inpatient admission for management. • We administer pulse IV methylprednisolone at 3 to 5 mg/kg daily for three to five doses, with a maximum daily dose of 500 mg. • After a glucocorticoid pulse, oral glucocorticoids are tapered immediately to the maintenance dose of oral prednisone the patient had been taking prior to the episode.

• If there are no concerns for nonadherence, we augment the maintenance prednisone dose. • As an example, if the rejection occurred while the patient was taking 5 mg/day, we would increase the maintenance prednisone to 10 mg/day.

In patients with Banff grade IB TCMR and few or no chronic histologic lesions who present less than one year posttransplant, we give r. ATG-Thymoglobulin in addition to pulse glucocorticoids at 1. 5 to 3 mg/kg per dose over one to three days for a total dose of 3 to 6 mg/kg in the first year.

Banff grade II or III rejection ØIn patients with biopsy-proven Banff grade IIA, IIB, or III TCMR, we typically advocate inpatient admission for management. We administer pulse IV methylprednisolone 3 to 5 mg/kg daily for three to five doses (with a maximum daily dose of 500 mg), followed by a short tapered dose of oral prednisone.

• A typical glucocorticoid taper would be to start with prednisone 40 mg daily and to reduce the daily dose by 10 mg every five days until a dose of 10 mg daily is reached, after which the daily dose is reduced to 5 mg daily after another five days.

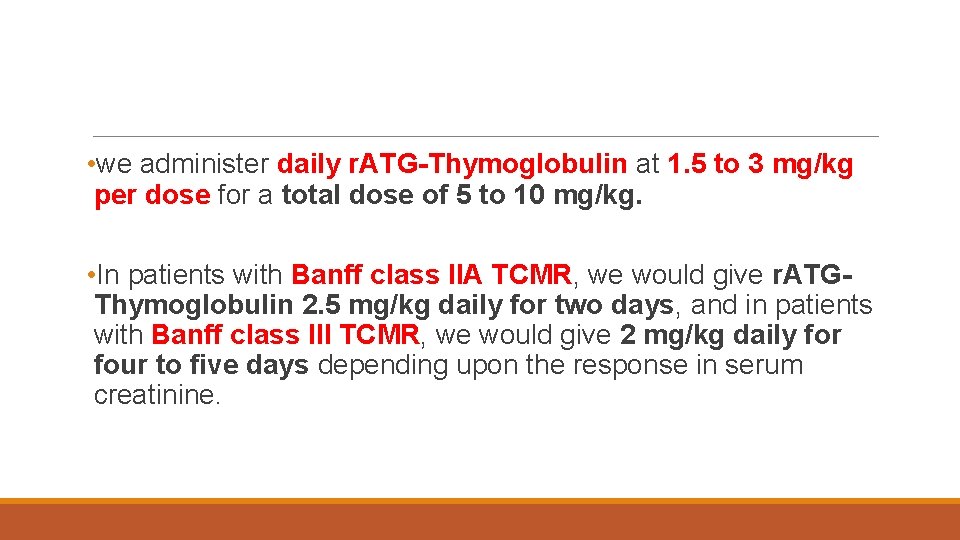
• we administer daily r. ATG-Thymoglobulin at 1. 5 to 3 mg/kg per dose for a total dose of 5 to 10 mg/kg. • In patients with Banff class IIA TCMR, we would give r. ATGThymoglobulin 2. 5 mg/kg daily for two days, and in patients with Banff class III TCMR, we would give 2 mg/kg daily for four to five days depending upon the response in serum creatinine.

In patients who cannot receive r. ATG-Thymoglobulin, we give alemtuzumab as a single IV dose of 30 mg.

Such patients include those with a known history of allergic reaction to r. ATG-Thymoglobulin (such as during induction therapy or during previous treatment of rejection) or those with a white blood cell count less than 2000/micro. L or a platelet coun tless than 75, 000/micro. L.

We also give alemtuzumab, rather than r. ATG-Thymoglobulin, to patients who have a previous history of significant rabbit exposure (ie, history of having raised or ingested rabbits) and, therefore, may be at risk for developing serum sickness after treatment with r. ATG-Thymoglobulin.

In all patients who are treated with r. ATG-Thymoglobulin (or alemtuzumab) and highdose glucocorticoids, we recommence antimicrobial and antiviral prophylaxis for at least three months with a regimen that is identical to that administered in the immediate posttransplant period.

Antimicrobial and Antiviral prophylaxis This includes prophylaxis against Pneumocystis pneumonia (PCP), cytomegalovirus (CMV) infection and disease, and herpes simplex infection (in patients who are at low-CMV risk). In addition, we also administer antifungal prophylaxis.

Active (acute) antibody-mediated rejection (ABMR)

INTRODUCTION

ØAntibody-mediated rejection (ABMR) is the most common cause of allograft failure after kidney transplantation.

The revised Banff 2017 classification of ABMR defines active (previously called acute) and chronic active ABMR as conditions in which histologic evidence of acute and chronic injury is associated with evidence of current/recent antibody interaction with vascular endothelium and serologic evidence of donor-specific antibodies (DSA) to human leukocyte antigen (HLA) or non-HLA antigens.

TREATMENT

ØThe primary goal of treating ABMR is to remove existing donor-specific antibodies (DSAs) and to eradicate the clonal population of B cells or plasma cells that is responsible for their production. • In general, we treat all patients who have evidence of active ABMR on biopsy.

we would treat patients who are discovered to have subclinical ABMR by surveillance biopsy. In addition, we treat patients with C 4 d-negative ABMR with the same approach that we use in patients with C 4 d-positive ABMR.

Approach to initial therapy Our recommendations for the treatment of ABMR are primarily based upon Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines and the 2019 Transplantation Society Working Group Expert Consensus.

• We typically advocate inpatient admission for patients because of the complexity of the treatment regimen. Our approach to the initial treatment of active ABMR depends upon the timing of the diagnosis of ABMR (algorithm 1)

Initial treatment of active antibodymediated rejection of the renal allograft

Within the first year posttransplant In patients who are diagnosed with active ABMR within the first year posttransplant, we treat with a combination of glucocorticoids, plasmapheresis and intravenous (IV) immune globulin (IVIG), and, in some patients, rituximab as follows:

ØWe give IV methylprednisolone at a dose of 300 to 500 mg daily for three to five days, followed by a rapid oral prednisone taper to the patient's previous maintenance dose of prednisone.

ØPlasmapheresis is performed daily or every other day for a maximum of six sessions or until the serum creatinine is within 20 to 30 percent of the baseline. • The initial treatment is typically a one-and-one-half-volume exchange with albumin, and subsequent treatments are a one-volume exchange with albumin.

• We prefer an every-other-day plasmapheresis schedule as albumin alone can often be administered for replacement with interval recovery of the prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen to acceptable levels without the need to administer fresh frozen plasma. • This avoids the risk of antigen sensitization; however, one to two units of fresh frozen plasma may be used for replacement at the end of a plasmapheresis treatment if indicated

ØWe administer IVIG at a dose of 100 mg/kg after each session of plasmapheresis. We typically give 500 mg/kg per day for one to two days after the final session of plasmapheresis, with a total cumulative target dose of at least 1000 mg/kg of IVIG.
![ØIn patients with evidence of microvascular inflammation on biopsy ie Banff glomerulitis score g ØIn patients with evidence of microvascular inflammation on biopsy (ie, Banff glomerulitis score [g]](https://slidetodoc.com/presentation_image_h2/0ea14e873dce0ac514d3949387eae525/image-46.jpg)
ØIn patients with evidence of microvascular inflammation on biopsy (ie, Banff glomerulitis score [g] + peritubular capillary score [ptc] >0), we administer rituximab as a single dose of 200 to 375 mg/m 2 after completion of plasmapheresis and IVIG.

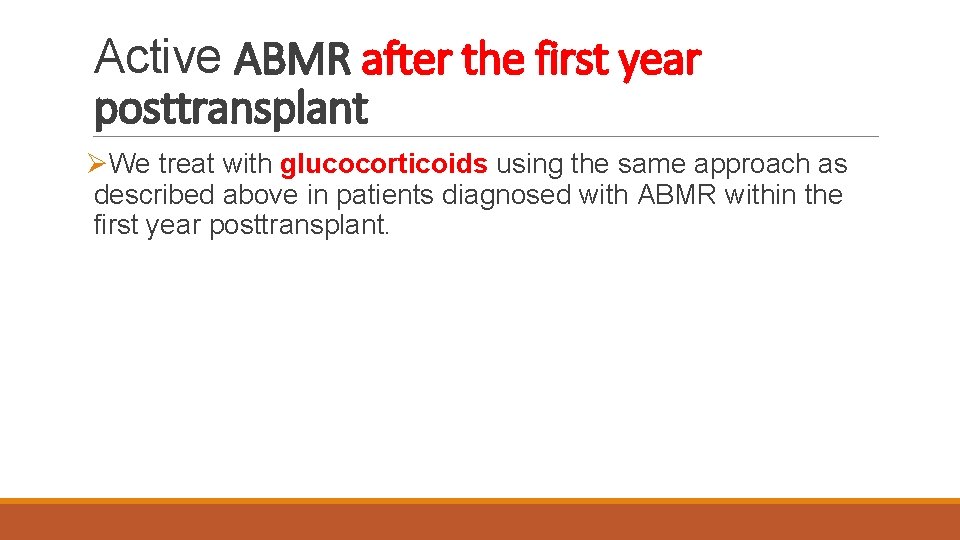
Active ABMR after the first year posttransplant ØWe treat with glucocorticoids using the same approach as described above in patients diagnosed with ABMR within the first year posttransplant.

Øwe do not perform plasmapheresis in such patients because of the lack of evidence supporting the safety and efficacy of plasmapheresis in late-onset ABMR.

ØWe administer IVIG at a dose of 200 mg/kg every two weeks for three doses.

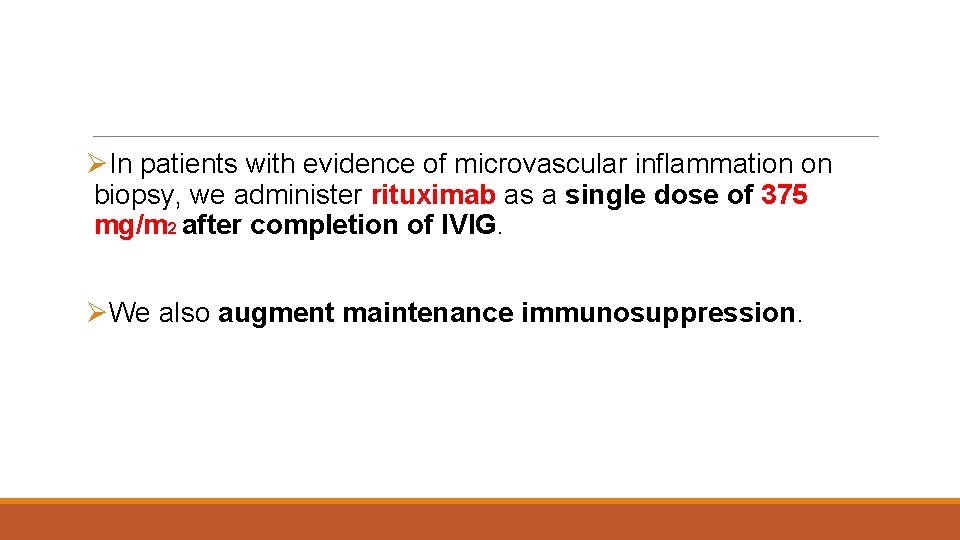
ØIn patients with evidence of microvascular inflammation on biopsy, we administer rituximab as a single dose of 375 mg/m 2 after completion of IVIG. ØWe also augment maintenance immunosuppression.

ØWe do not routinely use immunoadsorption, bortezomib, tocilizumab, eculizumab, or splenectomy in the initial treatment of patients with ABMR. • However, some of these therapies can be considered in patients who do not respond to initial treatment.

Antimicrobial and Antiviral prophylaxis ØIn all patients who are treated for active ABMR, we recommence antimicrobial and antiviral prophylaxis with a regimen that is identical to that administered in the immediate posttransplant period.

This includes prophylaxis against Pneumocystis pneumonia (PCP), cytomegalovirus (CMV) infection and disease, and herpes simplex infection (in patients who are at low CMV risk) for three months. In addition, we also administer antifungal prophylaxis and a prophylactic histamine-2 (H 2) blocker for prevention of peptic ulcer disease.

Monitoring the response to therapy

ØPatients are considered to have a successful reversal of ABMR if they meet all of the following parameters within three months of treatment: • Decrease in serum creatinine to within 20 to 30 percent of the baseline level • Decrease in proteinuria to the baseline level • Decrease in immunodominant DSA by >50 percent • Resolution of changes associated with ABMR on repeat kidney biopsy

Second-line agents in patients who have failed initial therapy

Most patients with active ABMR will respond to a combination of glucocorticoids, plasmapheresis, IVIG, and rituximab. However, in patients who do not respond to initial treatment with this combination, the following agents can be considered as rescue therapy.

Bortezomib ØBortezomib is a potent, reversible proteasome inhibitor that has been approved by the US Food and Drug Administration (FDA) as first-line therapy for multiple myeloma since 2008. • Bortezomib reduces intracellular protein degradation by inhibiting proteasomal activity and results in apoptosis, mainly via inhibition of nuclear factor kappa-B (NFk. B)-induced survival signals.

• The drug is particularly effective against differentiated plasma cells because of the high rate of protein synthesis in these cells

• Several case reports/series have demonstrated the effectiveness of bortezomib in treating ABMR, successfully reversing acute rejection, and/or reducing DSAs. However, bortezomib-treated patients experienced higher rates of gastrointestinal and hematologic toxicity.

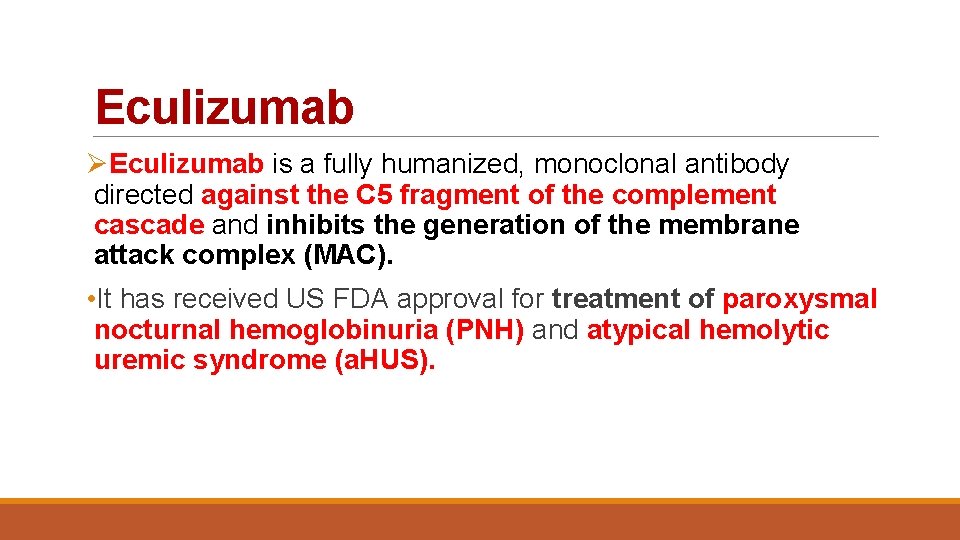
Eculizumab ØEculizumab is a fully humanized, monoclonal antibody directed against the C 5 fragment of the complement cascade and inhibits the generation of the membrane attack complex (MAC). • It has received US FDA approval for treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (a. HUS).

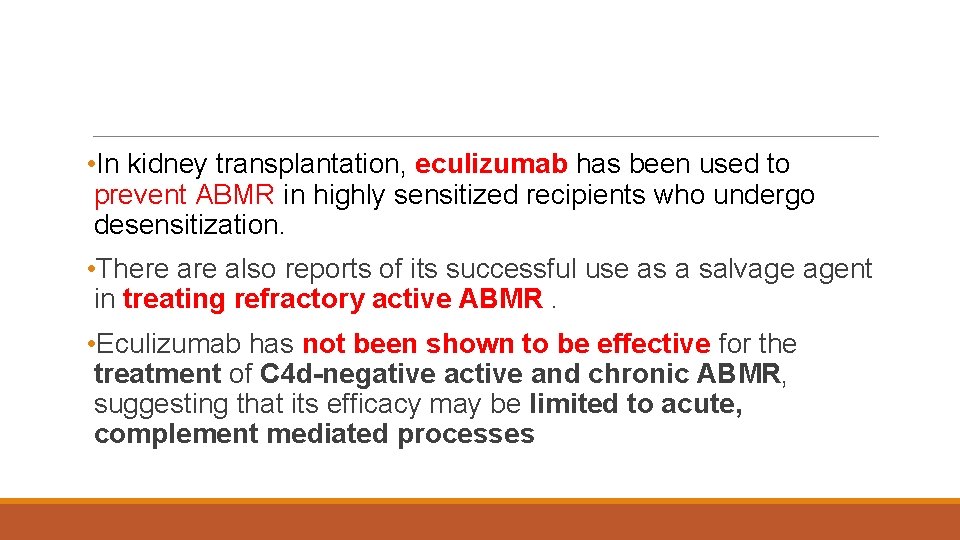
• In kidney transplantation, eculizumab has been used to prevent ABMR in highly sensitized recipients who undergo desensitization. • There also reports of its successful use as a salvage agent in treating refractory active ABMR. • Eculizumab has not been shown to be effective for the treatment of C 4 d-negative active and chronic ABMR, suggesting that its efficacy may be limited to acute, complement mediated processes

Less frequently used therapies

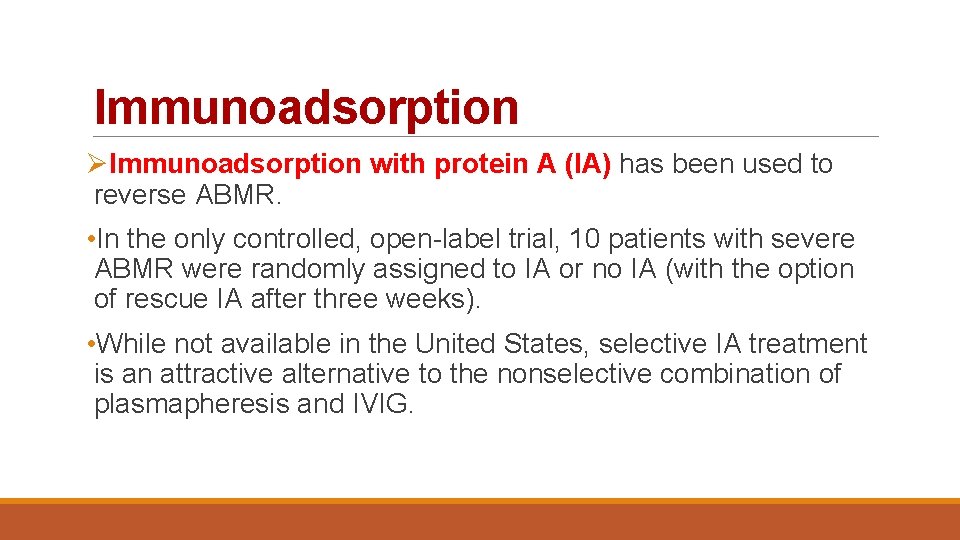
Immunoadsorption ØImmunoadsorption with protein A (IA) has been used to reverse ABMR. • In the only controlled, open-label trial, 10 patients with severe ABMR were randomly assigned to IA or no IA (with the option of rescue IA after three weeks). • While not available in the United States, selective IA treatment is an attractive alternative to the nonselective combination of plasmapheresis and IVIG.

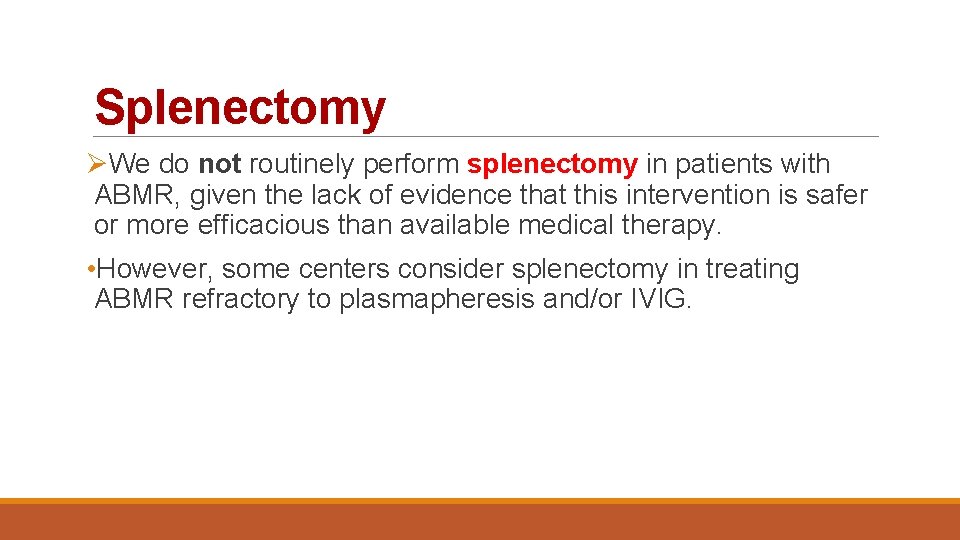
Splenectomy ØWe do not routinely perform splenectomy in patients with ABMR, given the lack of evidence that this intervention is safer or more efficacious than available medical therapy. • However, some centers consider splenectomy in treating ABMR refractory to plasmapheresis and/or IVIG.
 Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Res extra commercium
Res extra commercium Dfdba graft
Dfdba graft Autogenous vs allograft
Autogenous vs allograft Peritubular capillaries and vasa recta difference
Peritubular capillaries and vasa recta difference Treatments for acute renal failure
Treatments for acute renal failure Acute abdomen causes
Acute abdomen causes Acute gout attack treatment
Acute gout attack treatment Parietal pain
Parietal pain Pancreatitis film
Pancreatitis film Gallstone anatomy
Gallstone anatomy Chronic rejection
Chronic rejection Chronic rejection
Chronic rejection Test summary report template
Test summary report template Rejection ultrasound physics
Rejection ultrasound physics Chronic rejection
Chronic rejection Insulation coordination in high voltage engineering
Insulation coordination in high voltage engineering Paraprashing
Paraprashing Acceptance and rejection region
Acceptance and rejection region Rejection sensitive dysphoria
Rejection sensitive dysphoria Mc vs ac
Mc vs ac Sample rejection criteria
Sample rejection criteria Luke 4 rejection at nazareth
Luke 4 rejection at nazareth Rejection attachment
Rejection attachment Image frequency rejection ratio
Image frequency rejection ratio Chronic rejection
Chronic rejection Refifs
Refifs Dependency rejection
Dependency rejection Elisha goodman prayers for financial breakthrough
Elisha goodman prayers for financial breakthrough Sample rejection criteria
Sample rejection criteria Chronic rejection
Chronic rejection Cooling tower approach temperature
Cooling tower approach temperature Theories of fashion movement
Theories of fashion movement Are sugar gliders legal in maine
Are sugar gliders legal in maine Rejection revenge
Rejection revenge Viral load sample collection
Viral load sample collection Số nguyên tố là gì
Số nguyên tố là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tia chieu sa te
Tia chieu sa te Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Tư thế worm breton là gì
Tư thế worm breton là gì ưu thế lai là gì
ưu thế lai là gì Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Thẻ vin
Thẻ vin Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Hát lên người ơi
Hát lên người ơi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Phép trừ bù
Phép trừ bù Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Cong thức tính động năng
Cong thức tính động năng Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi