CMV Infection and Allograft Rejection Are we missing









- Slides: 47

CMV Infection and Allograft Rejection : Are we missing the point? Jeremy Chapman Westmead Hospital, Sydney



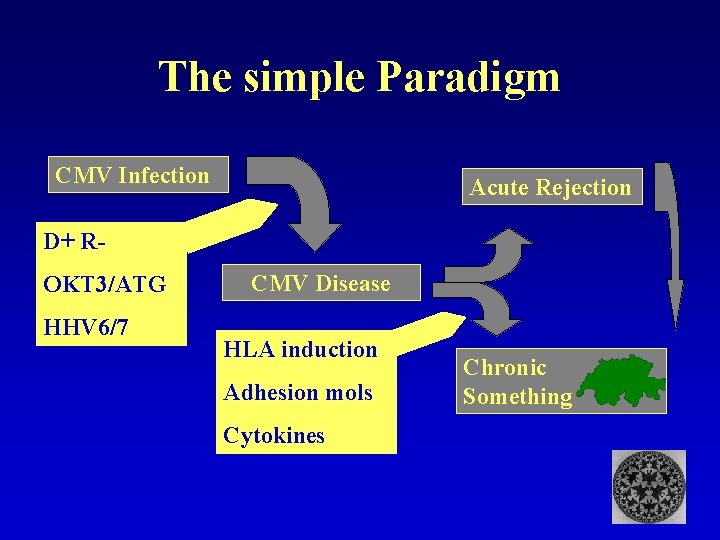
The simple Paradigm CMV Infection Acute Rejection D+ ROKT 3/ATG HHV 6/7 CMV Disease HLA induction Adhesion mols Cytokines Chronic Rejection Something

Evidence that the relationship with CMV may be more complex • • • Biology of CMV infection/disease Effects of CMV prophylaxis Relationships between CMV and rejection Effects of Biopsy identified Cellular infiltrate Mechanisms of damage Questions not answers

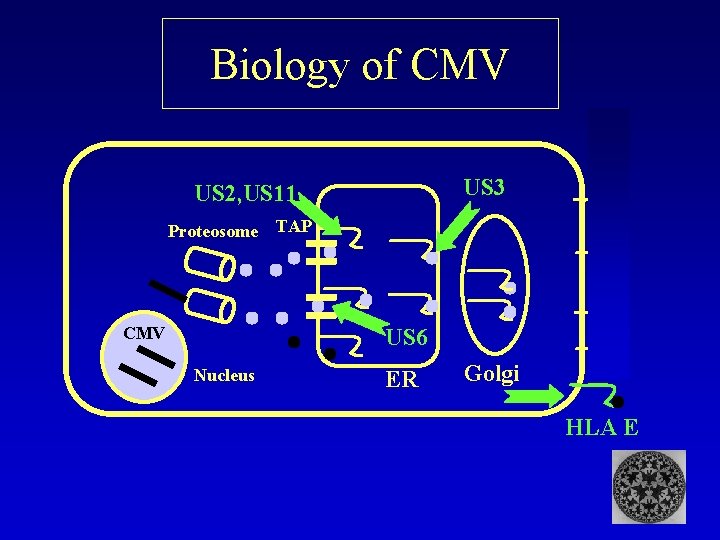
Biology of CMV US 3 US 2, US 11 Proteosome CMV TAP US 6 Nucleus ER Golgi HLA E

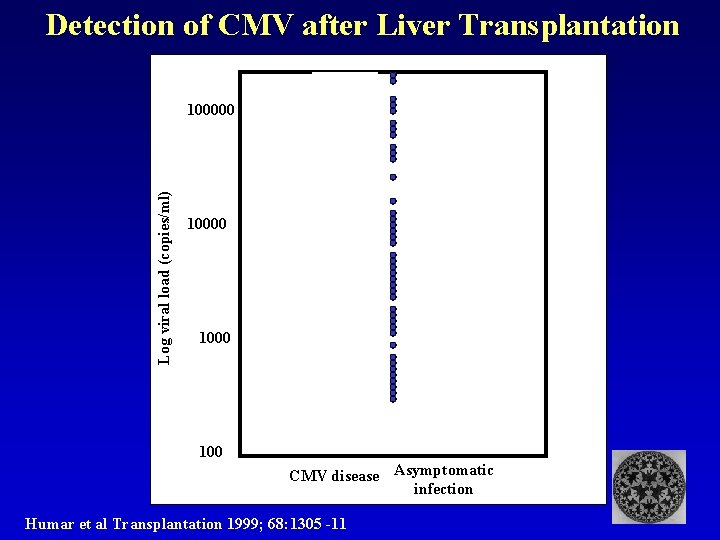
Detection of CMV after Liver Transplantation Log viral load (copies/ml) 100000 1000 100 CMV disease Asymptomatic infection Humar et al Transplantation 1999; 68: 1305 -11

Control of CMV Why isn’t the graft lost to CMV? SELF ALLO

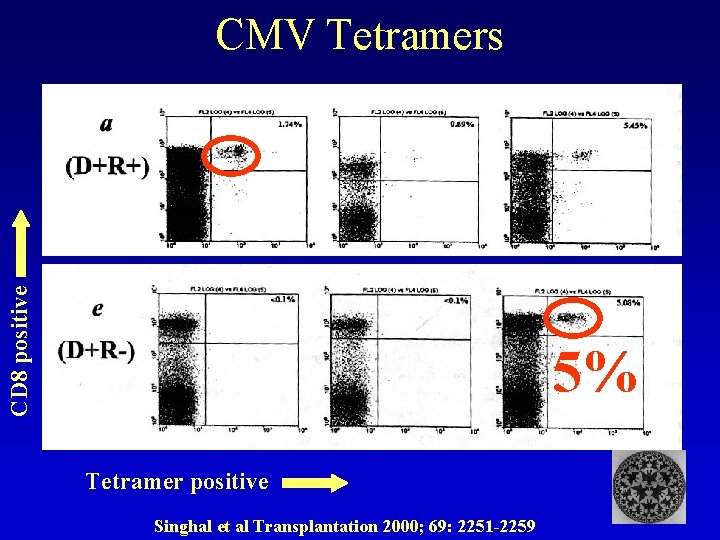
CD 8 positive CMV Tetramers 5% Tetramer positive Singhal et al Transplantation 2000; 69: 2251 -2259

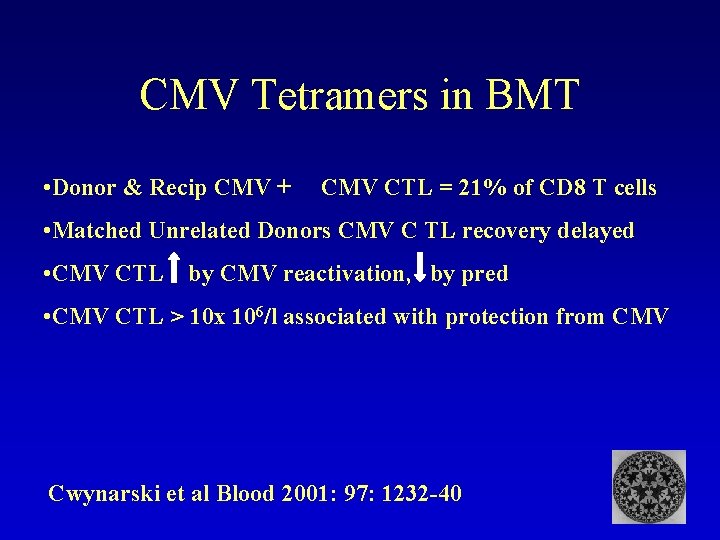
CMV Tetramers in BMT • Donor & Recip CMV + CMV CTL = 21% of CD 8 T cells • Matched Unrelated Donors CMV C TL recovery delayed • CMV CTL by CMV reactivation, by pred • CMV CTL > 10 x 106/l associated with protection from CMV Cwynarski et al Blood 2001: 97: 1232 -40

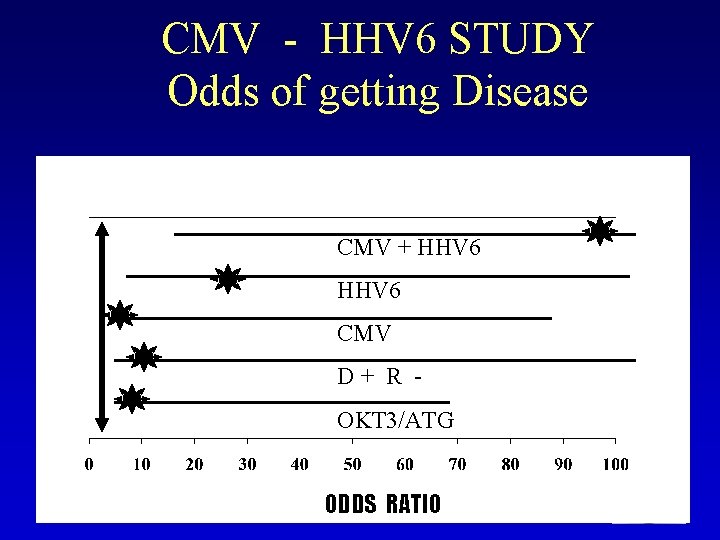
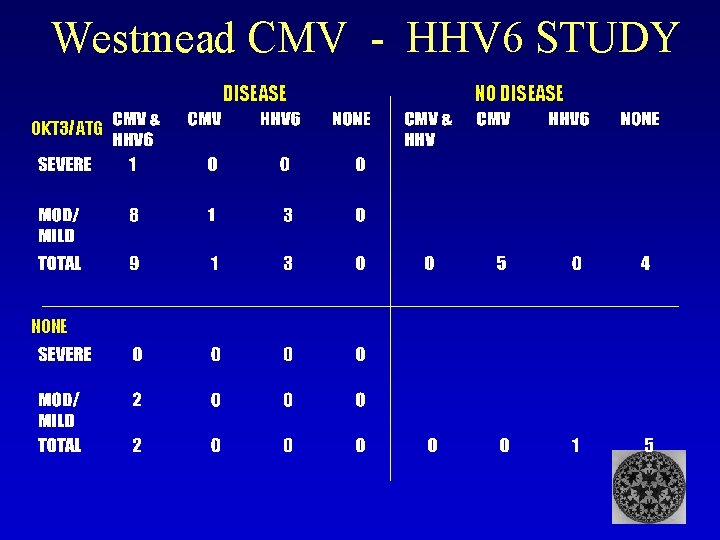
CMV - HHV 6 STUDY Odds of getting Disease CMV + HHV 6 CMV D+ R OKT 3/ATG ODDS RATIO

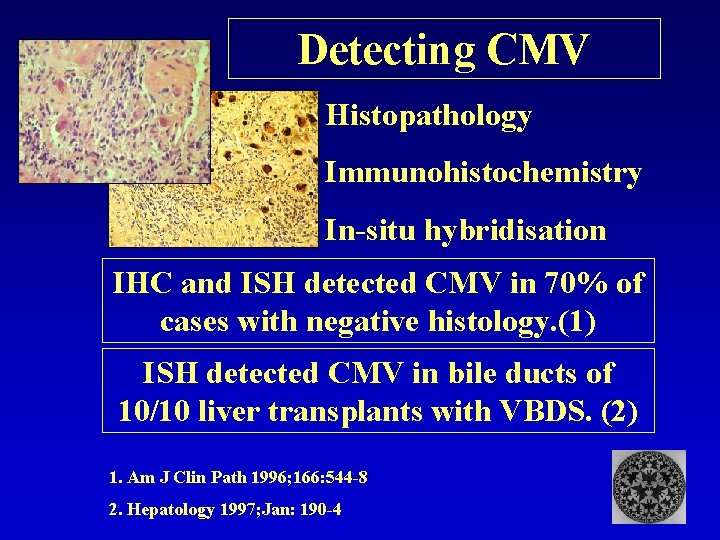
Detecting CMV Histopathology Immunohistochemistry In-situ hybridisation IHC and ISH detected CMV in 70% of cases with negative histology. (1) ISH detected CMV in bile ducts of 10/10 liver transplants with VBDS. (2) 1. Am J Clin Path 1996; 166: 544 -8 2. Hepatology 1997; Jan: 190 -4

Prevention of CMV disease after Transplantation: Effects on incidence of rejection Evidence from clinical trials

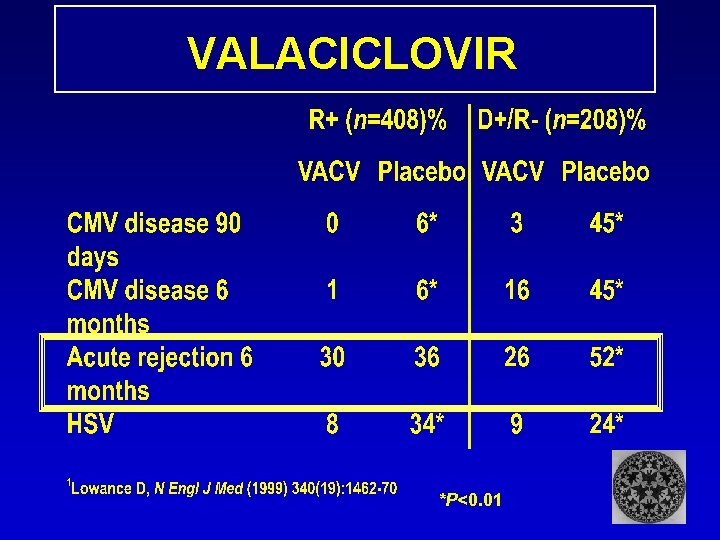
VALACICLOVIR *P<0. 01

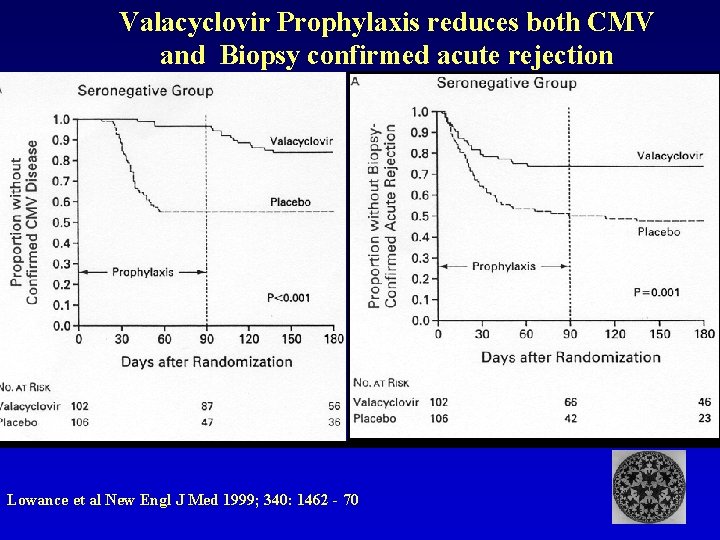
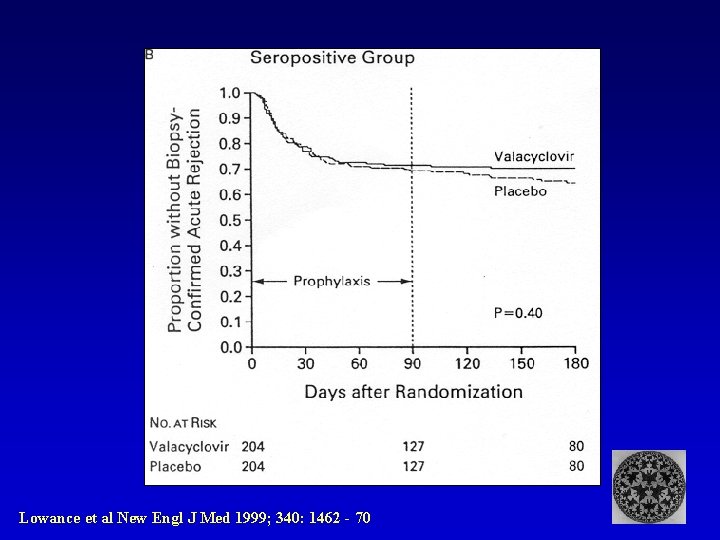
Valacyclovir Prophylaxis reduces both CMV and Biopsy confirmed acute rejection Lowance et al New Engl J Med 1999; 340: 1462 - 70

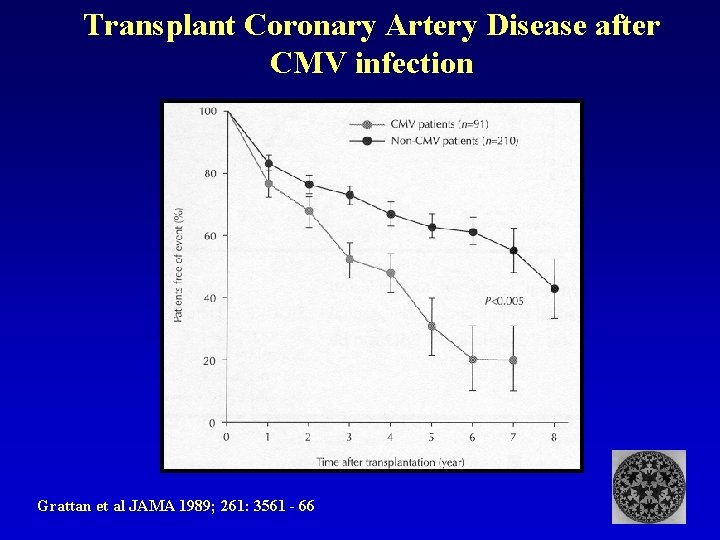
Transplant Coronary Artery Disease after CMV infection Grattan et al JAMA 1989; 261: 3561 - 66

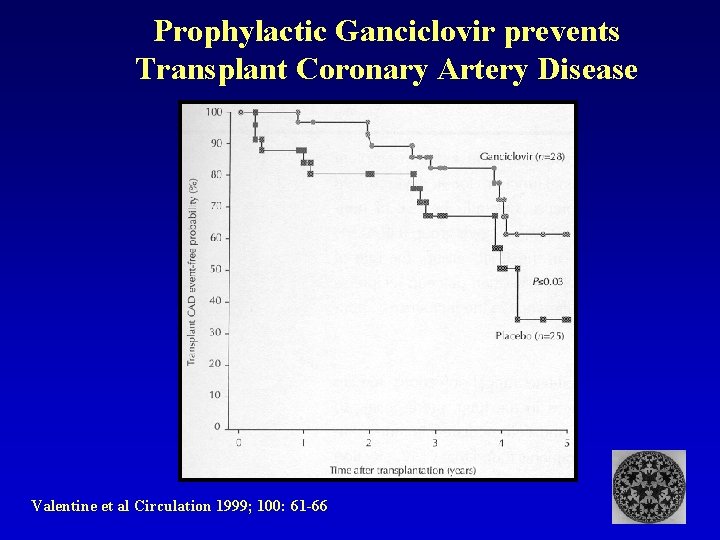
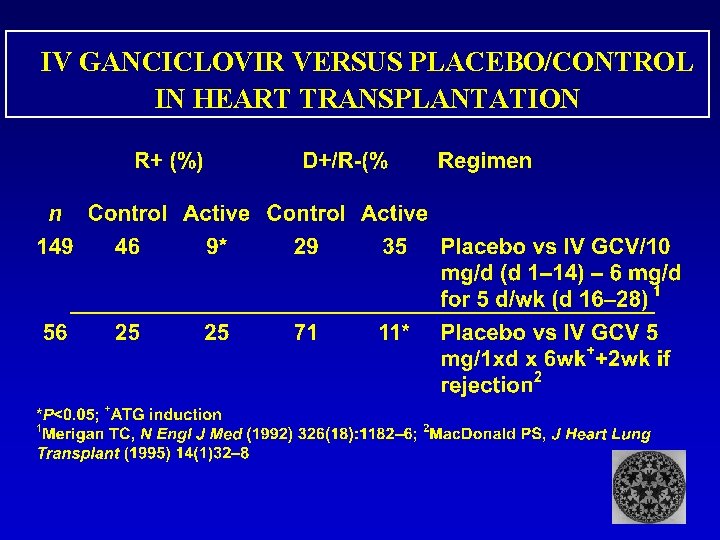
Prophylactic Ganciclovir prevents Transplant Coronary Artery Disease Valentine et al Circulation 1999; 100: 61 -66

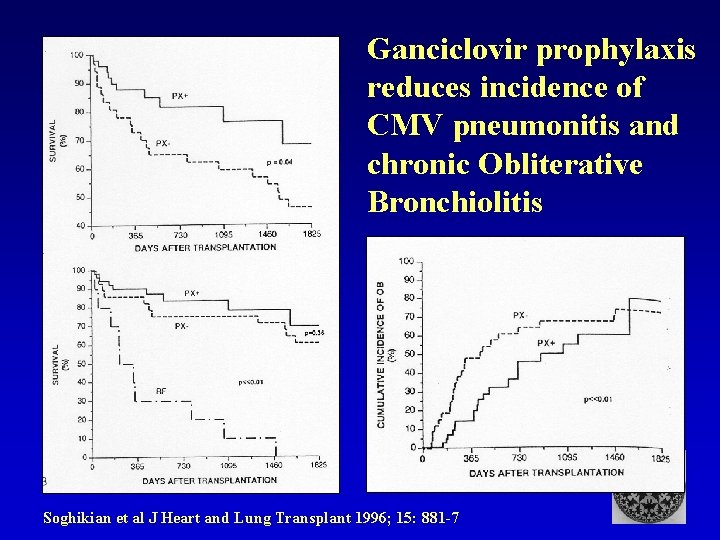
Ganciclovir prophylaxis reduces incidence of CMV pneumonitis and chronic Obliterative Bronchiolitis Soghikian et al J Heart and Lung Transplant 1996; 15: 881 -7

Messages from Protocol Histology Determinants of long term damage to renal allografts Nankivell et al Transplantation 2001; 71: 515 -523

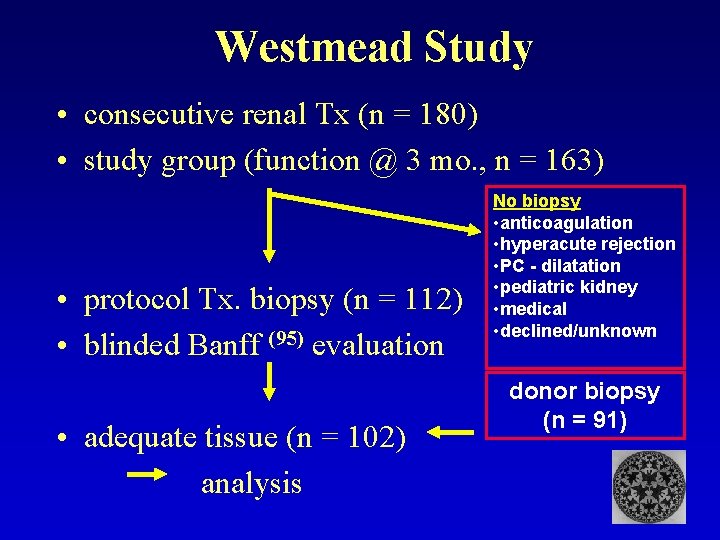
Westmead Study • consecutive renal Tx (n = 180) • study group (function @ 3 mo. , n = 163) • protocol Tx. biopsy (n = 112) • blinded Banff (95) evaluation • adequate tissue (n = 102) analysis No biopsy • anticoagulation • hyperacute rejection • PC - dilatation • pediatric kidney • medical • declined/unknown donor biopsy (n = 91)

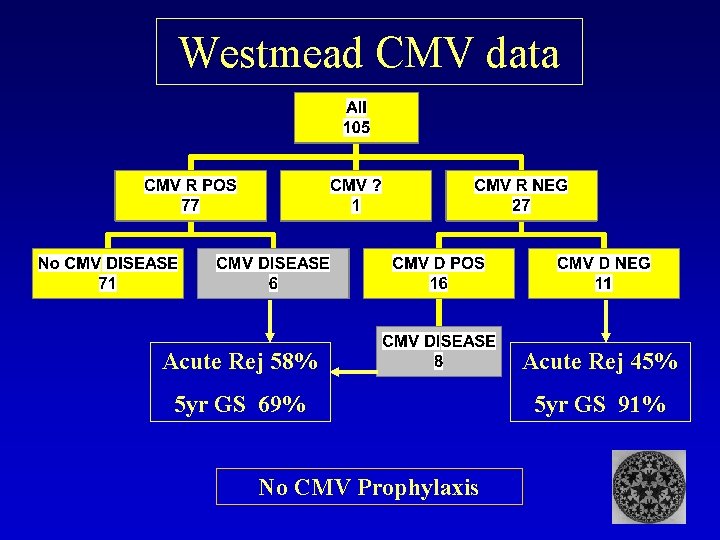
Westmead CMV data Acute Rej 58% Acute Rej 45% 5 yr GS 69% 5 yr GS 91% No CMV Prophylaxis

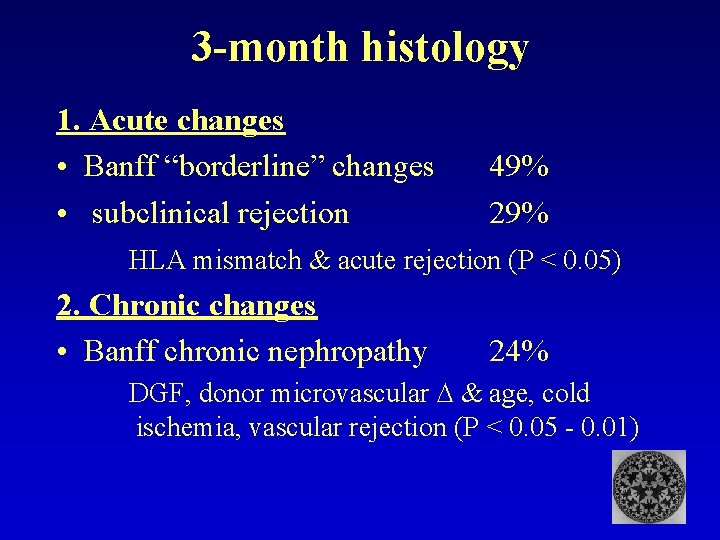
3 -month histology 1. Acute changes • Banff “borderline” changes • subclinical rejection 49% 29% HLA mismatch & acute rejection (P < 0. 05) 2. Chronic changes • Banff chronic nephropathy 24% DGF, donor microvascular & age, cold ischemia, vascular rejection (P < 0. 05 - 0. 01)

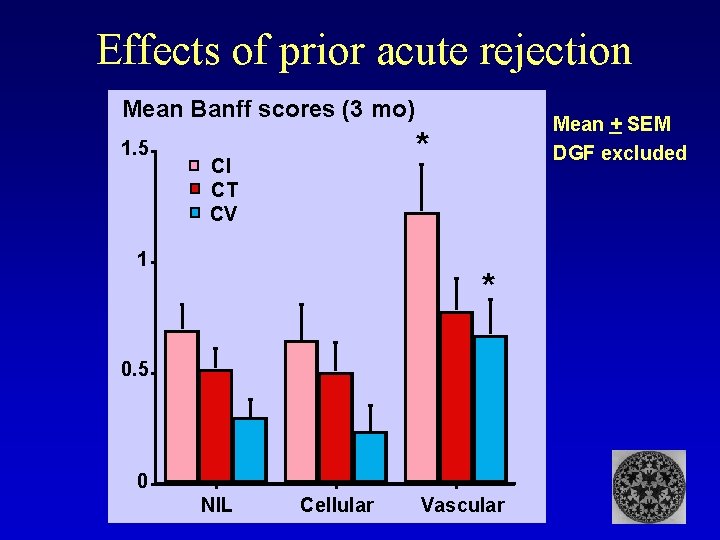
Effects of prior acute rejection Mean Banff scores (3 mo) 1. 5 Mean + SEM DGF excluded * CI CT CV 1 * 0. 5 0 NIL Cellular Vascular

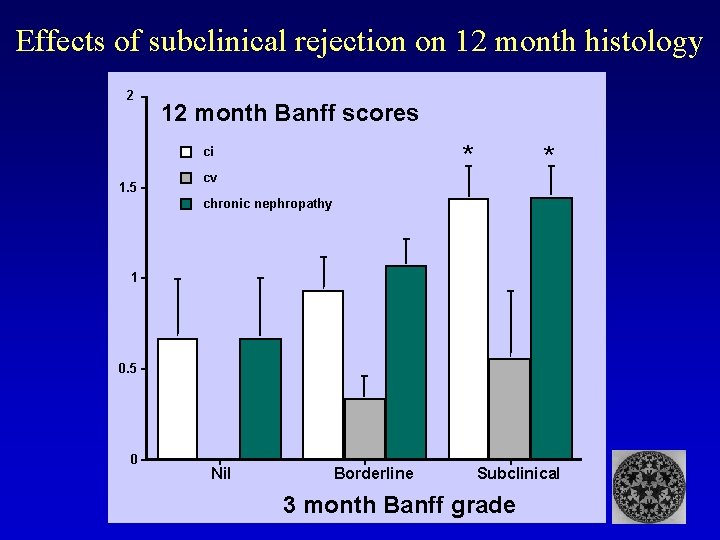
Effects of subclinical rejection on 12 month histology 2 12 month Banff scores * ci 1. 5 * cv chronic nephropathy 1 0. 5 0 Nil Borderline Subclinical 3 month Banff grade

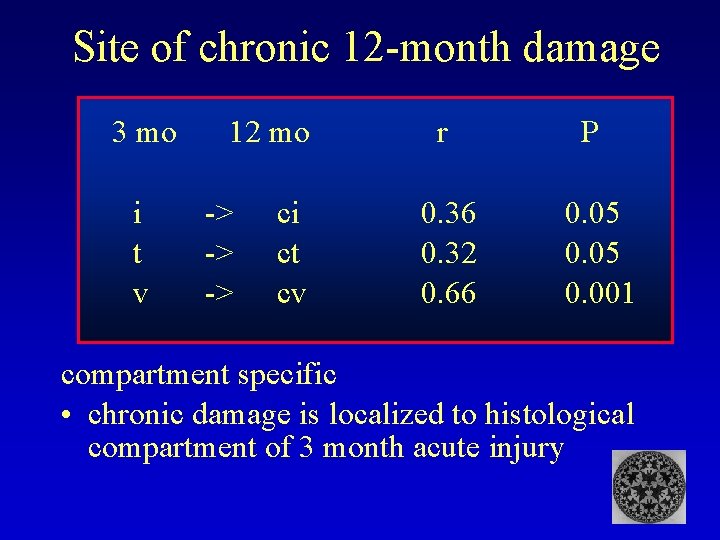
Site of chronic 12 -month damage 3 mo i t v 12 mo -> -> -> ci ct cv r 0. 36 0. 32 0. 66 P 0. 05 0. 001 compartment specific • chronic damage is localized to histological compartment of 3 month acute injury

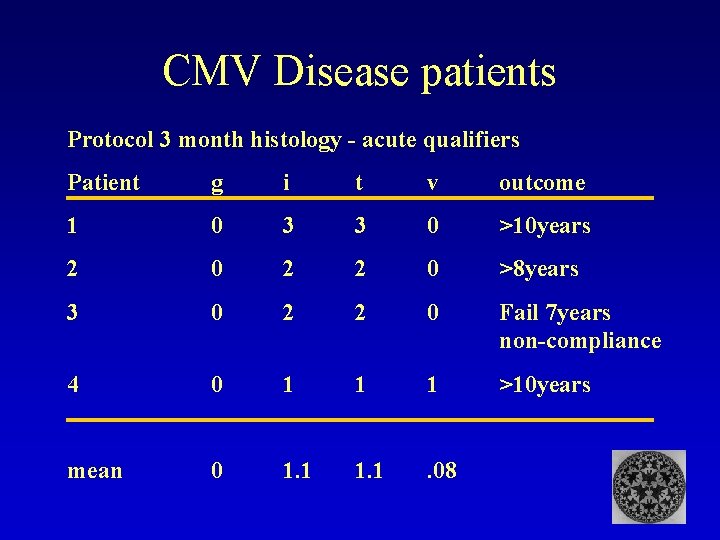
CMV Disease patients Protocol 3 month histology - acute qualifiers Patient g i t v outcome 1 0 3 3 0 >10 years 2 0 2 2 0 >8 years 3 0 2 2 0 Fail 7 years non-compliance 4 0 1 1 1 >10 years mean 0 1. 1 . 08

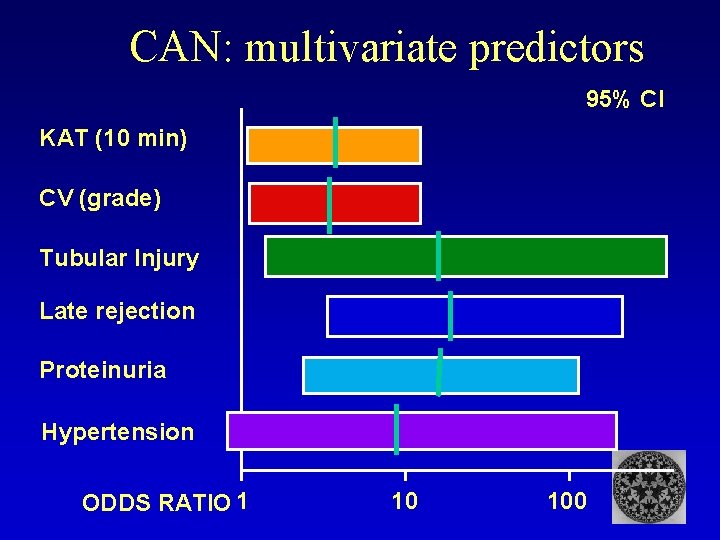
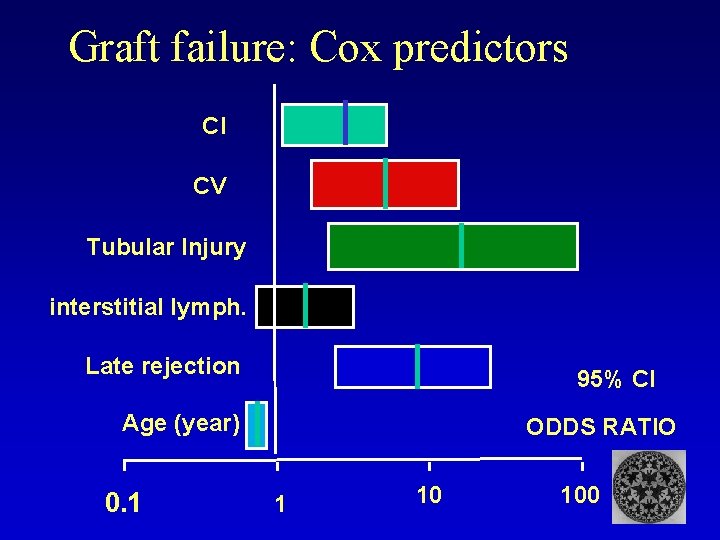
CAN: multivariate predictors 95% CI KAT (10 min) CV (grade) Tubular Injury Late rejection Proteinuria Hypertension ODDS RATIO 1 10 100

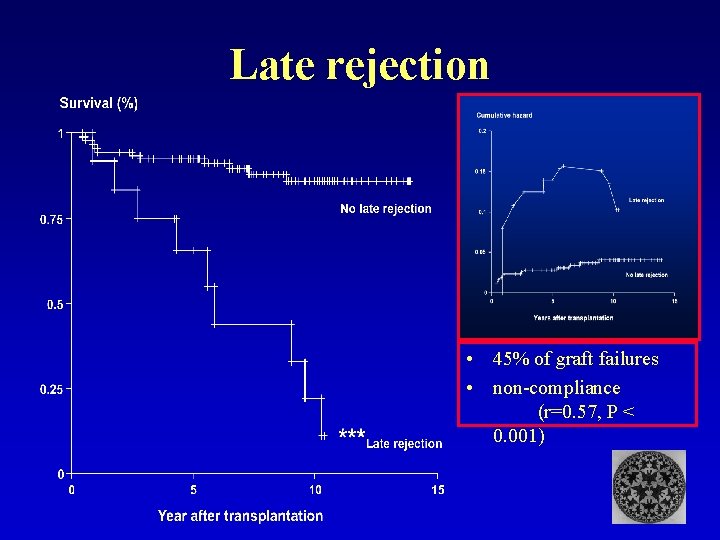
Late rejection • 45% of graft failures • non-compliance (r=0. 57, P < 0. 001)

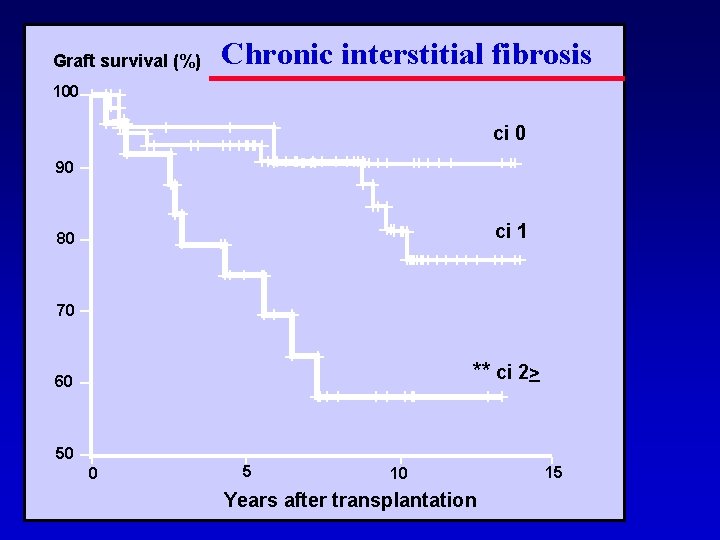
Graft survival (%) Chronic interstitial fibrosis 100 ci 0 90 ci 1 80 70 ** ci 2> 60 50 0 5 10 Years after transplantation 15

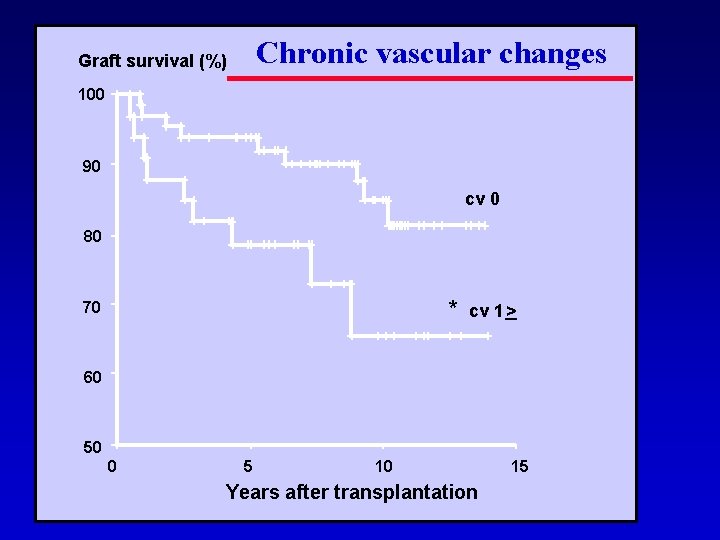
Chronic vascular changes Graft survival (%) 100 90 cv 0 80 * 70 cv 1 > 60 50 0 5 10 Years after transplantation 15

Graft failure: Cox predictors CI CV Tubular Injury interstitial lymph. Late rejection 95% CI Age (year) 0. 1 ODDS RATIO 1 10 100

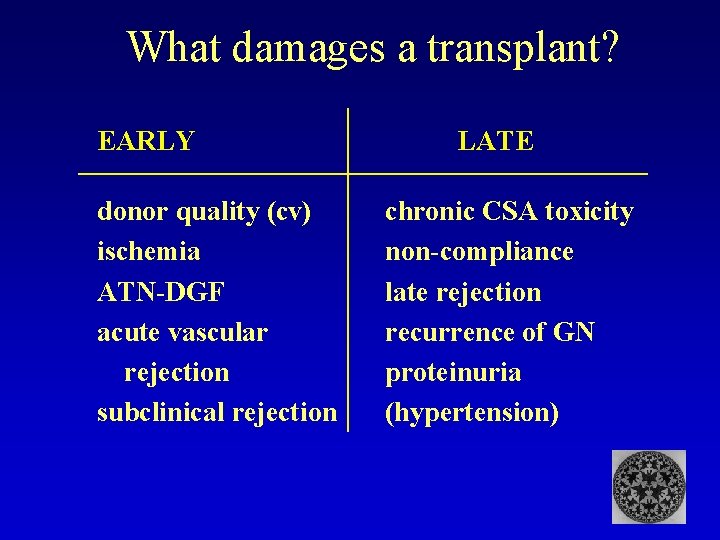
What damages a transplant? EARLY donor quality (cv) ischemia ATN-DGF acute vascular rejection subclinical rejection LATE chronic CSA toxicity non-compliance late rejection recurrence of GN proteinuria (hypertension)

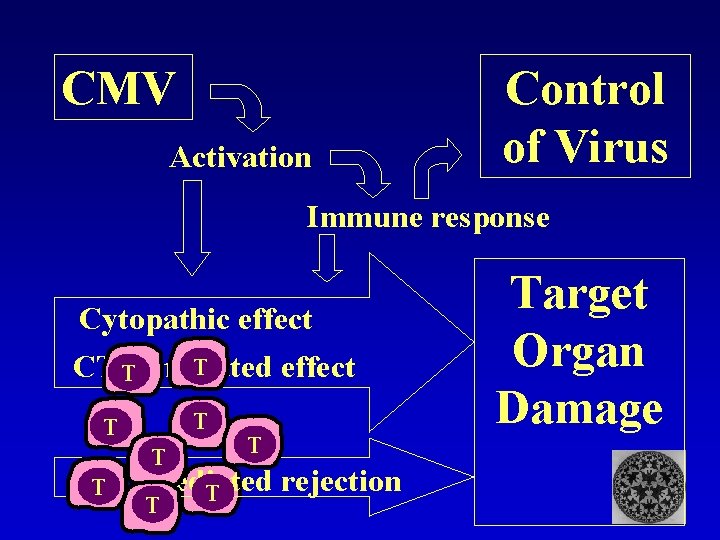
CMV Activation Control of Virus Immune response Cytopathic effect T CTL effect T mediated T T CTL mediated rejection T Target Organ Damage

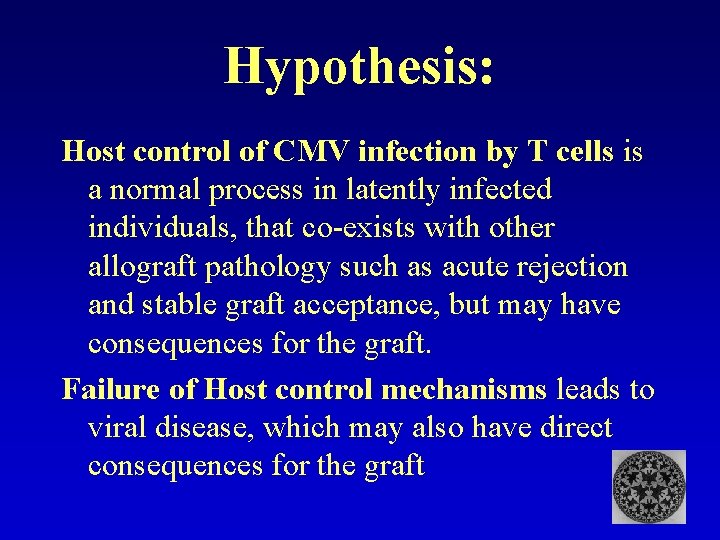
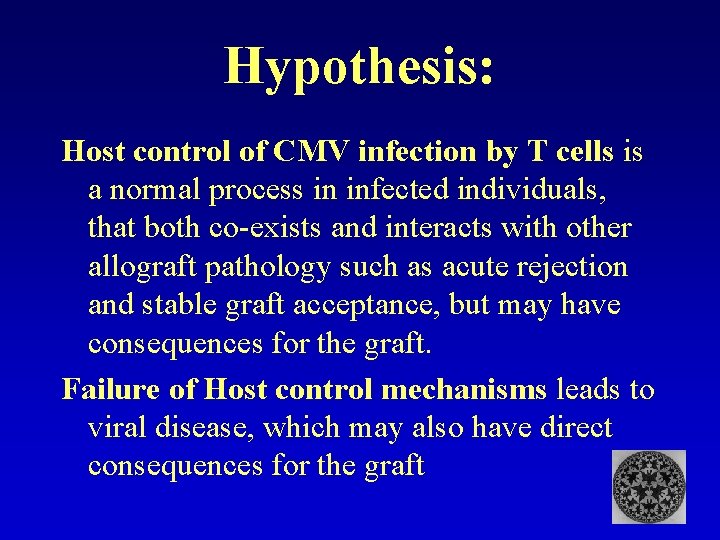
Hypothesis: Host control of CMV infection by T cells is a normal process in latently infected individuals, that co-exists with other allograft pathology such as acute rejection and stable graft acceptance, but may have consequences for the graft. Failure of Host control mechanisms leads to viral disease, which may also have direct consequences for the graft

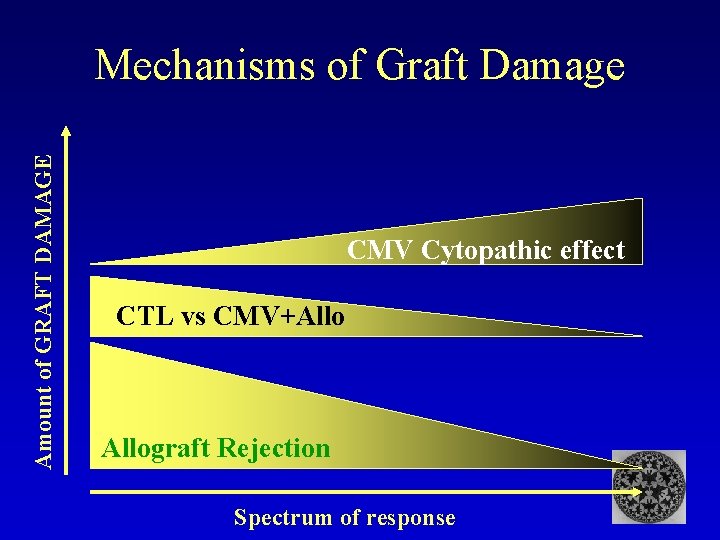
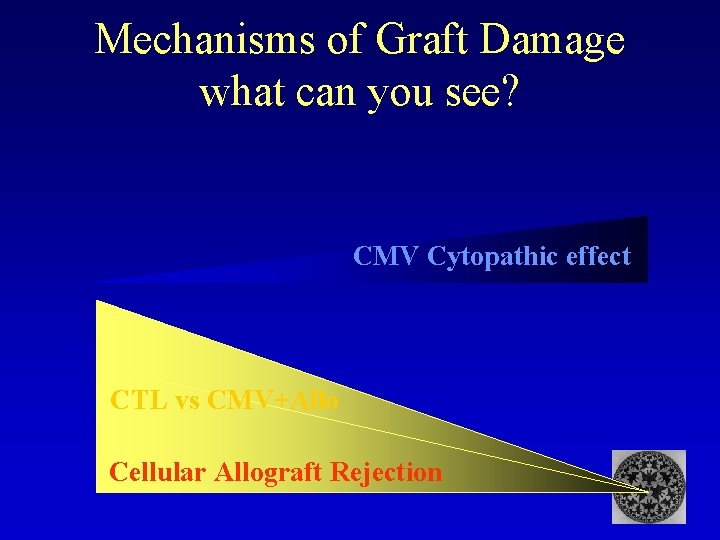
Amount of GRAFT DAMAGE Mechanisms of Graft Damage CMV Cytopathic effect CTL vs CMV+Allograft Rejection Spectrum of response

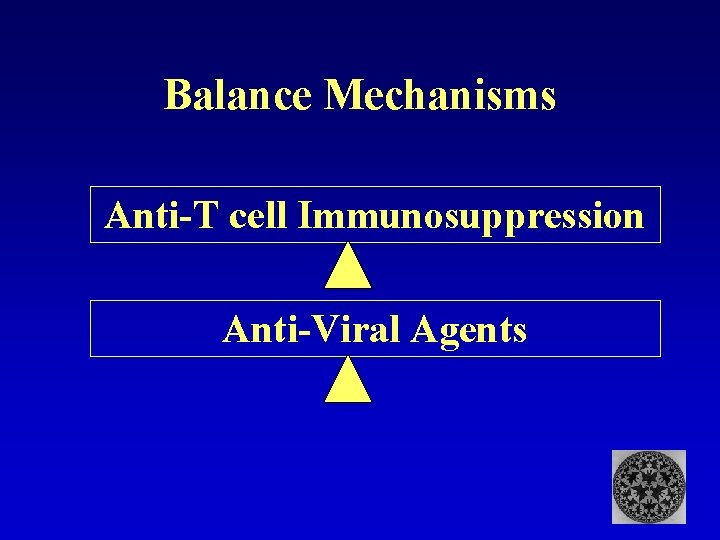
Balance Mechanisms Anti-T cell Immunosuppression Anti-Viral Agents

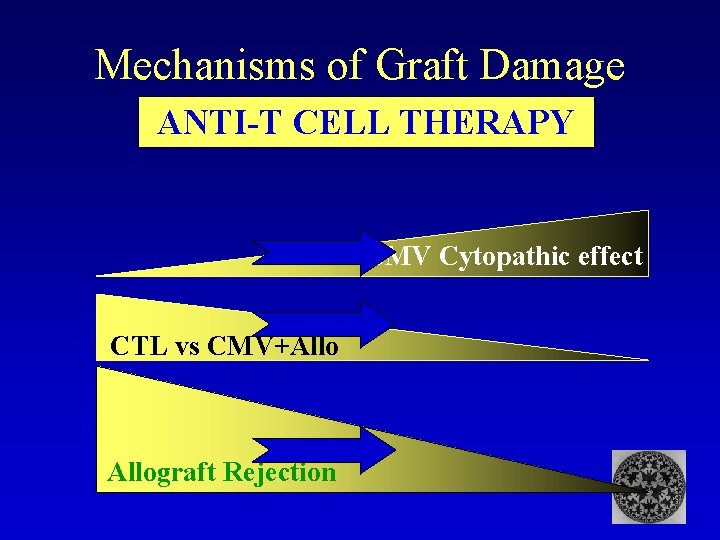
Mechanisms of Graft Damage ANTI-T CELL THERAPY CMV Cytopathic effect CTL vs CMV+Allograft Rejection

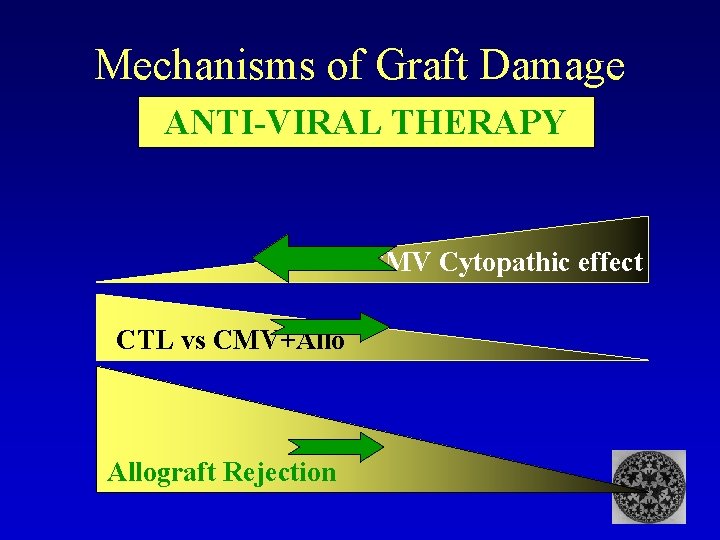
Mechanisms of Graft Damage ANTI-VIRAL THERAPY CMV Cytopathic effect CTL vs CMV+Allograft Rejection

Mechanisms of Graft Damage what can you see? CMV Cytopathic effect CTL vs CMV+Allo Cellular Allograft Rejection

Hypothesis: Host control of CMV infection by T cells is a normal process in infected individuals, that both co-exists and interacts with other allograft pathology such as acute rejection and stable graft acceptance, but may have consequences for the graft. Failure of Host control mechanisms leads to viral disease, which may also have direct consequences for the graft



Does it matter? • If CMV infection is controlled by T cells and damages the graft then anti-T cell therapy will increase the damage, while anti CMV therapy will reduce the damage • When a cellular infiltrate is due to T cells directed at Allo Ag then increased anti T cell therapy will be effective. But when the T cells are directed at CMV/Allo anti T cell therapy will lead to uncontrolled CMV

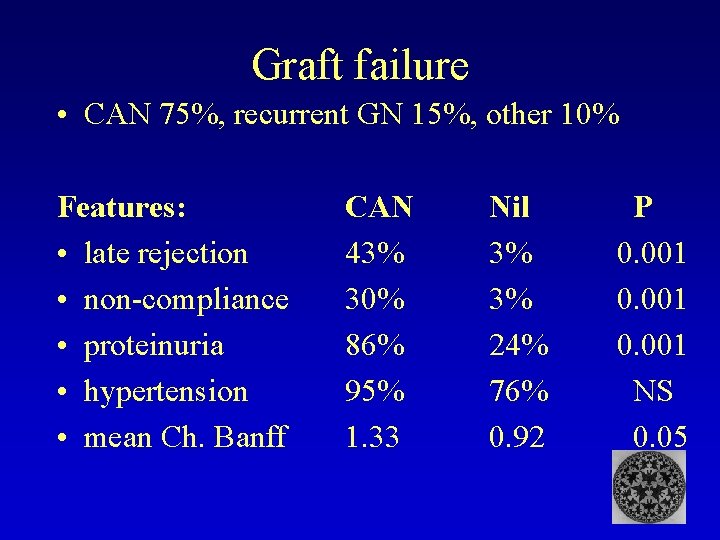
Graft failure • CAN 75%, recurrent GN 15%, other 10% Features: • late rejection • non-compliance • proteinuria • hypertension • mean Ch. Banff CAN 43% 30% 86% 95% 1. 33 Nil 3% 3% 24% 76% 0. 92 P 0. 001 NS 0. 05

Lowance et al New Engl J Med 1999; 340: 1462 - 70

Westmead CMV - HHV 6 STUDY DISEASE OKT 3/ATG NONE NO DISEASE

IV GANCICLOVIR VERSUS PLACEBO/CONTROL IN HEART TRANSPLANTATION