Transplantation LEC 6 REFIF S ALSHAWK PHD Transplantation
















![Test [cross-matching] is the most important level of compatibility testing that occurs prior Test [cross-matching] is the most important level of compatibility testing that occurs prior](https://slidetodoc.com/presentation_image_h/f03ac065f721788df911c37b0707aefe/image-28.jpg)



- Slides: 34

Transplantation LEC 6 REFIF S. AL-SHAWK (PHD)

Transplantation: the process of taking cells, tissues, or organs from one individual and placing them into a different individual or different site of the same individual • Graft: transplanted cells, tissues, or organs. • Donor: the individual who provides the graft. • Recipient: the individual who receives the graft. Also called the host.

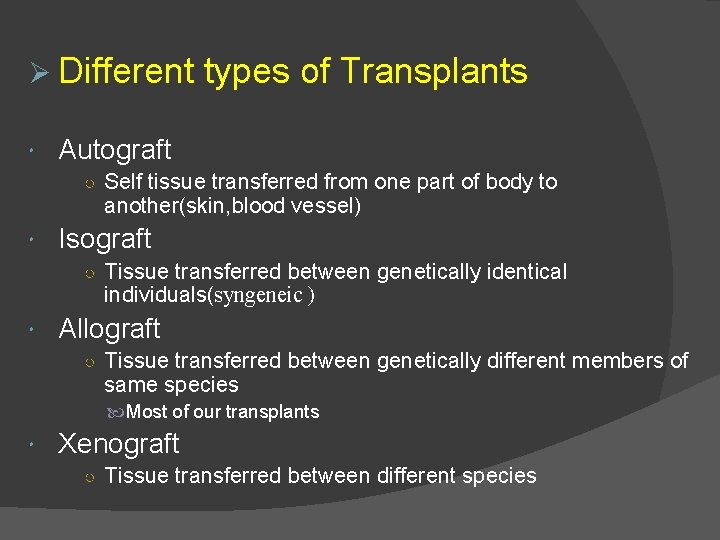
Ø Different types of Transplants Autograft ○ Self tissue transferred from one part of body to another(skin, blood vessel) Isograft ○ Tissue transferred between genetically identical individuals(syngeneic ) Allograft ○ Tissue transferred between genetically different members of same species Most of our transplants Xenograft ○ Tissue transferred between different species


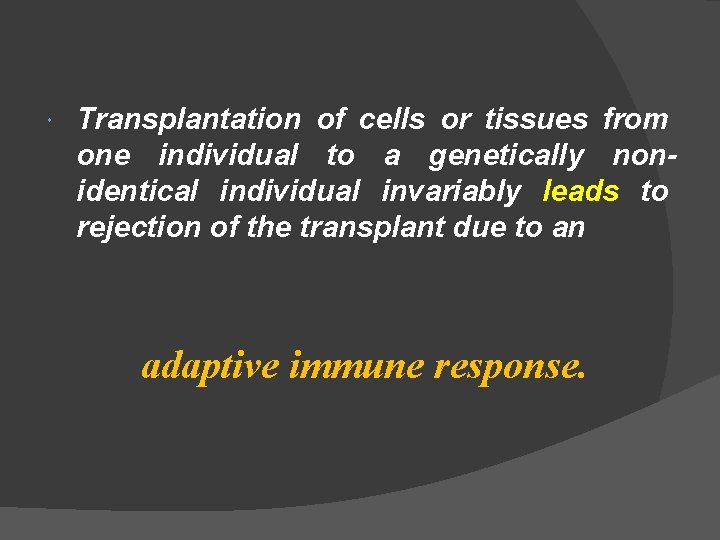
Transplantation of cells or tissues from one individual to a genetically nonidentical individual invariably leads to rejection of the transplant due to an adaptive immune response.

ADAPTIVE IMMUNE RESPONSES TO ALLOGRAFTS What is the target for rejection? Major histocompatibility antigens (MHC molecules) are expressed on all nucleated cells (class I) and on B cells APC, cells, monocytes/macrophages (class II) They are targets for rejection, Minor histocompatibility antigens: They are peptides derived from polymorphic cellular proteins bound to MHC class I molecules Other alloantigens : Human ABO blood group antigens and Some tissue specific antigens except identical twins

Schematic diagrams of the process of graft acceptance and rejection. (Specificity & Memory)

Recognition of Alloantigens by T Cells Allogeneic MHC molecules of a graft can be presented for recognition by the recipient’s T cells in two fundamentally different ways, called the direct and indirect pathways 1. Direct Recognition of MHC Alloantigens on Donor Cells : In the case of direct recognition, intact MHC molecules displayed by cells in the graft are recognized by recipient T cells without a need for processing by host APCs. Indirect Recognition of Alloantigens : In the indirect pathway, donor (allogeneic) MHC molecules are captured and processed by recipient APCs, and peptides derived from the allogeneic MHC molecules are presented in association with self MHC molecules. 2.


Activation and Effector Functions of Alloreactive T Lymphocytes When lymphocytes recognize alloantigens, they become activated to proliferate, differentiate, and perform effector functions that can damage grafts. 1) Activation of Alloreactive T Lymphocytes : The T cell response to an organ graft may be initiated in the lymph nodes that drain the graft. (sensitization )

doner APCs express donor MHC molecules as well as costimulators. migrate to regional lymph nodes and present, on their surface, unprocessed allogeneic MHC molecules to the recipient’s T cells Host dendritic cells from the recipient may also migrate into the graft, pick up graft alloantigens, and transport these back to the draining lymph nodes, where they are displayed


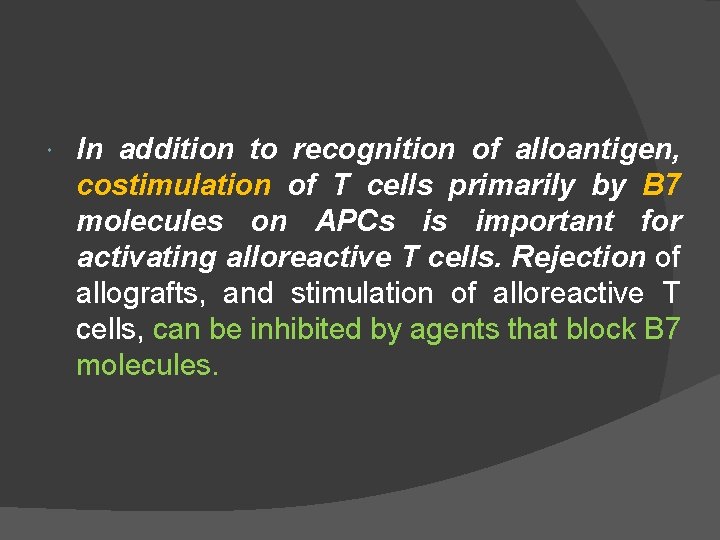
In addition to recognition of alloantigen, costimulation of T cells primarily by B 7 molecules on APCs is important for activating alloreactive T cells. Rejection of allografts, and stimulation of alloreactive T cells, can be inhibited by agents that block B 7 molecules.

Effector Functions of Alloreactive T Cells Alloreactive CD 4+ and CD 8+ T cells that are activated by graft alloantigens cause rejection by distinct mechanisms 2) Effector T cells migrate back into the graft and mediate rejection.

CD 8+ CTLs that are generated by direct allorecognition of donor MHC molecules on donor APCs can recognize the same MHC molecules on parenchymal cells in the graft and kill those cells by CTL-mediated killing of graft cells. CD 8+ CTLs that are generated by the indirect pathway are self MHC restricted, and they will not be able to kill the foreign graft cells because these cells do not express self MHC alleles displaying allogeneic peptides. (the principal mechanism of rejection is inflammation caused by the cytokines produced by the effector T cells.

Activation of Alloreactive B Cells and Production and Functions of Alloantibodies. Antibodies against graft antigens also contribute to rejection. Most high-affinity alloantibodies are produced by helper T cell–dependent activation of alloreactive B cells, much like antibodies against other protein antigens(donor HLA molecules, including both class I and class II MHC proteins). Engage effector mechanisms, including complement activation, and targeting and activation of neutrophils, macrophages, and NK cells through Fc receptor binding.

Acute graft rejection is mediated mostly by direct recognition of alloantigens, primarily by CD 8+ T cells that directly destroy the graft chronic graft rejection has a larger component of indirect recognition, resulting in activation of CD 4+ T cells that induce rejection mainly by triggering cytokine-mediated inflammation, and by helping B cells to make antibodies against alloantigens.


The acceptance or rejection of a transplant is determined by: *1 Class I & II MHC proteins on the donor cells, with class II playing the major role, especially DR locus. These allo. Ag activate Tcells(both helper & cytotoxic). The activated T-cell proliferate & then react against the allo. Ag on the donor cells. CD 8 +ve cytotoxic cells do the most of the killing of the allograft cells. Foreign MHC proteins activate more T-cells. *2 Both CD 4 & CD 8 T-cells have involved in allograft rejection, of which removal of them by using monoclonal Ab (anti. CD 4 & anti. CD 8) result in long-term survival of allograft. *3 Dendritic cells play a role in rejection as well, of which, it can present Ag in the context of class I MHC molecules, giving CD 8 +ve T-cell the opportunity to recognize allo. Ag. *4 *5 Difference in minor histocompatibility loci. ABO blood group. Note: difference in blood group and major histocompatibility Ags are responsible for the most intense graft rejection reaction.

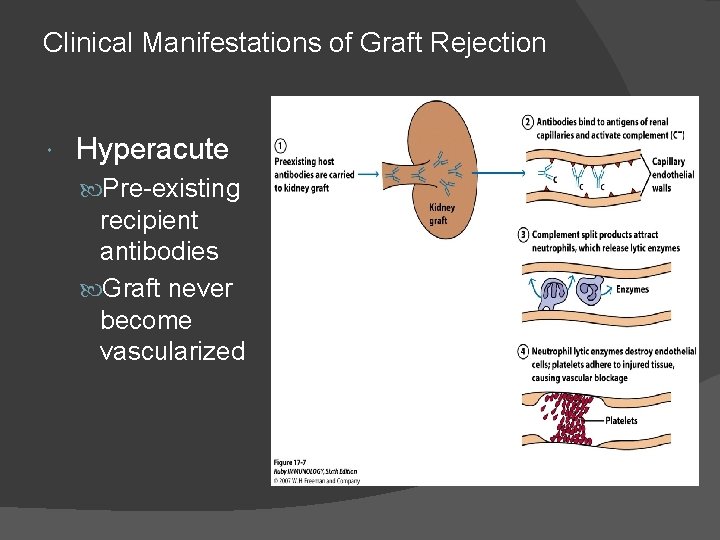
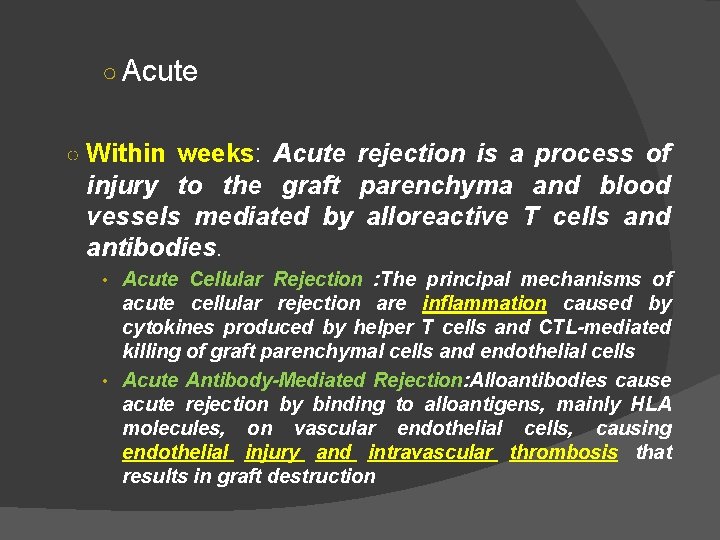
Clinical Manifestations of Graft Rejections: different classes of allograft rejection phenomena are classified according to their time of activation & the type of effector mechanism that predominates. These are: Hyperacute ○ Within hours: Hyperacute rejection is characterized by thrombotic occlusion of the graft vasculature that begins within minutes to hours after host blood vessels are anastomosed (joined) to graft vessels and is mediated by preexisting antibodies in the host circulation that bind to donor endothelial antigens

Clinical Manifestations of Graft Rejection Hyperacute Pre-existing recipient antibodies Graft never become vascularized

○ Acute ○ Within weeks: Acute rejection is a process of injury to the graft parenchyma and blood vessels mediated by alloreactive T cells and antibodies. • Acute Cellular Rejection : The principal mechanisms of acute cellular rejection are inflammation caused by cytokines produced by helper T cells and CTL-mediated killing of graft parenchymal cells and endothelial cells • Acute Antibody-Mediated Rejection: Alloantibodies cause acute rejection by binding to alloantigens, mainly HLA molecules, on vascular endothelial cells, causing endothelial injury and intravascular thrombosis that results in graft destruction


Chronic As therapy for acute rejection has improved, the major cause of the failure of vascularized organ allografts has become chronic rejection. A dominant lesion of chronic rejection in vascularized grafts is arterial occlusion as a result of the proliferation of intimal smooth muscle cells, and the grafts eventually fail mainly because of the resulting ischemic damage HOW? Months to years. Due to various mechanisms: cellmediated, deposition of antibodies or antigen antibody complexes with subsequent obliteration of blood vessels and interstitial fibrosis

What is graft versus Host Reaction ? When grafted tissue has mature T cells, they will attack host tissue leading to GVHR. § Major problem for bone marrow transplant. § Because it is the source of pluripotent hematopoietic stem cells, it can be used to reconstitute myeloid, erythroid, & lymphoid cells in a recipient who has lost these cells as a result of malignancy or chemotherapeutic regimens. § Because B. M. is a source of some mature T lymphocytes, it is necessary to remove these cells before transplantation to avoid the appearance of graft-versus-host disease in the recipient. In this special case of rejection, any mature T cells remaining in the B. M. inoculums can attack allogeneic MHCbearing cells of the recipient & cause widespread §

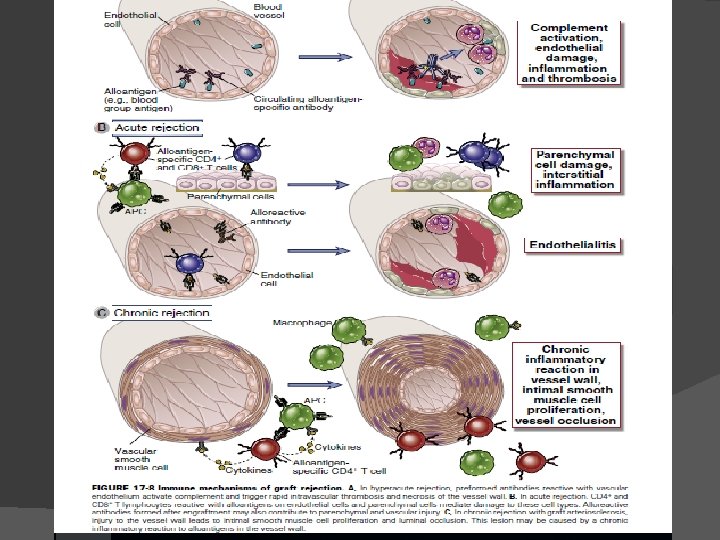
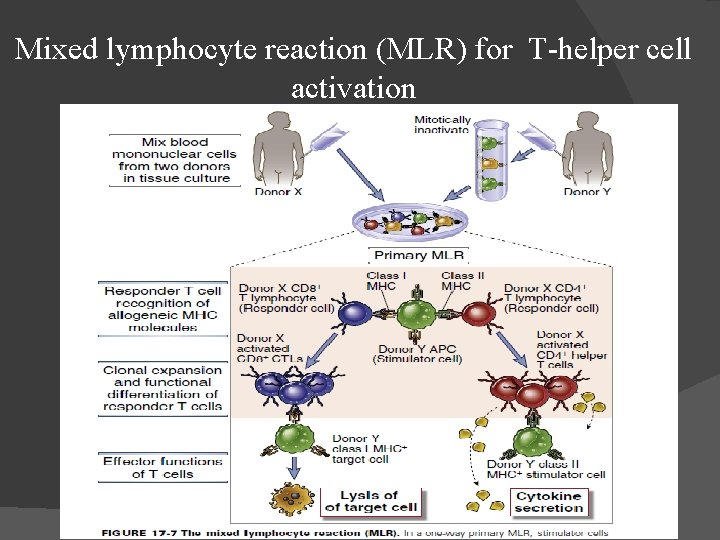
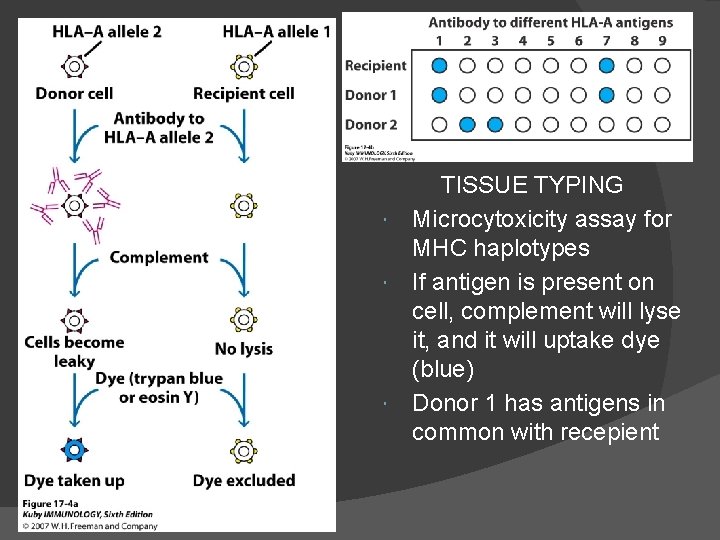
Tests for donor & recipient: Blood typing & ABO compatibility. HLA typing by microcytotoxicity test. Mixed lymphocyte reaction (MLR) for T-helper cell activation. Screening for preformed antibody and crossmatching. In cases where a living donor may be used for tissue transplantation, tissue-typing to match the HLA antigens of the recipient & donor is needed. Routine HLA typing focuses only on HLA-A, HLA-B, & HLA-DR. Patients awaiting organ transplants are screened for the presence of preformed antibodies reactive with allogeneic HLA molecules. These can arise because of previous pregnancies, transfusion, or transplantation & can mediate hyperacute graft rejection if they exist.

Mixed lymphocyte reaction (MLR) for T-helper cell activation
![Test crossmatching is the most important level of compatibility testing that occurs prior Test [cross-matching] is the most important level of compatibility testing that occurs prior](https://slidetodoc.com/presentation_image_h/f03ac065f721788df911c37b0707aefe/image-28.jpg)
Test [cross-matching] is the most important level of compatibility testing that occurs prior to solid organ transfer. The most common method used today is the [Luminex assay], which employs fluorochrome-labeled microbeads impregnated (loaded) with specific HLA proteins

TISSUE TYPING Microcytoxicity assay for MHC haplotypes If antigen is present on cell, complement will lyse it, and it will uptake dye (blue) Donor 1 has antigens in common with recepient

Prevention of rejection: General immunosuppressive therapy as, Mitotic inhibitors that inhibit T-cell proliferation. Anti-inflammatory agents as corticosteroid. Fungal metabolite that act as immunosuppressive agents. D. Total lymphoid irradiation, for all organ except B. M. The goal of immunosuppression is to block cell proliferation. 2. Specific treatment: A. Monoclonal Ab as * anti. CD 3, so ↓mature T-cell. * Ab to surface adhesion molecule , so no signal. * Ab to cytokine as anti IL-2. B. Block co-stimulatory signal e. g. Ab to CD 40 L so block binding of CD 40 -CD 40 L. 1. A. B. C.

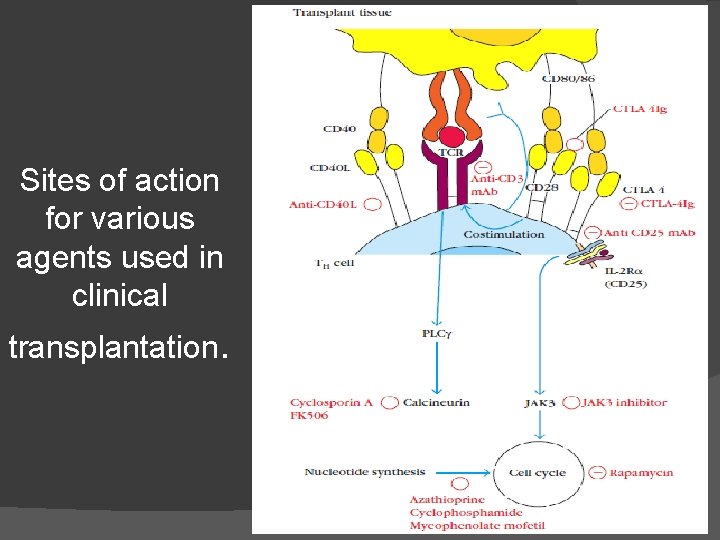
Sites of action for various agents used in clinical transplantation.


Xenotransplantation ○ Shortage of human donors ○ Obstacles with immune system ○ Closely related species have more success - However, taking risk of creating new viruses by recombination in graft
