CHIMES STUDY CONGENITAL CYTOMEGALOVIRUS INFECTION AND HEARING MULTICENTER











































- Slides: 62

CHIMES STUDY: CONGENITAL CYTOMEGALOVIRUS INFECTION AND HEARING MULTICENTER SCREENING STUDY Karen Fowler, Dr. PH University of Alabama in Birmingham Faye P. Mc. Collister, Ed. D University of Alabama, Emeritus Diane Sabo, Ph. D University of Pittsburgh

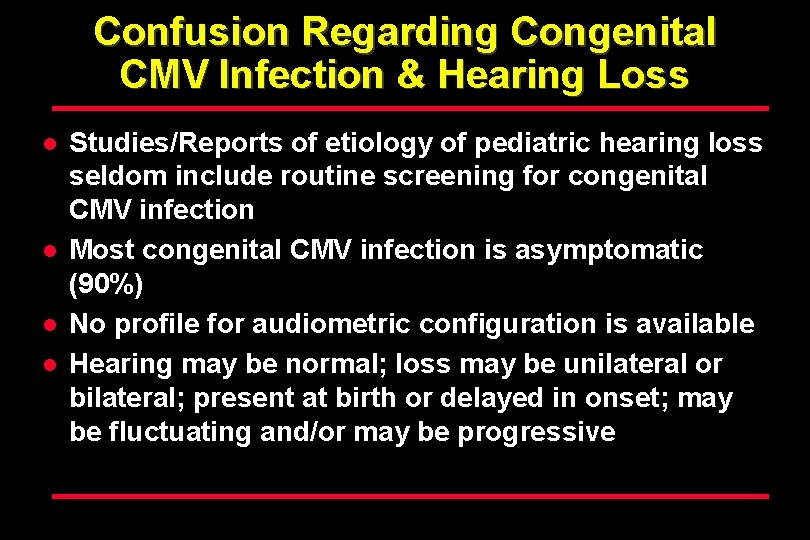
Confusion Regarding Congenital CMV Infection & Hearing Loss l l Studies/Reports of etiology of pediatric hearing loss seldom include routine screening for congenital CMV infection Most congenital CMV infection is asymptomatic (90%) No profile for audiometric configuration is available Hearing may be normal; loss may be unilateral or bilateral; present at birth or delayed in onset; may be fluctuating and/or may be progressive

Review of Congenital CMV Infection • Common virus although not easily spread person to person • Diagnosis needs to be made in the first 3 weeks of life • Clinical observation of infection in the newborn period identifies < 5% of all infants with congenital CMV infection • Delayed onset sequelae may occur over a long period with sensorineural hearing loss being the most common

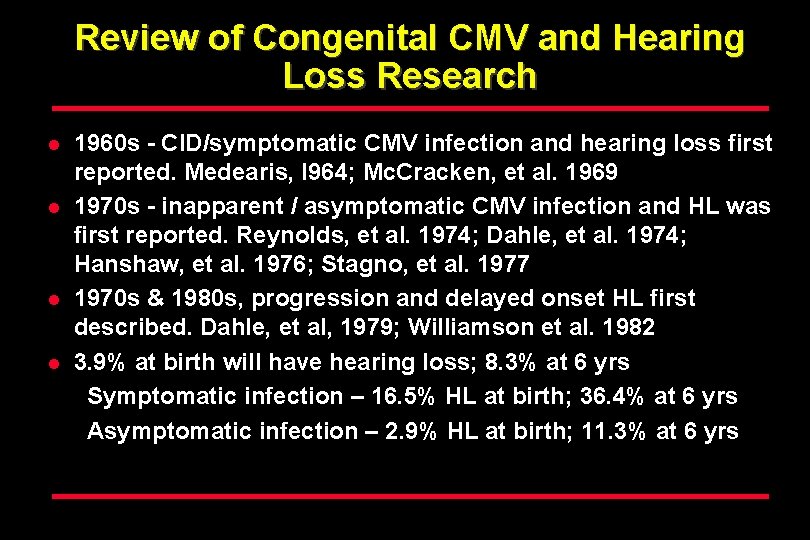
Review of Congenital CMV and Hearing Loss Research l l 1960 s - CID/symptomatic CMV infection and hearing loss first reported. Medearis, l 964; Mc. Cracken, et al. 1969 1970 s - inapparent / asymptomatic CMV infection and HL was first reported. Reynolds, et al. 1974; Dahle, et al. 1974; Hanshaw, et al. 1976; Stagno, et al. 1977 1970 s & 1980 s, progression and delayed onset HL first described. Dahle, et al, 1979; Williamson et al. 1982 3. 9% at birth will have hearing loss; 8. 3% at 6 yrs Symptomatic infection – 16. 5% HL at birth; 36. 4% at 6 yrs Asymptomatic infection – 2. 9% HL at birth; 11. 3% at 6 yrs

NIH/NIDCD Contract The Natural History of CMV-Related Hearing Loss and the Feasibility of CMV Screening as Adjunct to Hearing in the Newborn

CHIMES Study

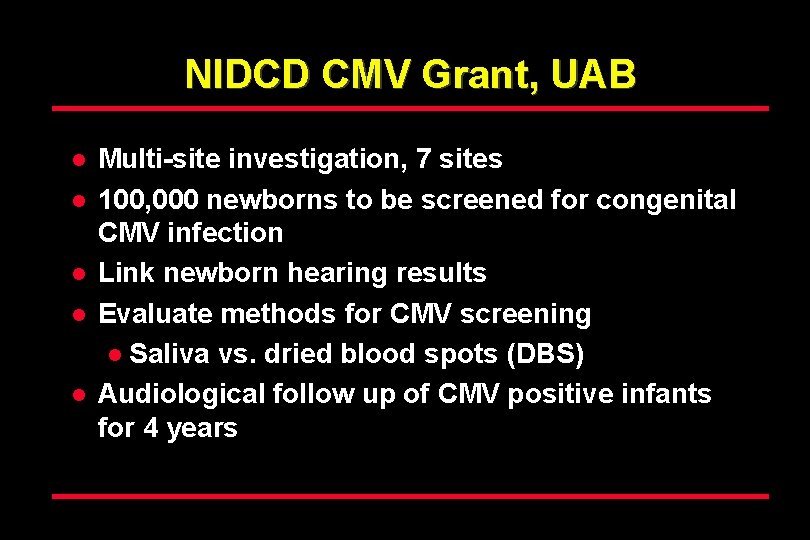
NIDCD CMV Grant, UAB l l l Multi-site investigation, 7 sites 100, 000 newborns to be screened for congenital CMV infection Link newborn hearing results Evaluate methods for CMV screening l Saliva vs. dried blood spots (DBS) Audiological follow up of CMV positive infants for 4 years

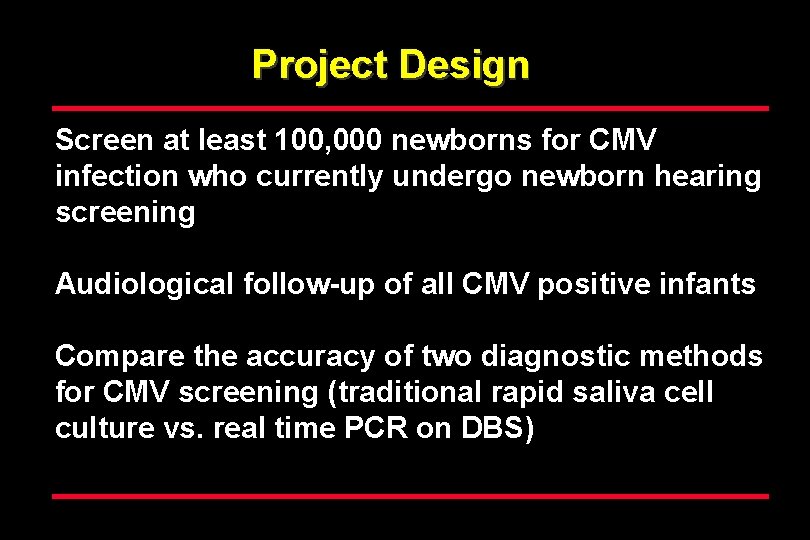
Project Design Screen at least 100, 000 newborns for CMV infection who currently undergo newborn hearing screening Audiological follow-up of all CMV positive infants Compare the accuracy of two diagnostic methods for CMV screening (traditional rapid saliva cell culture vs. real time PCR on DBS)

Objectives Define the long-term audiologic/otologic outcome in children with congenital CMV infection Determine the clinical validity and utility of CMV screening: Øin the detection of hearing impairment in the newborn Øin the prediction of hearing impairment with onset during infancy or in the early years of life

Project Staff l The project is directed by Drs. Suresh Bopanna & Karen Fowler l Each site will have a person designated as a co-principal investigator l Site audiologists direct audiological assessments l Project consultants: Faye Mc. Collister, Judy Gravel, Karl White

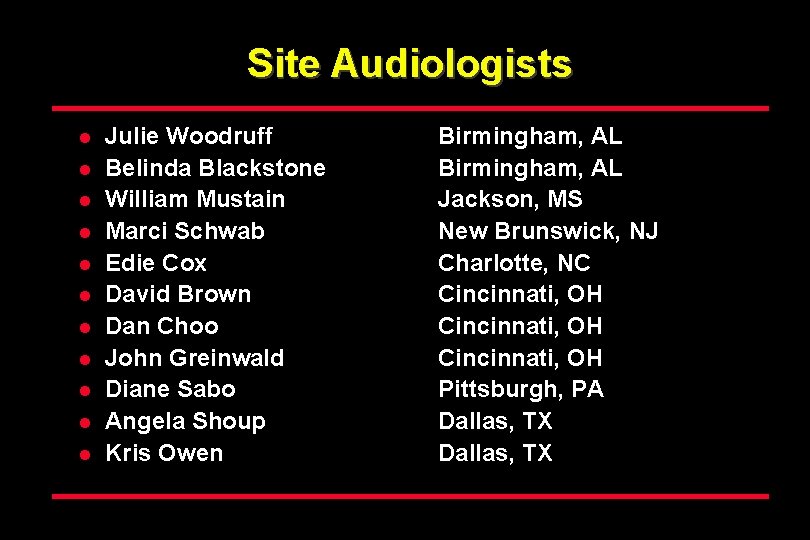
Site Audiologists l l l Julie Woodruff Belinda Blackstone William Mustain Marci Schwab Edie Cox David Brown Dan Choo John Greinwald Diane Sabo Angela Shoup Kris Owen Birmingham, AL Jackson, MS New Brunswick, NJ Charlotte, NC Cincinnati, OH Pittsburgh, PA Dallas, TX

Participating Hospitals Birmingham, AL University Hospital & Cooper Green Hospital Jackson, MS University of Mississippi Medical Center New Brunswick, NJ Saint Peters University Hospital Charlotte, NC Carolinas Medical Center Cincinnati, OH Good Samaritan Hospital Pittsburgh, PA Magee Women’s Hospital Dallas, TX Parkland Memorial Hospital

Selected Hospital Populations 38% Caucasian, Non Hispanic 34% Caucasian, Hispanic 24% African American 4% Asian

Challenges to the CHIMES Study (1) l l Establishing consistency in protocols across 7 sites, getting everyone on the same page Challenges inherent in longitudinal investigation: 6 year period of study, equipment changes, staff changes, protocol changes Age of subjects, birth through age 4 years, more difficult to evaluate audiologically Multiple disabilities, including developmental, vision, and motor for children with symptomatic infection

Challenges to the CHIMES Study (2) l l l Variability of hearing loss: progression, delay in onset, and fluctuation Subject ethnic background with language differences, cultural differences, social differences Otitis media resulting in conductive overlay for sn hearing loss, delay in getting assessment data Subject compliance with study protocol Subject retention

Challenges to the CHIMES Study (3) l l Specified protocol modified to accommodate clinical needs with rapidly progressive hearing loss Accuracy/consistency in data collection, recording, transfer, and storage Interpretation of data and clinical judgment Ensuring client confidentiality

Addressing the Challenges to the CHIMES Study l l l Standardized collection of data: developed data forms using barcoded labels with unique study identifier for all data sent from 7 sites (scanned and processed) Developed detailed Manual of Procedures (MOP) for study policies and procedures Developed audiology protocols establishing optimal and minimal goals for audiology data at visits Site Visits and Review of protocols Detailed retention plan with computerized database at each site and data forms (Visit Forms & Missed Visit Forms) to collect information on amount of contact each site had with a study participant during the intervals between visits

Addressing the Challenges to the CHIMES Study


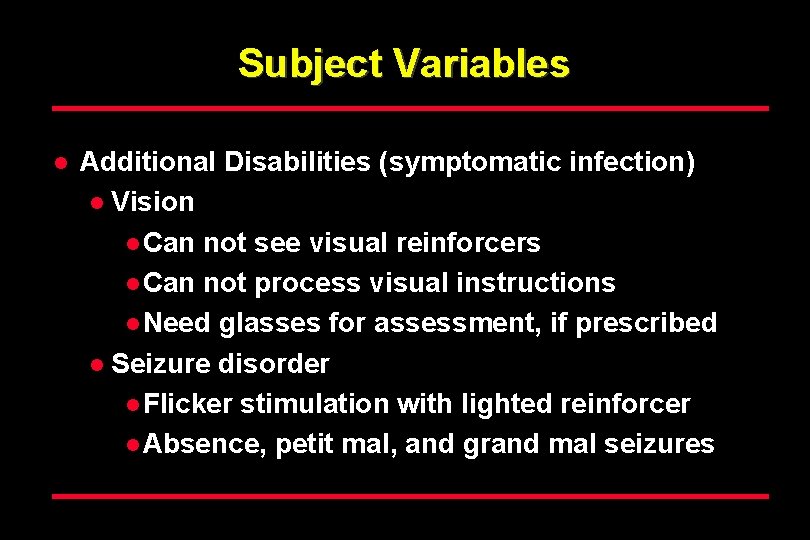
Subject Variables l Additional Disabilities (symptomatic infection) l Vision l Can not see visual reinforcers l Can not process visual instructions l Need glasses for assessment, if prescribed l Seizure disorder l Flicker stimulation with lighted reinforcer l Absence, petit mal, and grand mal seizures

Cultural Diversity l l Racial, cultural, socioeconomic differences exist among individuals from same country Interpreters may have difficulty explaining medical / technical information l May be difficult for family to understand l will not qualify for public assistance medical and technical services (hearing aids) l finding financial assistance challenging, at best

Cultural Diversity l Project informational materials will be provided in English and Spanish for parents and at understandable reading levels. l Communication options chosen by families for their child will be respected and supported.

CHIMES STUDY Audiological Assessment Protocol Diane Sabo, Ph. D University of Pittsburgh

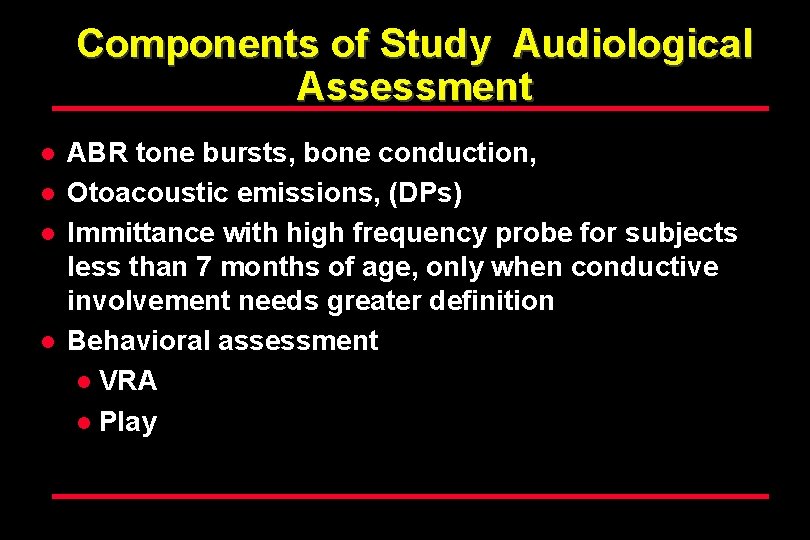
Components of Study Audiological Assessment l l ABR tone bursts, bone conduction, Otoacoustic emissions, (DPs) Immittance with high frequency probe for subjects less than 7 months of age, only when conductive involvement needs greater definition Behavioral assessment l VRA l Play

Screening Data Obtained l Physiologic data l ABR l Automated-ABR l OAEs l Automated OAEs l Combinations of the tests

Objective Hearing Diagnostic Methods l l Auditory Brainstem Response (ABR) l Tone Bursts –air and bone conduction Auditory Steady State Response (ASSR) not required, optional Acoustic Immittance Evoked Otoacoustic Emissions (EOAEs) l Distortion Product OAEs (DPOAEs)

Entry or Baseline Data l Physiologic tests l ABR l Tone bursts at. 5, 1, 2 and 4 k Hz l Bone conduction if air conduction abnormal (>25) l DPOAEs l 2 -8 k Hz l 60/45 l Signal and noise levels recorded

Physiologic Assessment with ABR and EOAEs • Advantages “Objective” • Reliable • Correlate well with hearing • Age not a factor (but sometimes an issue) •

Physiologic Assessment with ABR and EOAEs l Limitations l Not completely “objective” l Not tests of hearing l Not always good at predicting threshold l Not independent of infant’s behavior, state, or other factors

Click Spectrum Frequency

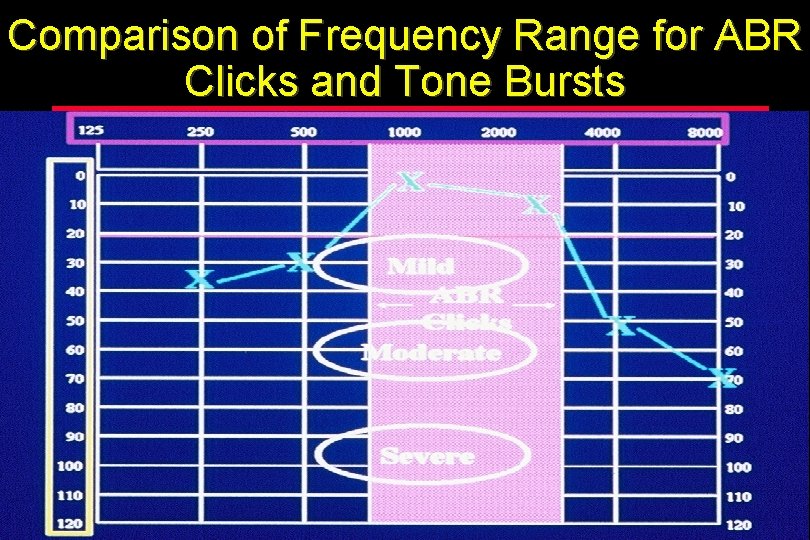
Comparison of Frequency Range for ABR Clicks and Tone Bursts


What We Know About ABR Clicks l Click commonly used, but……can give misleading information Click has energy at all frequencies transduced by earphone l Within limits, regions of normal hearing will generate ABR, even with regions of hearing loss l ABR tests avoid problems associated with reverse middle ear transmission, but potential still exists for missing hearing loss with click-evoked ABR l

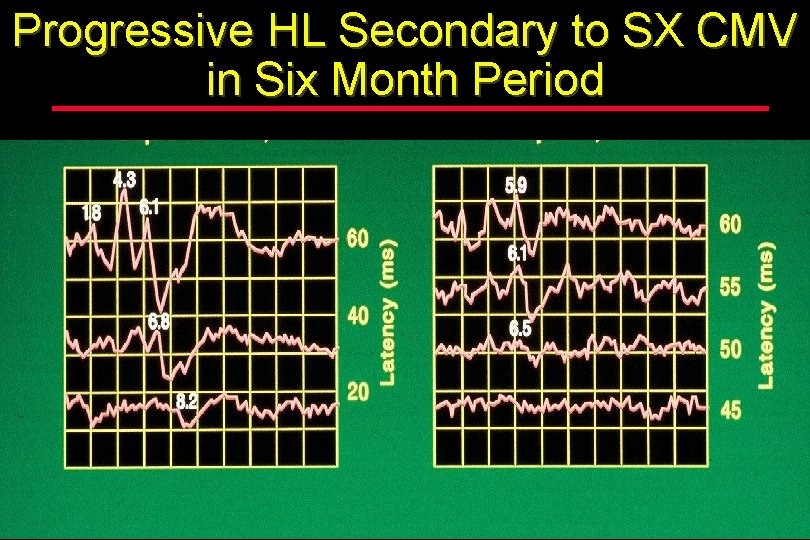
Progressive HL Secondary to SX CMV in Six Month Period

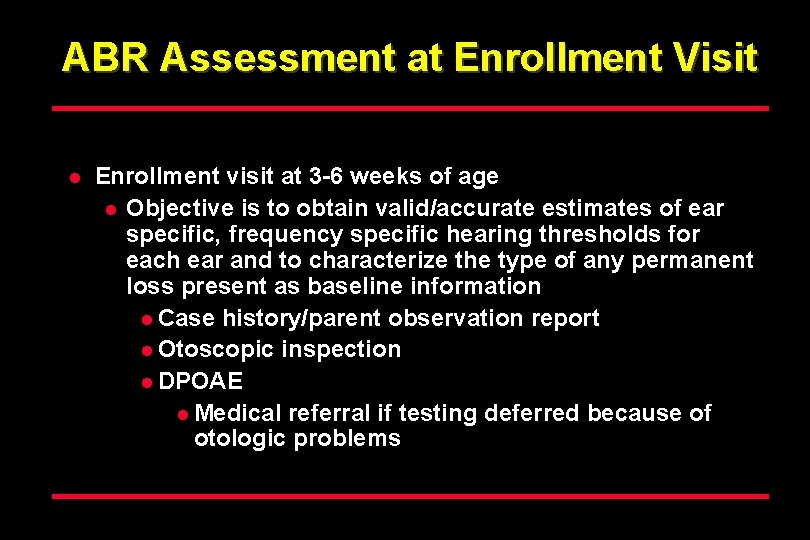
ABR Assessment at Enrollment Visit l Enrollment visit at 3 -6 weeks of age l Objective is to obtain valid/accurate estimates of ear specific, frequency specific hearing thresholds for each ear and to characterize the type of any permanent loss present as baseline information l Case history/parent observation report l Otoscopic inspection l DPOAE l Medical referral if testing deferred because of otologic problems

Physiologic Assessment with ABR and EOAEs • Advantages “Objective” • Reliable • Correlate well with hearing • Age not a factor (but sometimes an issue) •

Optimal Goal for ABR Assessment at Enrollment Visit (3 -6 Weeks) l l l ABR minimum response levels at. 5, 1. 0, 2. 0, and 4. 0 k. Hz for each ear DPOAE information at five frequency bands centered at 2, 3, 4, 6, and 8 Bone conduction, tympanometry, and ipsilateral reflexes may be required if loss suspected Record on ABR data form CF 107 Record on OAE data form Record on immittance data form CF 110

Minimal Goal for ABR Assessment at Enrollment Visit at 3 -6 Weeks of Age l Due to the importance of obtaining baseline information that is a clear and accurate reflection of infant’s hearing level a minimum goal was set: l Obtain MRL at 0. 5, 2. 0, and 4. 0 k. Hz for each ear l Bone conduction, tympanometry, and ipsilateral acoustic reflexes may be necessary if loss is suspected l If additional assessment apt necessary, use sequence system of 1, 2, and 3 on forms for enrollment visit l Prolonged delays between assessments avoided if possible

ABR Protocol l l l l Order for tone bursts: 2000, 4000, 500 and 1000 Use 2 -0 -2 for 500 Hz Use 4 -0 -4 for Rate 27 -29 Filter setting of 30 -3000 Two replications <1000 sweeps for 2. 0 and 4. 0 No less than 2000 sweeps for 0. 5 and 1. 0 k. Hz

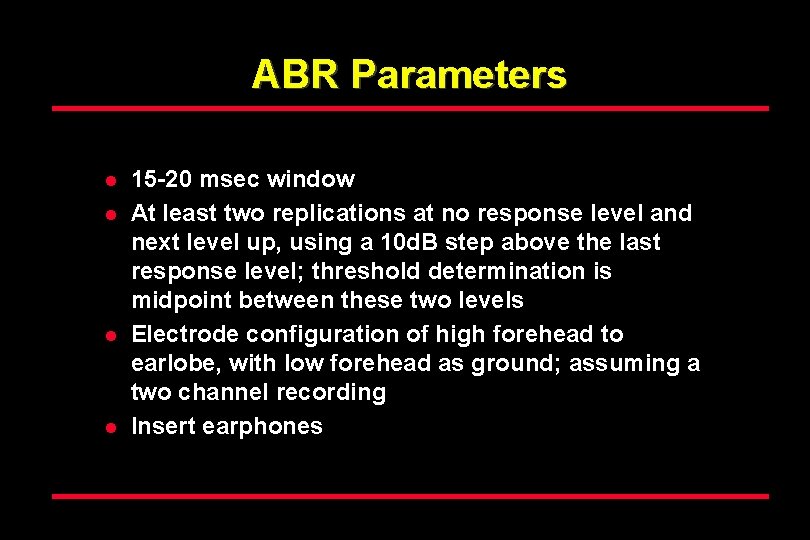
ABR Parameters l l 15 -20 msec window At least two replications at no response level and next level up, using a 10 d. B step above the last response level; threshold determination is midpoint between these two levels Electrode configuration of high forehead to earlobe, with low forehead as ground; assuming a two channel recording Insert earphones

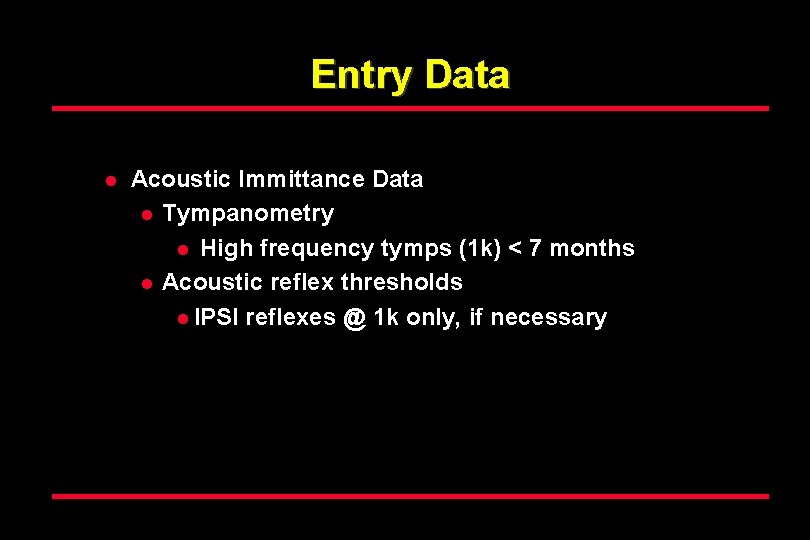
Entry Data l Acoustic Immittance Data l Tympanometry l High frequency tymps (1 k) < 7 months l Acoustic reflex thresholds l IPSI reflexes @ 1 k only, if necessary

Use Insert Earphones l l l Separation of stimulus artifact from the onset of the response makes wave I more visible Prevent ear canal collapse Increase interaural attenuation Provide greater comfort for long periods of time Can attenuate surrounding environmental noise more efficiently l Absolute latencies delayed by 0. 9 ms l Affect amplitude of wave I, lower

CHIMES STUDY Faye P. Mc. Collister, Ed. D University of Alabama, Emeritus Behavioral Audiological Assessment Protocols

Request Parental Help in Preparing for Behavioral Diagnostic Assessment l l Try to have child rested and feed, comfortable and attentive for test Schedule appointment away from nap time l Bring diapers for diaper change l Bring bottle for comforting l Bring pacifier, if used l Bring familiar car seat for test

Manage Baby/Mom/Diagnostic Environment l l l Quietest environment possible, no talking, no noisy toys Reduce undesirable visual, auditory, tactile distractions calm, alert child preferred, but not after heavy feeding, will fall asleep Do not separate child from parent and create crying, agitated child Wait for child to calm prior to initiating test

Child/Parent Handling Issues l l l Child State l Activity level l Comfort level Infection control l Clinic protocol l Gloves, disposables, cross contamination Parent inclusion l Pre-test information sharing l Time during testing l Time spent informing about procedure l Informing about the results

Visual Reinforcement Audiometry l l Developmentally appropriate technique that gives valid estimate of hearing Gives ear specific, frequency specific information Reliable method for early years Practical for repeat use

Schedule for Behavioral Audiological Assessment l Visual Reinforcement Audiometry scheduled at 7, 12, 18, and 24 month follow-up visits l Play Audiometry scheduled at 24, 30, 36, 42, and 42 month follow-up visit

VRA Assessment Information l l Beginning stimulus type l warble tone, speech Beginning transducer l earphone, speaker l # beginning conditioning trials l # reconditioning trials l # stimulus trials l # control trials, if greater than 0, # correct

VRA Assessment Information l l l Test time Number of breaks taken DPs completed, 2, 3, 4, 6, and 8 Acoustic Immittance Measures Done? l Acoustic reflex threshold l tympanometry Follow up/ reschedule, no, yes- mark reason l Fussy scared, wax occluding canal, time, refused earphones/inserts, habituated, test reliability poor, failed to condition, mrl > 20, cnd if sn loss present, other- specify Medical Follow-up Recommended- yes, no- mark reason l Conductive component, tymps abnormal, ab gap, draining ear, hearing aid clearance

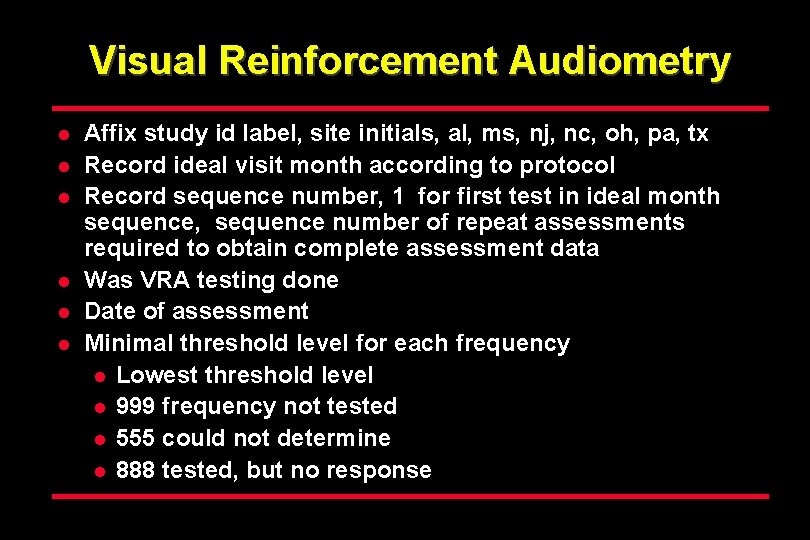
Visual Reinforcement Audiometry l l l Affix study id label, site initials, al, ms, nj, nc, oh, pa, tx Record ideal visit month according to protocol Record sequence number, 1 for first test in ideal month sequence, sequence number of repeat assessments required to obtain complete assessment data Was VRA testing done Date of assessment Minimal threshold level for each frequency l Lowest threshold level l 999 frequency not tested l 555 could not determine l 888 tested, but no response

VRA at Follow Up Visits at 8, 12, 18, and 24 Months of Age l l l l Optimal goal: obtain pure tone minimum response level of 15 d. B HL at 0. 5, 1. 0, 2. 0, and 4. 0 k. Hz If MRLs elevated above 20 d. B HL, tymps and/or bc will be needed to distinguish between sn, cond. Or mixed loss Record VRA data on CF 108 If immittance is administered, record data on CF 110 Minimal goal: Obtain MRLs at 0. 5, 2. 0, and 4. 0 k. Hz If MRLs elevated above 20 d. B HL, administer tymps and/or bc If MRLs for Minimal goal can not be obtained, schedule up to 3 additional visits and report any valid data collected using sequenced numbered data forms: visit 1, 2, or 3, for 8 month data

Visual Reinforcement Audiometry l l Optional, 8000 hz and speech awareness threshold, record if obtained Bc not required, leave field blank

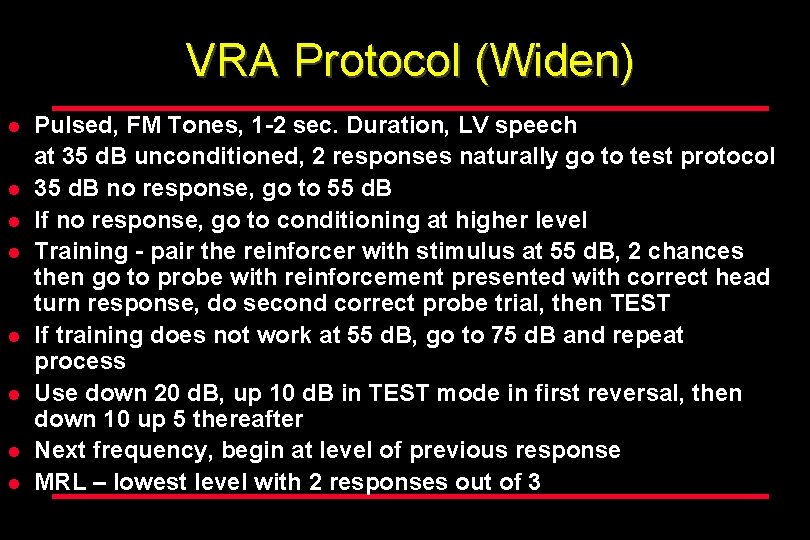
VRA Protocol (Widen) l l l l Pulsed, FM Tones, 1 -2 sec. Duration, LV speech at 35 d. B unconditioned, 2 responses naturally go to test protocol 35 d. B no response, go to 55 d. B If no response, go to conditioning at higher level Training - pair the reinforcer with stimulus at 55 d. B, 2 chances then go to probe with reinforcement presented with correct head turn response, do second correct probe trial, then TEST If training does not work at 55 d. B, go to 75 d. B and repeat process Use down 20 d. B, up 10 d. B in TEST mode in first reversal, then down 10 up 5 thereafter Next frequency, begin at level of previous response MRL – lowest level with 2 responses out of 3

Ensuring Stimulus Control For Behavioral Responses l l Control trials - observation intervals in which no stimulus was present, but examiner notes head turn behavior and no reinforcement provided to ensure head turn linked to stimulus presentation (inserted at a rate of 25%) >30%, test validity ? ? ? Probe trials - stimulus presentations at suprathreshold levels used to confirm conditioning at beginning and throughout session

When Child Does Not Condition l l l Change ear Change stimulus type- another frequency or speech Mode of presentation change – sound field Change reinforcer, strength of video reinforcer not strong enough Take a 10 minute break

Play Audiometry Data Form l l Complete at 24, 30, 36, 42, and 48 month follow up visits Complete Header l Affix study id label, complete site id with state initials l Was testing done- yes, no give reason, l Parental refusal, late for apt, missed slot, uncooperative, would not participate l Date of audiology evaluation – mm/dd/yy l Minimal threshold level, enter lowest threshold for each freq. for each ear. l Could not test, enter 555 l Was not tested, enter 999 l Was tested, no response , enter 888

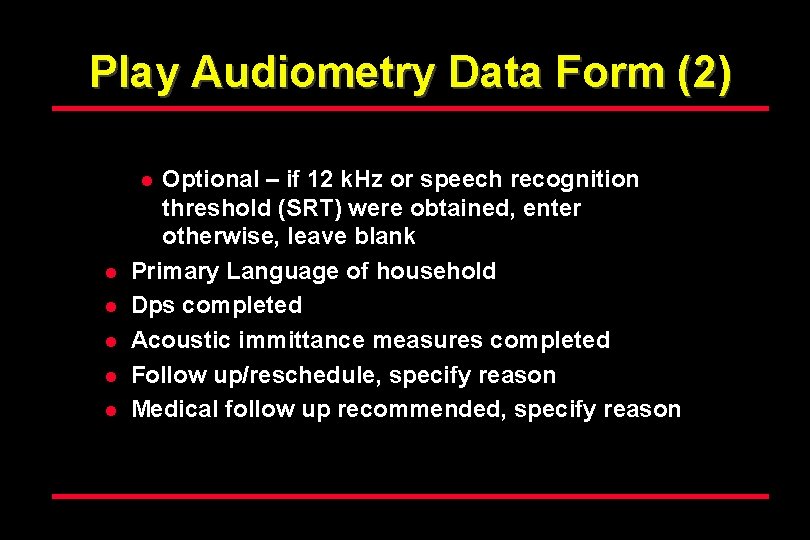
Play Audiometry Data Form (2) Optional – if 12 k. Hz or speech recognition threshold (SRT) were obtained, enter otherwise, leave blank Primary Language of household Dps completed Acoustic immittance measures completed Follow up/reschedule, specify reason Medical follow up recommended, specify reason l l l

Looks Good, But……. Visual alerting by examiner can give normal audiogram for child who is deaf l Results may look confusing, but be accurate, evaluate carefully l Hearing is dynamic, does not necessarily stay the same, be alert to progression l Use all sources of information l



Contact the CHIMES Study l www. uab. edu/chimesstudy Karen Fowler kfowler@uab. edu
 Multicenter study design
Multicenter study design Cytomegalovirus
Cytomegalovirus Tube xylophone
Tube xylophone Congenital pneumonia
Congenital pneumonia Congenital pneumonia
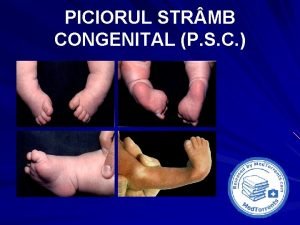
Congenital pneumonia Picior varus equin
Picior varus equin Stumped cornea
Stumped cornea Eisenmenger syndrome
Eisenmenger syndrome Congenital rubella syndrome
Congenital rubella syndrome Congenital malformations
Congenital malformations Congenital voice disorders
Congenital voice disorders Hypothyroidism classification
Hypothyroidism classification Murmur in asd
Murmur in asd Tga cxr
Tga cxr Congenital fibrosis of the extraocular muscles
Congenital fibrosis of the extraocular muscles Polyrhinia
Polyrhinia Most common congenital anomalies
Most common congenital anomalies Congenital adrenal hyperplasia electrolytes
Congenital adrenal hyperplasia electrolytes Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital cardiologist near me
Congenital cardiologist near me Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Potter face oligohydramnios
Potter face oligohydramnios Congenital hydronephrosis
Congenital hydronephrosis Congenital
Congenital Feminization tubes
Feminization tubes Michael addidle
Michael addidle Cyptorchism
Cyptorchism Congenital toxoplasmosis
Congenital toxoplasmosis Congenital anomaly
Congenital anomaly Canadian congenital heart alliance
Canadian congenital heart alliance Congenital glaucoma triad
Congenital glaucoma triad Vulvodynia
Vulvodynia Schlussel urology
Schlussel urology Congenital heart disease pda
Congenital heart disease pda Congenital diaphragmatic hernia
Congenital diaphragmatic hernia Congenital heart
Congenital heart Congenital rubella
Congenital rubella Biolateral macostomia
Biolateral macostomia Causes of congenital anomalies
Causes of congenital anomalies Congenital flat foot
Congenital flat foot Guyton
Guyton Graves disease mnemonic
Graves disease mnemonic Barlow ortolani
Barlow ortolani Alpha angle hip dysplasia
Alpha angle hip dysplasia Congenital heart defect
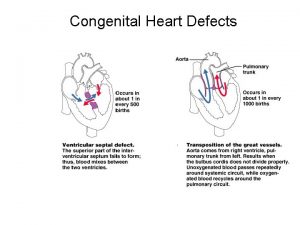
Congenital heart defect Congenital
Congenital Congenital amusia
Congenital amusia Congenital syphilis triad
Congenital syphilis triad Cold abscess stages
Cold abscess stages Disease penis
Disease penis Congenital
Congenital Congenital glaucoma
Congenital glaucoma Chapter 42 hearing speech and vision problems
Chapter 42 hearing speech and vision problems Hearing and equilibrium
Hearing and equilibrium Middle ear
Middle ear Beach side sight
Beach side sight Physical barriers to listening
Physical barriers to listening Proxemics in communication skills
Proxemics in communication skills Bsc speech
Bsc speech Puncture resistant container
Puncture resistant container Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Chapter 16 infection control and standard precautions
Chapter 16 infection control and standard precautions