INFECTION CONTROL CONTENTS INTRODUCTION INFECTION TRANSMISSION OF INFECTION






























































- Slides: 89

INFECTION CONTROL

CONTENTS • INTRODUCTION • INFECTION • TRANSMISSION OF INFECTION • MODE OF TRANSMISSION • MODE OF INFECTION CONTROL • INFECTION CONCERN IN DENTISTRY • OBJECTIVES OF INFECTION CONTROL • PERSONAL BARRIER PROTECTION • EMERGENCY & EXPOSURE INCIDENT PLAN • OPERATORY ASEPSIS • DISINFECTION • INSTRUMENT HANDLING & CLEANING • STERILIZATION • MONITORS OF STERILIZATON • CLINICAL WASTE DISPOSAL • STORAGE OF STERILIZED ITEMS • HANDPIECE ASEPSIS • CONCLUSION

INTRODUCTION • Microorganisms are ubiquitous. • Since pathogenic microorganisms cause contamination, infection and decay, it becomes necessary to remove or destroy them from materials and areas. • This is the objective of infection control and sterilization.

DEFINITIONS • INFECTION CONTROL – Also called “exposure control plan” by OSHA is a required office program that is designed to protect personnel against risks of exposure to infection. • STERILIZATION: Use of a physical or chemical procedure to destroy all microorganisms including substantial numbers of resistant bacterial spores. • Sterilization means the destruction of all life forms. (Ronald B Luftig) • Sterilization is the process of killing or removing all viable organisms. (MIMS – PLAYFAIR)

• STERILE: Free from all living microorganisms; usually described as a probability (e. g. , the probability of a surviving microorganism being 1 in 1 million). • DISINFECTION: Destruction of pathogenic and other kinds of microorganisms by physical or chemical means. Disinfection is less lethal than sterilization, because it destroys the majority of recognized pathogenic microorganisms, but not necessarily all microbial forms (e. g. , bacterial spores). • Disinfection is a process of removing or killing most, but not all, viable organisms. (MIMSPLAYFAIR) • Disinfection refers to the destruction of pathogenic organisms. (Ronald B

• DISINFECTANT: A chemical agent used on inanimate objects to destroy virtually all recognized pathogenic microorganisms, but not necessarily all microbial forms (e. g. , bacterial endospores). • ASEPSIS: prevention of microbial contamination of living tissues or sterile materials by excluding, removing or killing microorganisms.

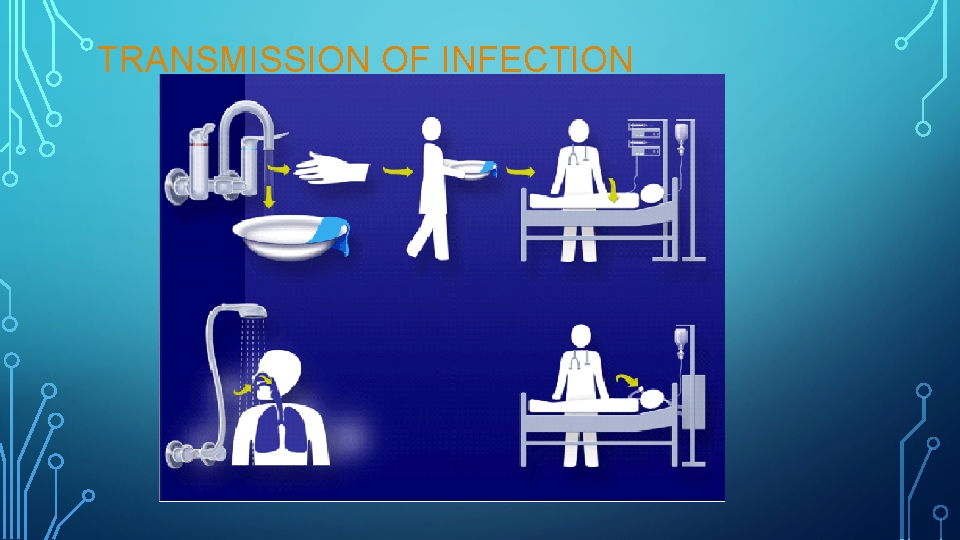
TRANSMISSION OF INFECTION

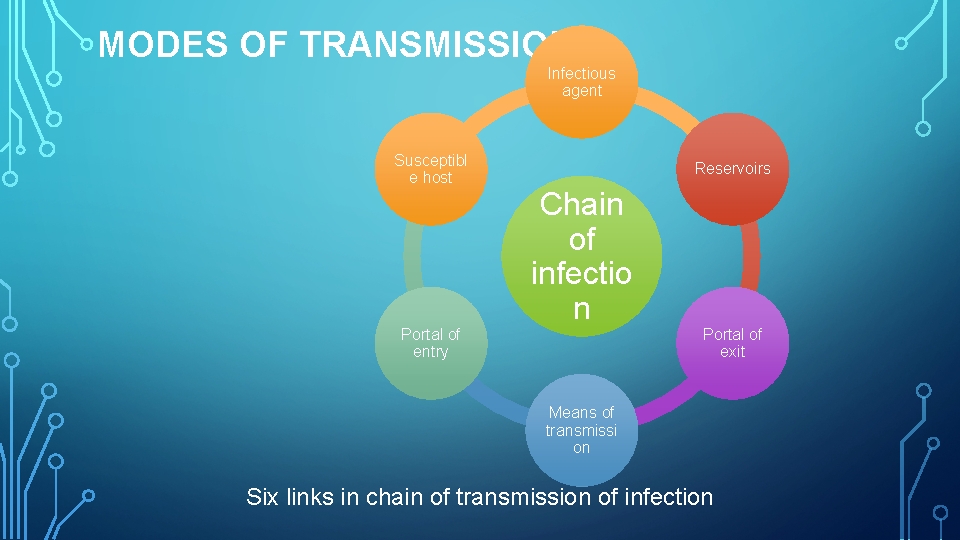
MODES OF TRANSMISSION: Infectious agent Susceptibl e host Reservoirs Chain of infectio n Portal of entry Portal of exit Means of transmissi on Six links in chain of transmission of infection

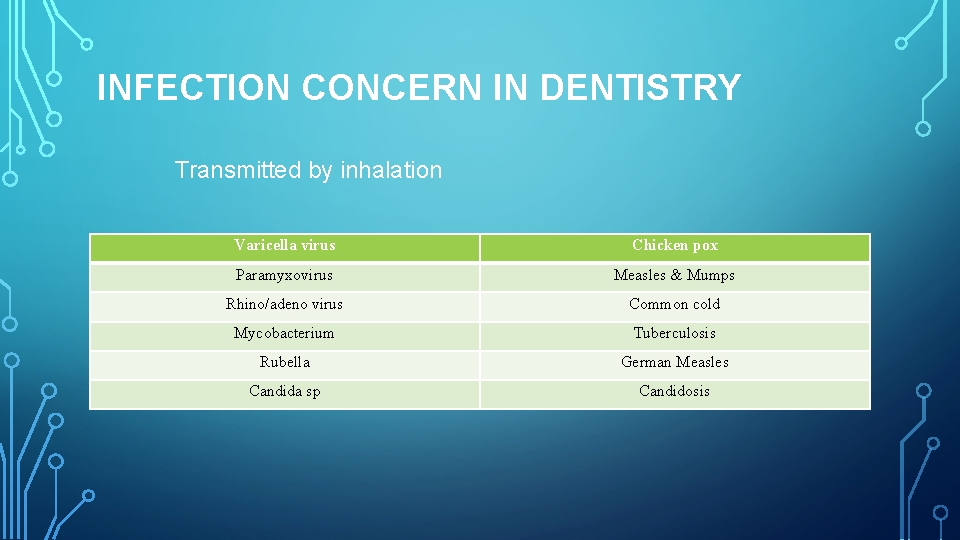
INFECTION CONCERN IN DENTISTRY Transmitted by inhalation Varicella virus Chicken pox Paramyxovirus Measles & Mumps Rhino/adeno virus Common cold Mycobacterium Tuberculosis Rubella German Measles Candida sp Candidosis

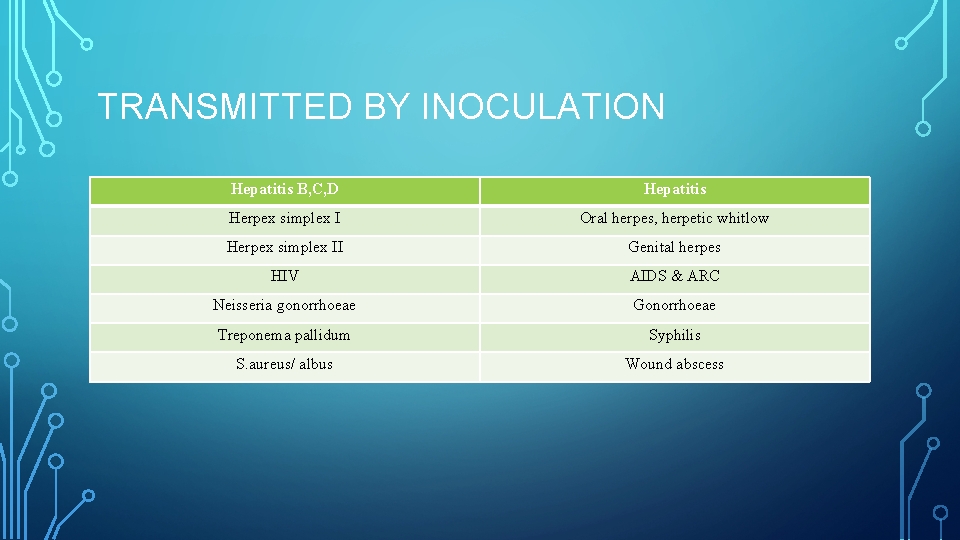
TRANSMITTED BY INOCULATION Hepatitis B, C, D Hepatitis Herpex simplex I Oral herpes, herpetic whitlow Herpex simplex II Genital herpes HIV AIDS & ARC Neisseria gonorrhoeae Gonorrhoeae Treponema pallidum Syphilis S. aureus/ albus Wound abscess

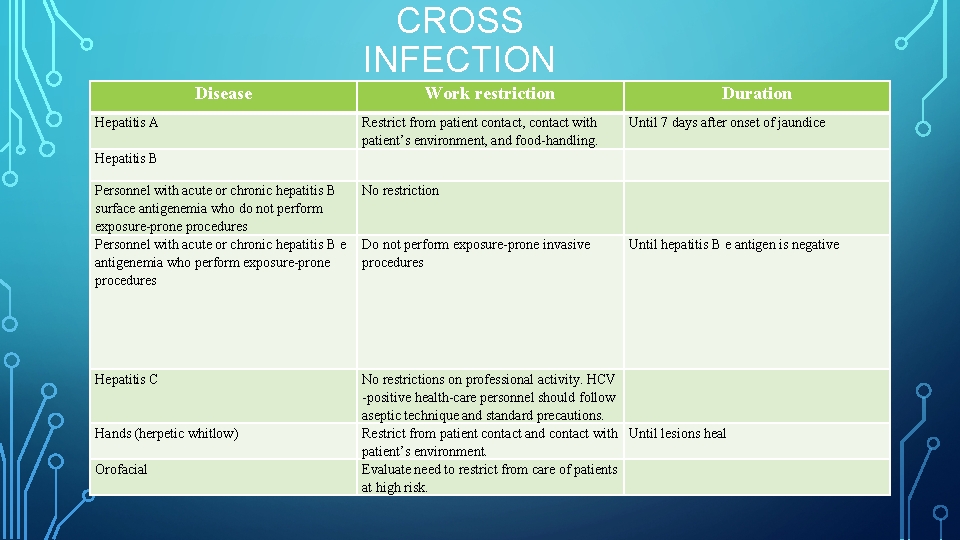
CROSS INFECTION Disease Hepatitis A Hepatitis B Work restriction Restrict from patient contact, contact with patient’s environment, and food handling. Personnel with acute or chronic hepatitis B No restriction surface antigenemia who do not perform exposure prone procedures Personnel with acute or chronic hepatitis B e Do not perform exposure prone invasive antigenemia who perform exposure prone procedures Hepatitis C Hands (herpetic whitlow) Orofacial Duration Until 7 days after onset of jaundice Until hepatitis B e antigen is negative No restrictions on professional activity. HCV positive health care personnel should follow aseptic technique and standard precautions. Restrict from patient contact and contact with Until lesions heal patient’s environment. Evaluate need to restrict from care of patients at high risk.

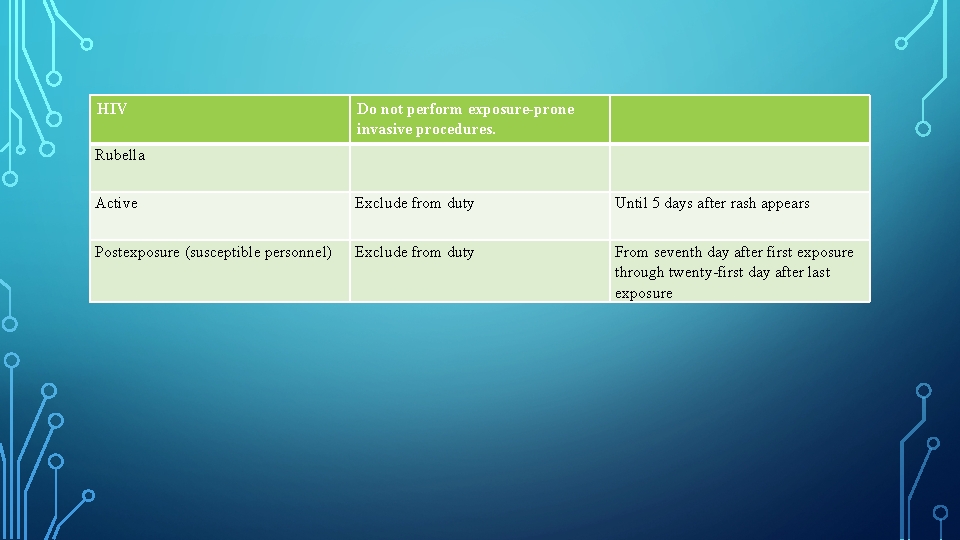
HIV Do not perform exposure-prone invasive procedures. Rubella Active Exclude from duty Until 5 days after rash appears Postexposure (susceptible personnel) Exclude from duty From seventh day after first exposure through twenty first day after last exposure

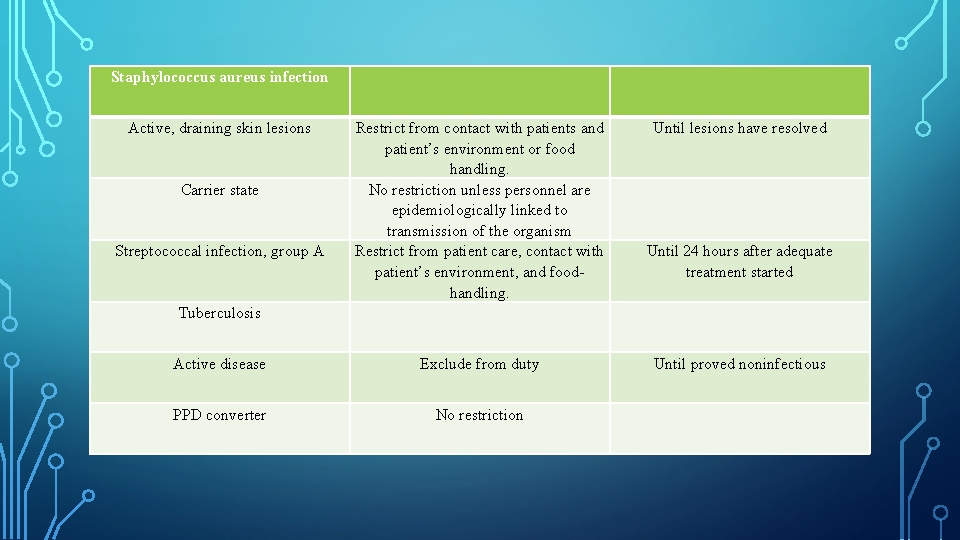
Staphylococcus aureus infection Active, draining skin lesions Until lesions have resolved Tuberculosis Restrict from contact with patients and patient’s environment or food handling. No restriction unless personnel are epidemiologically linked to transmission of the organism Restrict from patient care, contact with patient’s environment, and food handling. Active disease Exclude from duty Until proved noninfectious PPD converter No restriction Carrier state Streptococcal infection, group A Until 24 hours after adequate treatment started

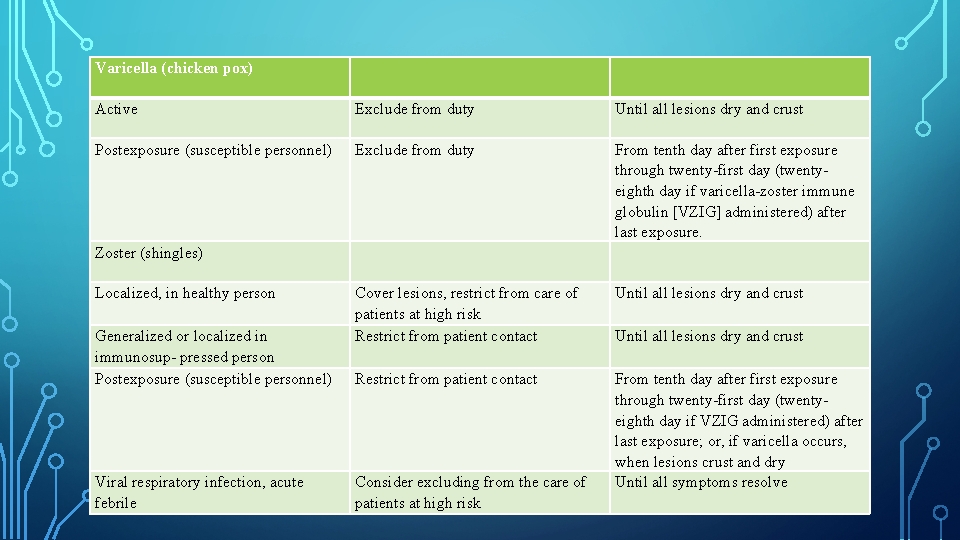
Varicella (chicken pox) Active Exclude from duty Until all lesions dry and crust Postexposure (susceptible personnel) Exclude from duty Zoster (shingles) From tenth day after first exposure through twenty first day (twenty eighth day if varicella zoster immune globulin [VZIG] administered) after last exposure. Localized, in healthy person Cover lesions, restrict from care of patients at high risk Restrict from patient contact Until all lesions dry and crust Restrict from patient contact From tenth day after first exposure through twenty first day (twenty eighth day if VZIG administered) after last exposure; or, if varicella occurs, when lesions crust and dry Until all symptoms resolve Generalized or localized in immunosup pressed person Postexposure (susceptible personnel) Viral respiratory infection, acute febrile Consider excluding from the care of patients at high risk Until all lesions dry and crust

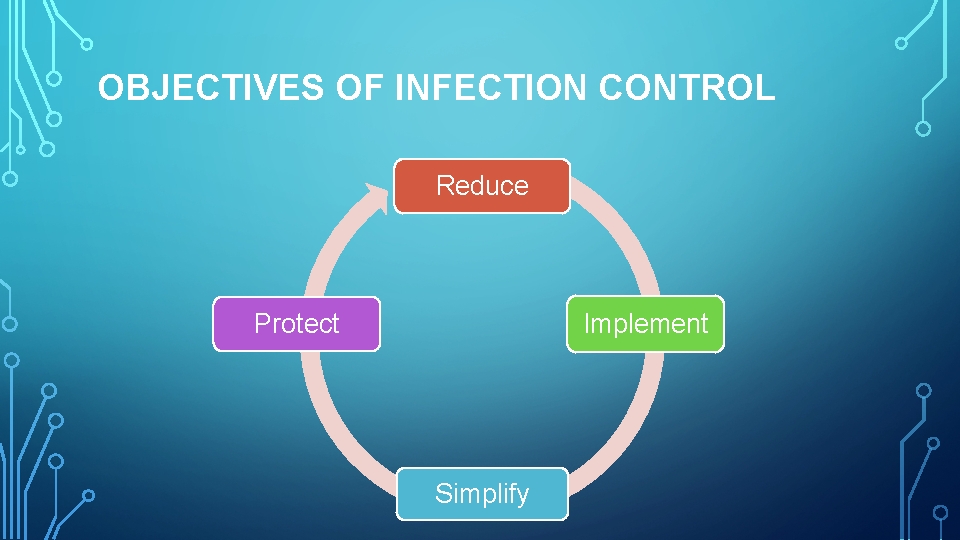
OBJECTIVES OF INFECTION CONTROL Reduce Protect Implement Simplify

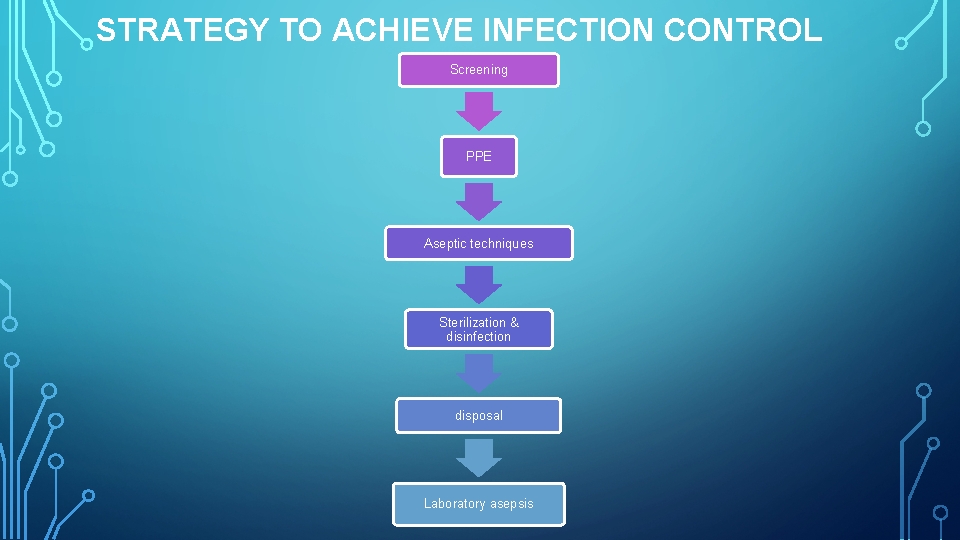
STRATEGY TO ACHIEVE INFECTION CONTROL Screening PPE Aseptic techniques Sterilization & disinfection disposal Laboratory asepsis

PREPROCEDURAL MOUTH RINSE Whe n Wher e How

CONTINUED… • Phenolic related essential oils • Bis-biguanides • Quaternary ammonium compounds • Halogens • Oxygenating agents • A commercial mouthrinse containing 0. 05 percent CPC when used as a preprocedural mouthrinse was equally effective as CHX in reducing the levels of spatter bacteria generated during ultrasonic scaling. Magda Feres et all, JADA 2010

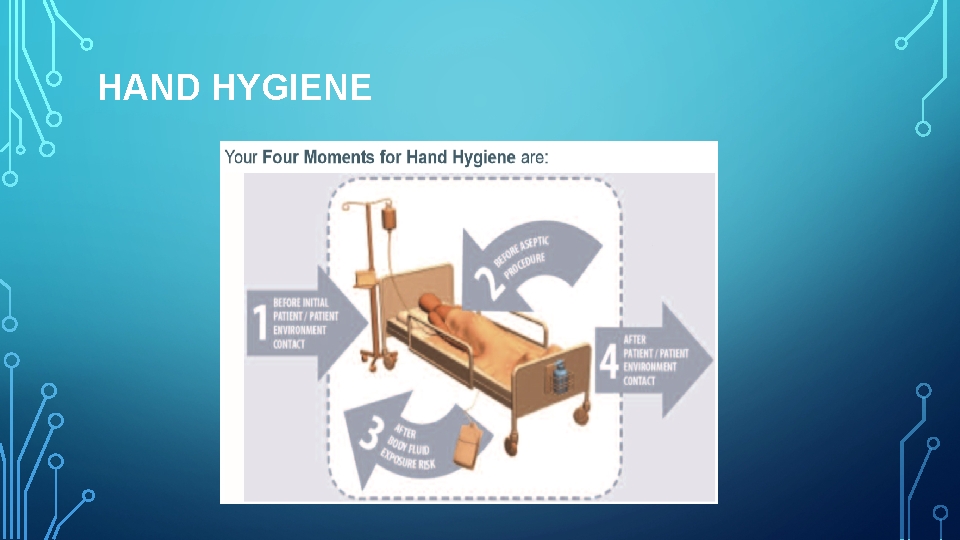
HAND HYGIENE

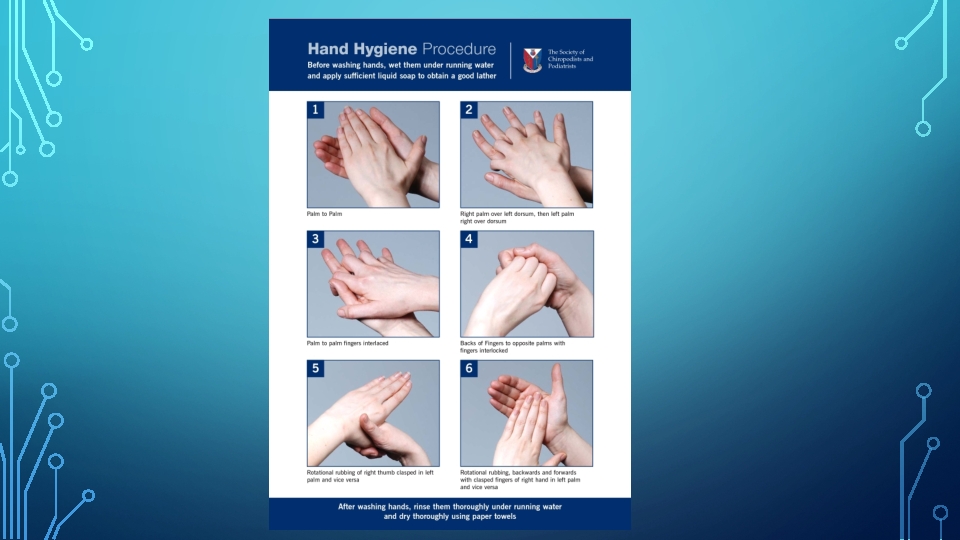
• For routine dental examination procedures, hand washing is achieved by using either a plain or antimicrobial soap and water. • The purpose of surgical hand antisepsis is to eliminate transient flora and reduce resident flora to prevent introduction of organisms in the operative wound, if gloves become punctured or torn. • At the beginning of a routine treatment period, watches and jewelry must be removed and hands must be washed with a suitable cleanser. • Hands must be lathered for at least 10 seconds, rubbing all surfaces and rinsed. • Clean brushes can be used to scrub under and around the nails. • Must be repeated at least once to remove all soil.

HAND CLEANSERS • CHLORHEXIDINE BASED – these contain 2 - 4% chlorhexidine gluconate with 4% isopropyl alcohol in a detergent solution with a p. H of 5. 0 to 6. 5. They have broader activity for special cleansing(e. g: for surgery, glove leaks, or when clinician experiences injury). But it can be hazardous to eyes. • POVIDONE IODONE – contain 7. 5 -10% povidone iodine, used as a surgical hand scrub. • PARACHLOROMETEXYLENOL(PCMX) – they are bactericidal and fungicidal at 2% concentration. Non irritating and recommended for routine use. • ALCOHOL HAND RUBS- ethyl alcohol and isopropyl alcohol are widely used at 70% concentration. They are rapidly germicidal when applied to the skin.


PERSONAL BARRIER PROTECTION • Personal protective equipment (PPE), or barrier precautions, are a major component of Standard precautions. • PPE is essential to protect the skin and the mucous membranes of personnel from exposure to infectious or potentially infectious materials. • The various barriers are gloves, masks, protective eye wear, surgical head cap & overgarments

GLOVES • Types: 1. Latex gloves

VINYL GLOVES

NITRILE GLOVES

NEOPRENE

GENERAL PURPOSE UTILITY GLOVES

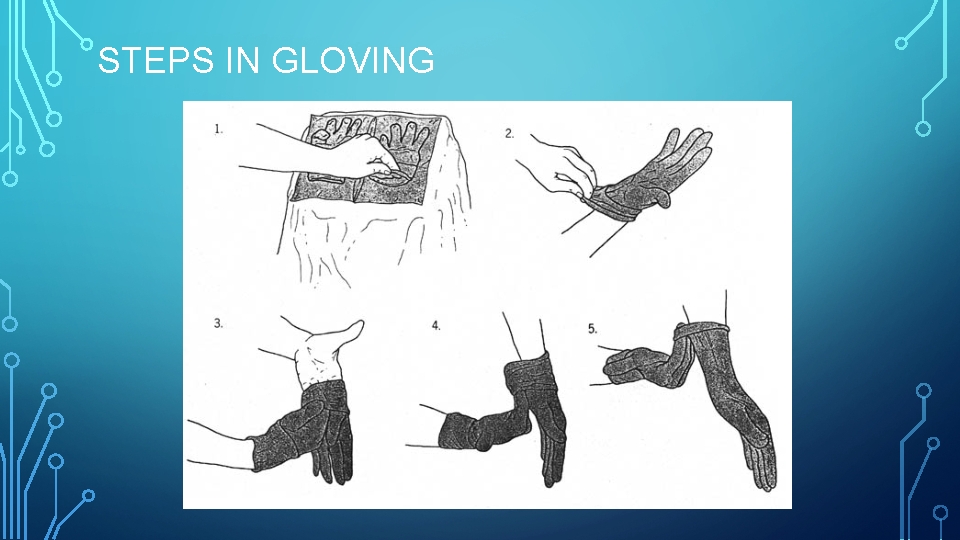
STEPS IN GLOVING

CONTACT DERMATITIS AND LATEX HYPERSENSITIVITY • Contact dermatitis is classified as 1. Irritant 2. Allergic. • Latex hypersensitivity

PRECAUTIONS TAKEN FOR LATEX ALLERGIC PATIENTS • Be aware that latent allergens in the ambient air can cause respiratory or anaphylactic symptoms among persons with latex hypersensitivity. • Patients with latex allergy can be scheduled for the first appointment of the day to mini- mize their inadvertent exposure to airborne latex particles. • Have emergency treatment kits with latex free products available at all times.

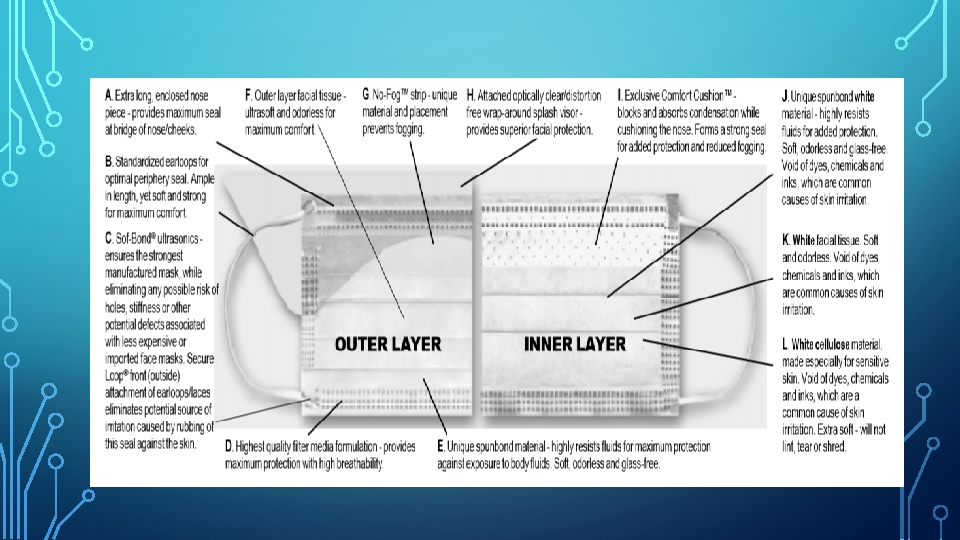
MASKS • Types: 1. Surgical masks (required to have fluid-resistant properties). 1. Procedure/isolation masks • Made up from a melt blown placed between non-woven fabric Layers of a Mask 1. an outer layer 2. a microfiber middle layer - filter large wearer-generated particles 3. a soft, absorbent inner layer - absorbs moisture. • Available in 2 sizes: regular and petite.


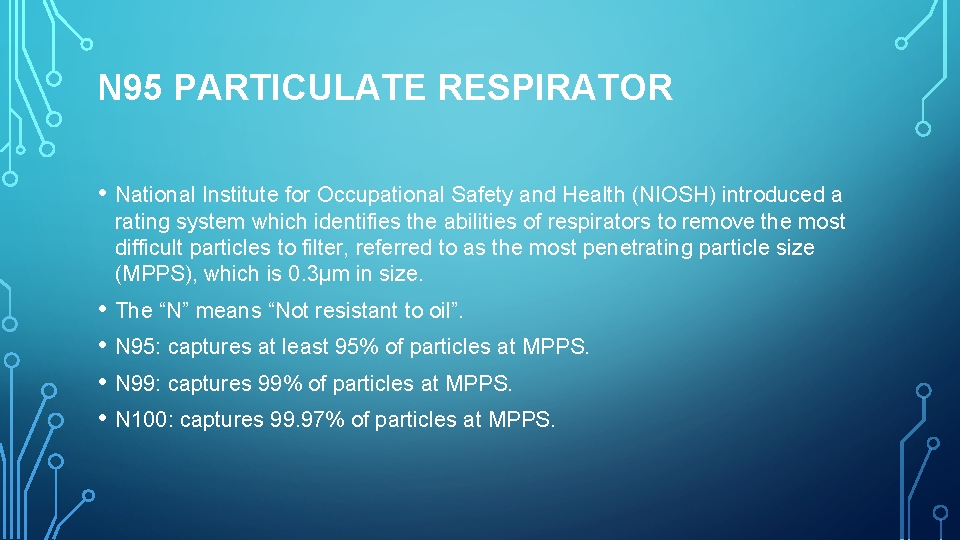
N 95 PARTICULATE RESPIRATOR • National Institute for Occupational Safety and Health (NIOSH) introduced a rating system which identifies the abilities of respirators to remove the most difficult particles to filter, referred to as the most penetrating particle size (MPPS), which is 0. 3µm in size. • The “N” means “Not resistant to oil”. • N 95: captures at least 95% of particles at MPPS. • N 99: captures 99% of particles at MPPS. • N 100: captures 99. 97% of particles at MPPS.

WHEN SHOULD I WEAR AN N 95 RESPIRATOR? N 95 particulate respirator

EYE WEAR • CAUSES OF EYE DAMAGE: 1. Aerosols and spatter may transmit infection 2. Sharp debris projected from mouth while using air turbine handpiece, ultrasonic scaler may cause eye injury. 3. Injuries to eyes of patients caused by sharp instruments especially in supine position

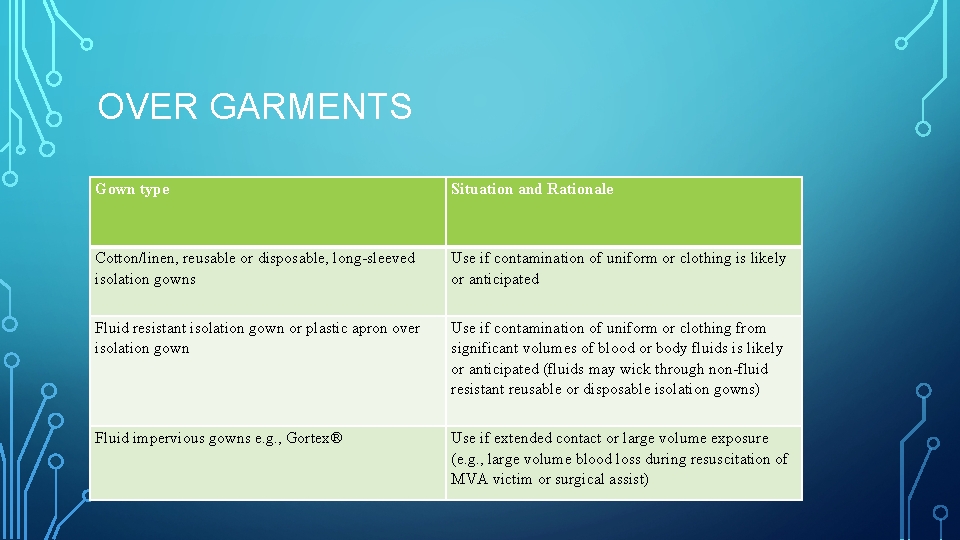
OVER GARMENTS Gown type Situation and Rationale Cotton/linen, reusable or disposable, long sleeved isolation gowns Use if contamination of uniform or clothing is likely or anticipated Fluid resistant isolation gown or plastic apron over isolation gown Use if contamination of uniform or clothing from significant volumes of blood or body fluids is likely or anticipated (fluids may wick through non fluid resistant reusable or disposable isolation gowns) Fluid impervious gowns e. g. , Gortex® Use if extended contact or large volume exposure (e. g. , large volume blood loss during resuscitation of MVA victim or surgical assist)

FOOTWEAR • Most hospitals have their own policies regarding footwear. • Footwear with open heels and/or holes across the top can increase the risk of harm to the person wearing them due to more direct exposure to blood/body fluids or of sharps being dropped for examples.

PRECAUTIONS TO AVOID INJURY EXPOSURE • Engineering controls are the primary method to reduce exposures to blood from sharp instruments and needles • Work-practice controls establish practices to protect personnel whose responsibilities include handling, using, or processing sharp devices. • Sharp end of instruments must be pointed away from the hand • Avoid handling large number of sharp devices.

EMERGENCY & EXPOSURE INCIDENT PLAN • Management of exposure includes: 1. General wound care and cleaning. 2. Counseling of the exposed worker regarding bloodborne pathogens. 3. Source patient testing for HBV, HCV and HIV (consent required). 4. Documentation of the incident and review. 5. Postexposure assessment and prophylaxis for the health care worker. 6. Baseline and follow up serology of the worker

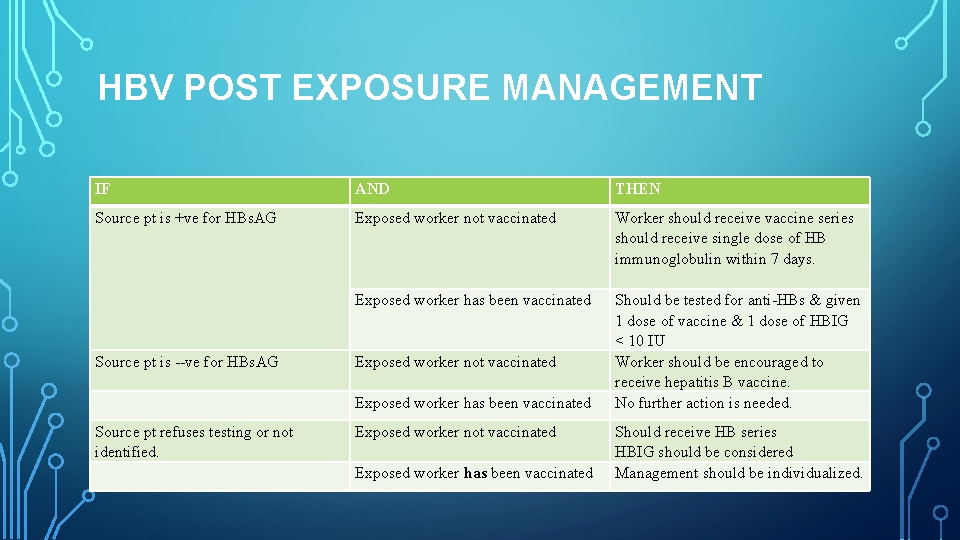
HBV POST EXPOSURE MANAGEMENT IF AND THEN Source pt is +ve for HBs. AG Exposed worker not vaccinated Worker should receive vaccine series should receive single dose of HB immunoglobulin within 7 days. Should be tested for anti HBs & given 1 dose of vaccine & 1 dose of HBIG < 10 IU Worker should be encouraged to receive hepatitis B vaccine. No further action is needed. Exposed worker has been vaccinated Source pt is ve for HBs. AG Exposed worker not vaccinated Source pt refuses testing or not identified. Exposed worker not vaccinated Exposed worker has been vaccinated Should receive HB series HBIG should be considered Management should be individualized.

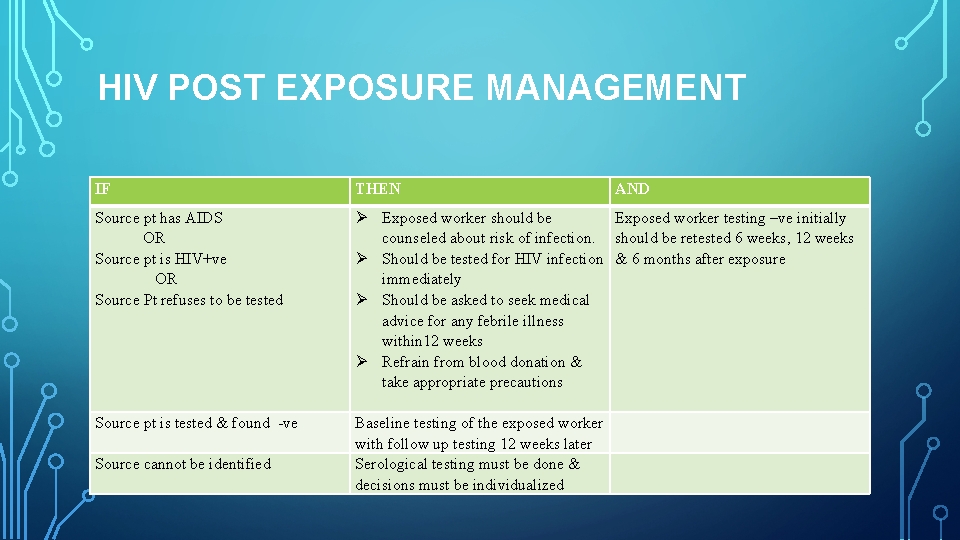
HIV POST EXPOSURE MANAGEMENT IF THEN AND Source pt has AIDS OR Source pt is HIV+ve OR Source Pt refuses to be tested Exposed worker should be counseled about risk of infection. Should be tested for HIV infection immediately Should be asked to seek medical advice for any febrile illness within 12 weeks Refrain from blood donation & take appropriate precautions Baseline testing of the exposed worker with follow up testing 12 weeks later Serological testing must be done & decisions must be individualized Exposed worker testing –ve initially should be retested 6 weeks, 12 weeks & 6 months after exposure Source pt is tested & found ve Source cannot be identified

OPERATORY ASEPSIS • In the dental operatory, environmental surfaces (i. e. , a surface or equipment that does not contact patients directly) can become contaminated during patient care. Certain surfaces, especially ones touched frequently (e. g. , light handles, unit switches, and drawer knobs) can serve as reservoirs of microbial contamination, although they have not been associated directly with transmission of infection to either personnel or patients. • Transfer of microorganisms from contaminated environmental surfaces to patients occurs primarily through personnel hand contact

• Almost 40(1939) years ago, Dr. E. H. Spaulding proposed a classification system for disinfecting and sterilizing medical and surgical instruments. This system, or variations of it, has been used in infection control over the years. Disinfection of surgical instruments in a chemical solution

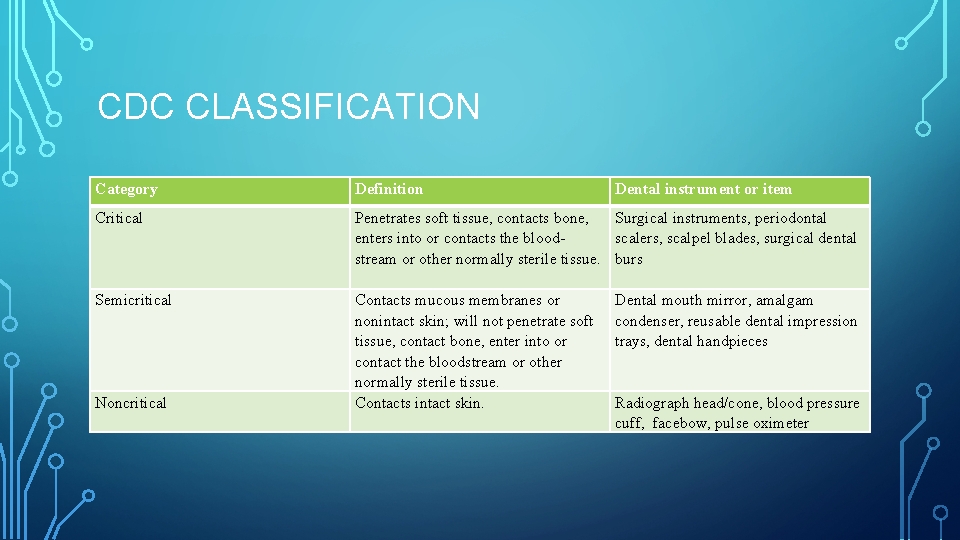
CDC CLASSIFICATION Category Definition Dental instrument or item Critical Penetrates soft tissue, contacts bone, enters into or contacts the blood stream or other normally sterile tissue. Contacts mucous membranes or nonintact skin; will not penetrate soft tissue, contact bone, enter into or contact the bloodstream or other normally sterile tissue. Contacts intact skin. Surgical instruments, periodontal scalers, scalpel blades, surgical dental burs Semicritical Noncritical Dental mouth mirror, amalgam condenser, reusable dental impression trays, dental handpieces Radiograph head/cone, blood pressure cuff, facebow, pulse oximeter

DISINFECTION • Disinfection is always at least a two-step procedure: • The initial step involves vigorous scrubbing of the surfaces to be disinfected and wiping them clean. • The second step involves wetting the surface with a disinfectant and leaving it wet for the time prescribed by the manufacturer.

• The ideal disinfectant has the following properties: 1. Broad spectrum of activity 2. Acts rapidly 3. Non corrosive 4. Environment friendly 5. Is free of volatile organic compounds 6. Nontoxic & nonstaining

• High-level disinfection: Disinfection process that inactivates vegetative bacteria, mycobacteria, fungi, and viruses but not necessarily high numbers of bacterial spores. • Intermediate-level disinfection: Disinfection process that inactivates vegetative bacteria, the majority of fungi, mycobacteria, and the majority of viruses (particularly enveloped viruses) but not bacterial spores. • Low-level disinfectant: Liquid chemical germicide. OSHA requires lowlevel hospital disinfectants also to have a label claim for potency against HIV and HBV. • Gigasept which contains succindialdehyde and dimethoxytetrahydrofuran are used for disinfection of plastic and rubber materials eg: dental chair

CLEANING AND DISINFECTION STRATEGIES FOR BLOOD SPILLS • Strategies for decontaminating spills of blood and other body fluids differ by setting and volume of the spill. • The person assigned to clean the spill should wear gloves and other PPE as needed. • Visible organic material should be removed with absorbent material (e. g. , disposable paper towels discarded in a leak-proof, appropriately labeled container). • Nonporous surfaces should be cleaned and then decontaminated with either an hospital disinfectant effective against HBV and HIV or an disinfectant with a tuberculocidal claim (i. e. , intermediate-level disinfectant). • However, if such products are unavailable, a 1: 100 dilution of sodium hypochlorite (e. g. , approximately ¼ cup of 5. 25% household chlorine bleach

PRINCIPLES AND PROCEDURES FOR HANDLING AND CLEANING INSTRUMENTS AFTER TREATMENT • The safest and most efficient instrument cleaning procedures involve ultrasonic cleaning of used instruments kept in a perforated basket or cassette throughout the cleaning procedure. • Used instruments are commonly placed in an anti microbial solution as this softens and loosens debris. • Next, move the or basket of instruments into an ultrasonic cleaning device, rinse them, and then carefully inspect the instruments for debris. • Dip instruments likely to rust into a rust inhibitor solution. Drain & dry instruments with absorbent towel.

ULTRASONIC CLEANERS AND SOLUTIONS • An ultrasonic cleaner uses sound waves, that are outside the human hearing range to form oscillating bubbles, a process called cavitation. • These bubbles act on debris to remove it from the instruments. • Ultrasonic cleaning is the safest and most efficient way to clean sharp instruments. • Operate the tank at one-half to three-fourths full of cleaning solution at all times- Use only cleaning solutions recommended by ultrasonic device manufacturers. • Operate the ultrasonic cleaner for 3 -6 minutes for loose instruments 10 -20 mins for cassettes or longer as directed by the manufacturer to give optimal

INSTRUMENT WASHER • Instrument washers use high-velocity hot water and a detergent to clean instruments. • These devices require personnel to either place instruments in a basket or to use instrument cassettes during the cleaning and drying cycles. • Types: 1. Counter top model 2. Resembles a kitchen dish washer

THERMAL DISINFECTORS • These devices may look like the instrument washers described above; however, there is one important difference. • The high temperature of the water and chemical additives in these devices cleans and disinfects the instruments. • Instruments can be more safely handled, and if the dental healthcare professional were to sustain a puncture injury, it would not require the follow-up that a contaminated exposure requires

NEW SOLAR ENERGY TECHNOLOGY: KILLING GERMS ON MEDICAL, DENTAL INSTRUMENTS • “It is completely off-grid, uses sunlight as the energy source, is not that large, kills disease-causing microbes effectively and relatively quickly and is easy to operate. • Halas and colleagues have prototypes of two solar steam machines. 1. The autoclave for sterilizing medical and dental instruments. 2. Autoclave for disinfecting human and animal wastes

• Metallic nanoparticles bits of material so small that hundreds would fit inside the period go into a container of water. • Sunlight focused into the water quickly heats the nanoparticles, which scientists are terming “nanoheaters. ” • A layer of steam forms on the nanoheaters and buoys them up to the water’s surface. They release the steam and sink back down into the water to repeat the process. • “Nanoheaters generate steam at a remarkably high efficiency, ” Halas said. “More than 80 percent of the energy they absorb from sunlight goes into production of steam. • The prototype autoclaves consist of a dish-like mirror that focuses sunlight into a container of water with the nanoheaters.

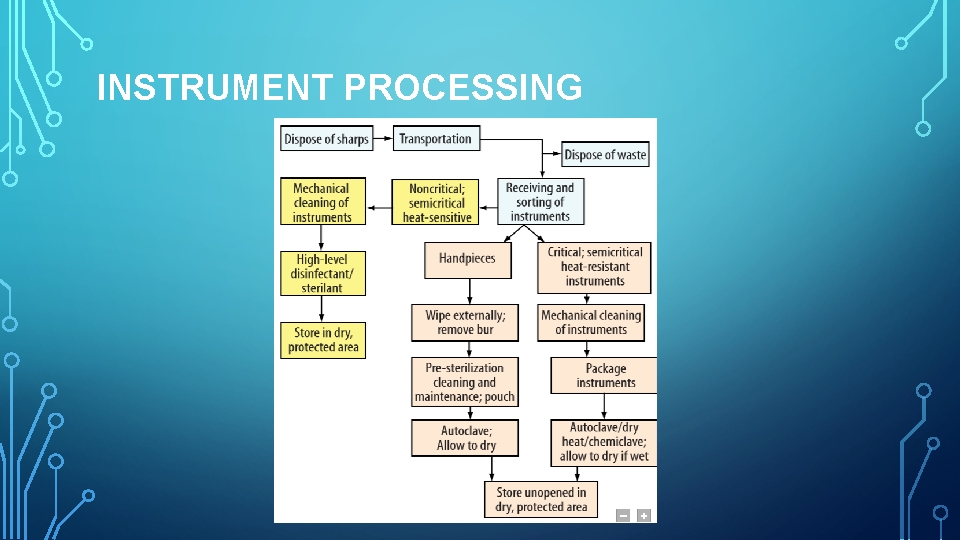
INSTRUMENT PROCESSING

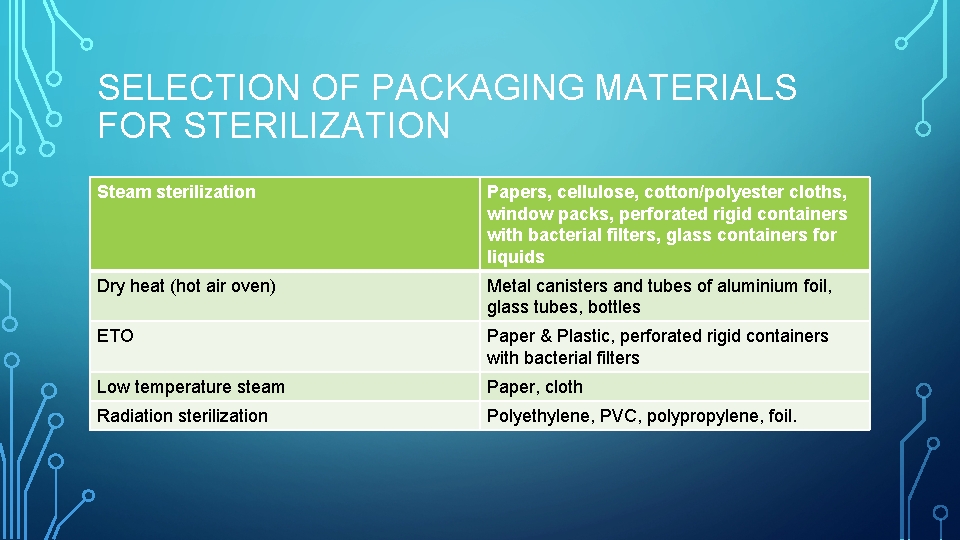
SELECTION OF PACKAGING MATERIALS FOR STERILIZATION Steam sterilization Papers, cellulose, cotton/polyester cloths, window packs, perforated rigid containers with bacterial filters, glass containers for liquids Dry heat (hot air oven) Metal canisters and tubes of aluminium foil, glass tubes, bottles ETO Paper & Plastic, perforated rigid containers with bacterial filters Low temperature steam Paper, cloth Radiation sterilization Polyethylene, PVC, polypropylene, foil.

STERILIZATION • Stages for instrument sterilization: 1. Presoaking 2. Cleaning 3. Corrosion control and lubrication 4. Packaging 5. Sterilization 6. Handling sterile instruments 7. Storage 8. Distribution

AGENTS USED IN STERILIZATION • Physical agents: 1. Sunlight 2. Drying 3. Dryheat: flaming, incineration, hot air 4. Moist heat: pasteurization, boiling, steam under pressure, steam under normal pressure. • Chemical agents: 1. Alcohols: ethyl, isopropyl, trichlorobutanol 2. Aldehydes: formaldehyde, glutaraldehyde 3. Dyes 4. Halogens Filtration: candles asbestos pads, membranes 5. Phenols Surface-active agents 6. Radiation Metallic salts 7. Ultrasonic and sonic vibrations 8. Gases: ethylene oxide, 5.

THE FOUR ACCEPTED METHODS OF STERILIZATION ARE : • Steam pressure sterilization (autoclave) • Chemical vapor pressure sterilization- (chemiclave) • Dry heat sterilization (dryclave) • Ethylene oxide sterilization

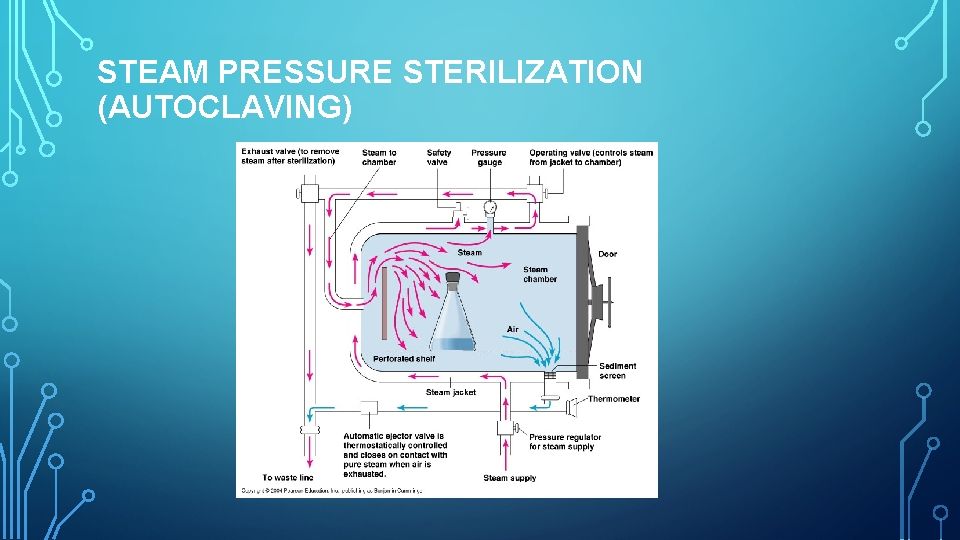
STEAM PRESSURE STERILIZATION (AUTOCLAVING)

• Advantages of Autoclaves. 1. Autoclaving is the most rapid and 2. 3. • Disadvantages of Autoclaves. 1. Items sensitive to the elevated effective method for sterilizing cloth surgical packs and towel packs. 2. Autoclaving tends to rust carbon steel instruments and burs. Is dependable and economical 3. Instruments must be air dried at completion of cycle Sterilization is verifiable. temperature cannot be autoclaved.

TYPES OF AUTOCLAVE DOWNWARD DISPLACEMENT • Also known as Gravity displacement unit. • This is because of the method of air removal in the sterilization chamber. POSITIVE PRESSURE DISPLACEMENT • It’s an improvement over downward displacement autoclave. • Steam is created in a second, separate chamber and held until the proper amount to displace all of the air in the sterilization chamber is accumulated. • The steam is then released into the sterilization chamber in a pressurized blast, forcing the air out through the drain hole and starting the sterilization process

• NEGATIVE PRESSURE DISPLACEMENT • one of the most accurate types of unit available • Once the sterilization chamber door is closed, a vacuum pump removes the air. • Steam is created in a second, separate chamber. • Once the air has been completely removed from the sterilization chamber, the steam is then released into the sterilization chamber in a pressurized blast much like that of a positive pressure displacement unit. • The negative pressure displacement unit is able to achieve a high "Sterility Assurance Level" (SAL), but the system can be quite large and costly.

• TRIPLE VACUUM AUTOCLAVE • A triple vacuum autoclave is set up/function in a similar fashion to a negative pressure displacement. • This is repeated three times, hence the name "triple vacuum" autoclave. This type of autoclave is suitable for all types of instruments and is very versatile

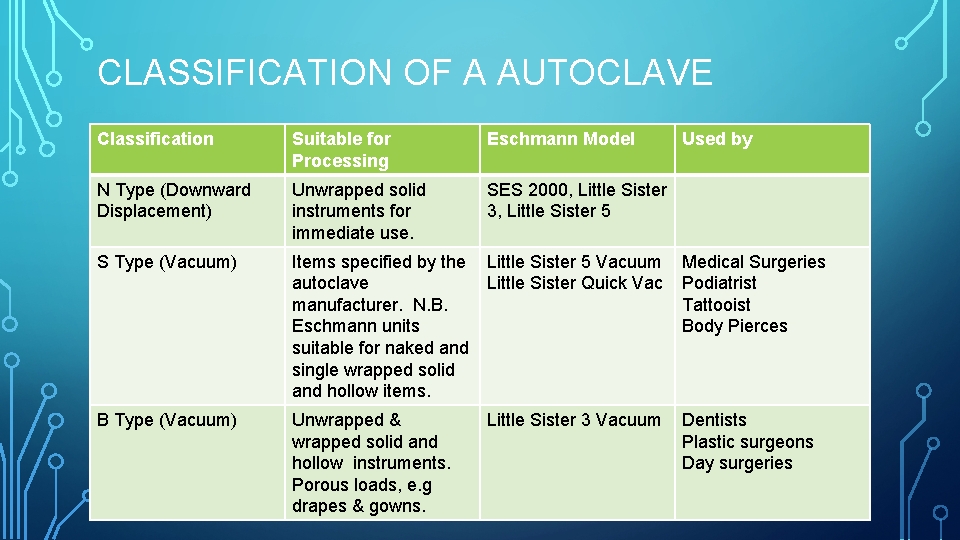
CLASSIFICATION OF A AUTOCLAVE Classification Suitable for Processing Eschmann Model Used by N Type (Downward Displacement) Unwrapped solid instruments for immediate use. SES 2000, Little Sister 3, Little Sister 5 S Type (Vacuum) Items specified by the Little Sister 5 Vacuum Medical Surgeries autoclave Little Sister Quick Vac Podiatrist manufacturer. N. B. Tattooist Eschmann units Body Pierces suitable for naked and single wrapped solid and hollow items. B Type (Vacuum) Unwrapped & wrapped solid and hollow instruments. Porous loads, e. g drapes & gowns. Little Sister 3 Vacuum Dentists Plastic surgeons Day surgeries

CHEMICAL VAPOR PRESSURE STERILIZATION (CHEMICLAVING)

The 1938 patent of dr. George hollenback and the work of hollenback and harvey in 1940 s culminated in the development of an unsaturated chemical vapor system , also called harvey chemiclave. • Advantages 1. Carbon steel and other corrosion- • Disadvantages 1. Items sensitive to the elevated sensitive instruments are said to be sterilized without rust. 2. 3. 4. Relatively quick turnaround time for instruments. Load comes out dry. Sterilization is verifiable. temperature will be damaged. Vapor odor is offensive, requires aeration. 2. Heavy cloth wrappings of surgical instruments may not be penetrated to provide sterilization.

DRY HEAT STERILIZATION • Conventional Dry Heat Ovens • Short-Cycle, High-Temperature Dry Heat Ovens

• Advantages of Dry Heat Sterilization 1. 2. • Disadvantages of Dry Heat Sterilization Carbon steel instruments and burs do not rust, corrode, if they are well dried before processing. 1. High temperatures may damage more heat-sensitive items, such as- rubber or plastic goods. Industrial forced-draft hot air ovens usually provide a larger capacity at a reasonable price. 2. Sterilization cycles are prolonged at the lower temperatures. 3. Must be calibrated and monitored 3. Rapid cycles are possible at high temperatures. 4. Low initial cost and sterilization is verifiable.

ETHYLENE OXIDE STERILIZATION (ETO) MOBILE FUMIGATOR

• Advantages: 1. Operates effectively at low • Disadvantages: 1. Potentially mutagenic and temperatures 2. 3. 4. carcinogenic. Gas is extremely penetrative 2. Can be used for sensitive equipment like handpieces. Requires aeration chamber , cycle time lasts hours 3. Usually only hospital based. Sterilization is verifiable

GAMMA RADIATION • The Nature of Gamma Radiation A form of pure energy that is generally characterized by its deep penetration and low dose rates, Gamma Radiation effectively kills microorganisms throughout. • Benefits of Gamma Radiation include: 1. precise dosing 2. rapid processing 3. uniform dose distribution 4. system flexibility 5. dosimetric release–the immediate availability of product after processing. • Penetrating Sterilization: Even with High-Density Products Gamma Radiation is a penetrating sterilant. • Substantial Decrease in Organism Survival: Gamma Radiation kills microorganisms by attacking the DNA molecule.

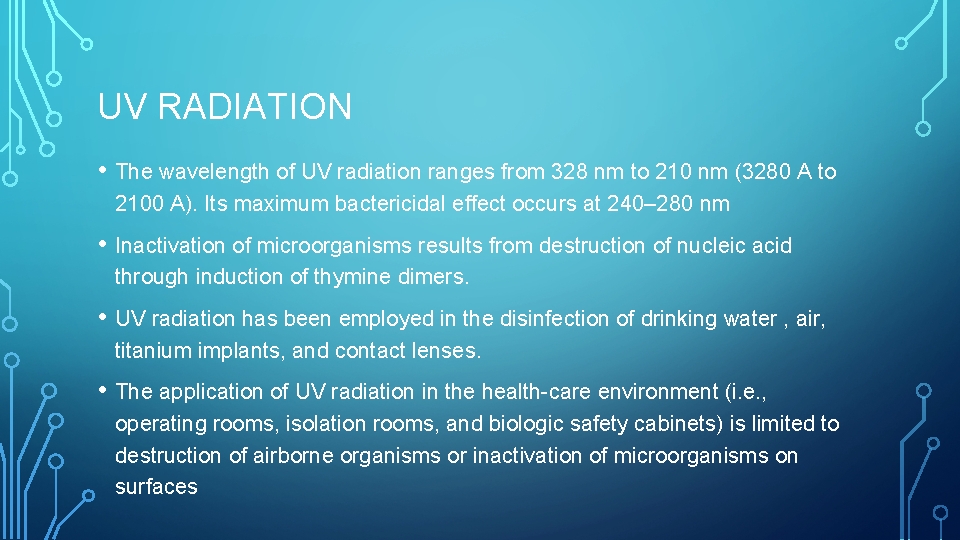
UV RADIATION • The wavelength of UV radiation ranges from 328 nm to 210 nm (3280 A to 2100 A). Its maximum bactericidal effect occurs at 240– 280 nm • Inactivation of microorganisms results from destruction of nucleic acid through induction of thymine dimers. • UV radiation has been employed in the disinfection of drinking water , air, titanium implants, and contact lenses. • The application of UV radiation in the health-care environment (i. e. , operating rooms, isolation rooms, and biologic safety cabinets) is limited to destruction of airborne organisms or inactivation of microorganisms on surfaces

FLASH STERILIZATION • “Flash” steam sterilization was originally defined by Underwood and Perkins as sterilization of an unwrapped object at 1320 C for 3 minutes at 27 -28 lbs. of pressure in a gravity displacement sterilizer. • Currently, the time required for flash sterilization depends on the type of sterilizer and the type of item (i. e. , porous vs non-porous items). • Uses: • Flash sterilization is considered acceptable for processing cleaned patientcare items that cannot be packaged, sterilized, and stored before use. • It also is used when there is insufficient time to sterilize an item by the preferred package method.

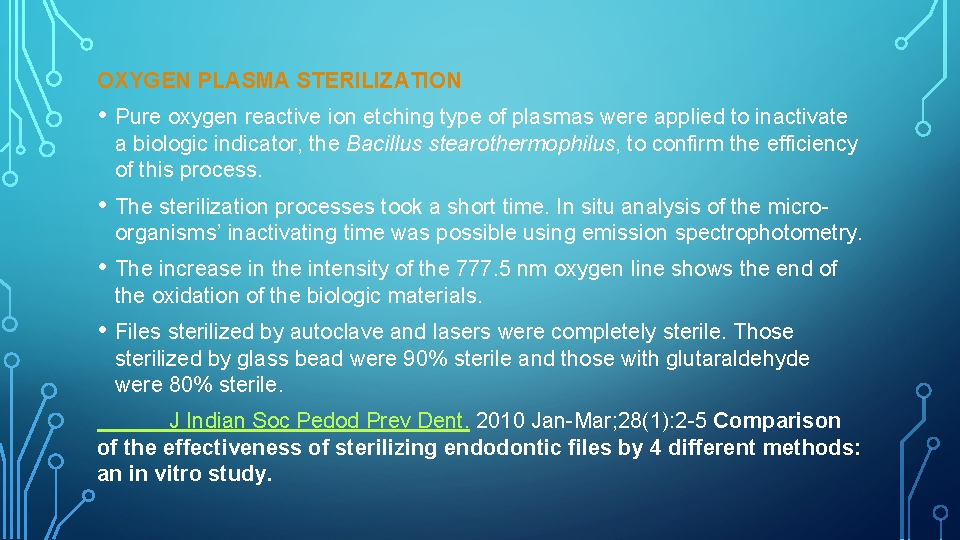
OXYGEN PLASMA STERILIZATION • Pure oxygen reactive ion etching type of plasmas were applied to inactivate a biologic indicator, the Bacillus stearothermophilus, to confirm the efficiency of this process. • The sterilization processes took a short time. In situ analysis of the microorganisms’ inactivating time was possible using emission spectrophotometry. • The increase in the intensity of the 777. 5 nm oxygen line shows the end of the oxidation of the biologic materials. • Files sterilized by autoclave and lasers were completely sterile. Those sterilized by glass bead were 90% sterile and those with glutaraldehyde were 80% sterile. J Indian Soc Pedod Prev Dent. 2010 Jan-Mar; 28(1): 2 -5 Comparison of the effectiveness of sterilizing endodontic files by 4 different methods: an in vitro study.

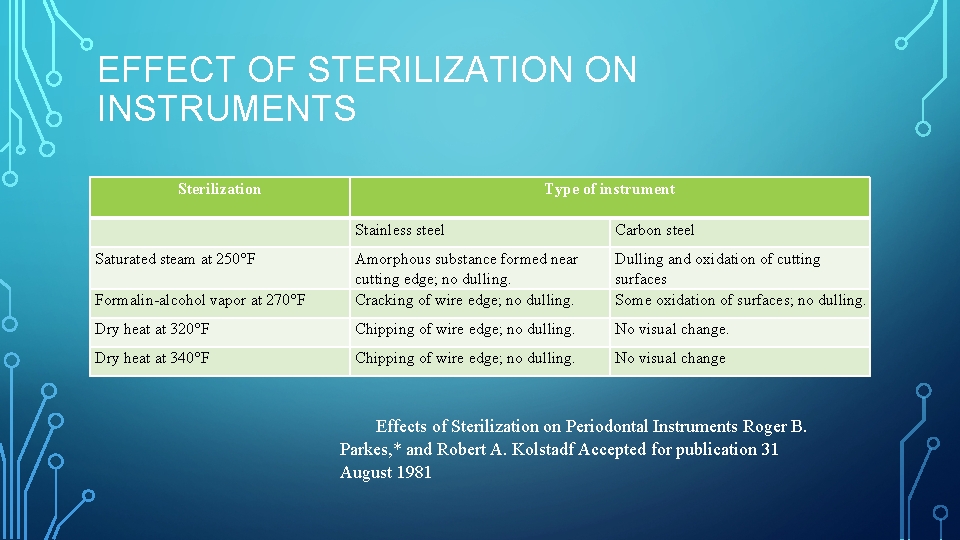
EFFECT OF STERILIZATION ON INSTRUMENTS Sterilization Type of instrument Carbon steel Stainless steel Saturated steam at 250°F Formalin alcohol vapor at 270°F Amorphous substance formed near cutting edge; no dulling. Cracking of wire edge; no dulling. Dulling and oxidation of cutting surfaces Some oxidation of surfaces; no dulling. Dry heat at 320°F Chipping of wire edge; no dulling. No visual change. Dry heat at 340°F Chipping of wire edge; no dulling. No visual change Effects of Sterilization on Periodontal Instruments Roger B. Parkes, * and Robert A. Kolstadf Accepted for publication 31 August 1981

OTHER STERILIZATION METHODS • Dry-Heat Sterilizers • Liquid Chemicals • Performic Acid • Filtration • Microwave • Glass Bead “Sterilizer” • Vaporized Hydrogen Peroxide • Formaldehyde Steam • Gaseous Chlorine Dioxide • Vaporized Peracetic Acid • Infrared radiation

NEW METHODS OF STERILIZATION • Various new methods of sterilization are under investigation and development. • Peroxide vapor sterilization - an aqueous hydrogen peroxide solution boils in a heated vaporizer and then flows as a vapor into a sterilization chamber containing a load of instruments at low pressure and low temperature • Ultraviolet light - exposes the contaminants with a lethal dose of energy in the form of light. The UV light will alter the DNA of the pathogens. Not effective against RNA viruses like HIV.

OZONE • Ozone sterilization is the newest low-temperature sterilization method recently introduced in the US and is suitable for many heat sensitive and moisture sensitive or moisture stable medical devices • Ozone sterilization is compatible with stainless steel instruments. • Ozone Parameters • The cycle time is approximately 4. 5 hours, at a temperature of 850 F – 940 F.

STORAGE AND CARE OF STERILE INSTRUMENTS • Storage areas should be dust proof, dry, well ventilated and easily accessible for routine dental use. • Sterile materials should be stored atleast 8 -10 inches from the floor, atleast 18 inches from the ceiling, and atleast 2 inches from the outside walls. • Items should be positioned so that packaged items are not crushed, bent, crushed, compressed or punctured. • • Items are not stored in any location where they can become wet. • Ultra violet chambers and formalin chambers are now commonly used for storage of instruments. Outside shipping containers and corrugated cartons should not be used as containers in sterile storage areas.

MONITORS OF STERILIZATION • There are 3 methods of monitoring sterilization: • Mechanical techniques • Chemical indicators 1. Internal 2. External • Biological indicators

STERILIZATION METHOD AUTOCLAVE SPORE TYPE INCUBATION TEMPERATURE Bacillus stearothemophilus 56°C Bacillus subtilis 37°C B. Pumilus E 601 370 C CHEMICAL VAPOR DRY HEAT ETHYLENE OXIDE Gamma radiation Sterilization monitoring has four components: 1. a sterilization indicator on the instrument bag, stamped with the date it is sterilized, 2. daily color change process indicator strips, 3. weekly biologic spore test, and 4. documentation notebook.

HANDPIECE ASEPSIS • Oral fluid contamination problems of rotary equipment and especially the high-speed handpiece involve: • • • contamination of hand-piece external surfaces and crevices, • • growth of environmental aquatic bacteria in water lines turbine chamber contamination that enters the mouth, water spray retraction and aspiration of oral fluids into the water lines of older dental units exposure of personnel to spatter and aerosols generated by intraoral use of rotary equipment.

HANDPIECE SURFACE CONTAMINATION CONTROL

ULTRASONIC SCALARS • Soak inserts in a container containing 70% isopropyl alcohol for removal of organic debris. • Rinse cleaned inserts thoroughly in warm water to remove all chemicals. As a final rinse, replace the insert into the scaler handpiece and operate the scaler for 10 seconds at the maximum water flow setting to flush out any retained chemicals • Dry inserts completely with air syringe • Package in proper wrap, bags, pouches, trays, or cassettes. Add spore tests and chemical indicators. • Ethylene Oxide is the preferred method of choice • Dry heat and chemical vapor methods of sterilization are considered ineffective methods with risk of damage to materials as per American Dental association Supplement to J. A. D. A. 8/92.

CLINICAL WASTE DISPOSAL • Red: Anatomical waste • Yellow: waste which requires disposal by incineration only • Black: Domestic waste minimum treatment/disposal required is landfill, municipal incineration. • Blue: medicinal waste for incineration • White: amalgam waste for recovery.

CONCLUSION • Pervasive increases in serious transmissible diseases over the last few decades have created global concern and impacted the treatment mode of all health care practitioners. • Emphasis has now expanded to assuring and demonstrating to patients that they are well protected from risks of infectious disease. • Infection control has helped to allay concerns of the health care personnel and instill confidence and in providing a safe environment for both patient and personnel.

• • • • REFERENCES: Pathways of the pulp, 9 th edition, armamentarium & sterilization. Cohen Operative dentistry, infection control, 4 edition, sturdevent. th Grossmans endodontic practice, 11 th edition, instrument sterilization. Textbook of microbiology, sterilization and disinfection, 7 th edition, Ananthanarayan Textbook of clinical periodontology, Newman, Takei, Carranza, 11 th edition. Introduction to sterilization disinfection & infection control, 2 nd edition, Joan F Gardner Sterilization and disinfection of dental instruments by ADA Disinfection & sterilization of dental instruments TB MED 266, 1995 CDC, guidelines for disinfection & sterilization in health care facilities 2008. Infection prevention and control, college of respiratory therapists Ontario, june 2011 Effects of sterilization on periodontal instruments, JOP, vol 53, no: 7, 1982. New CDC guidelines for selected infection control procedures, chris miller. CDC guidelines for infection control in dental health care settings, Dec 19, 2003/vol. 52. Sterilization of ultrasonic inserts.
 Puncture resistant container
Puncture resistant container Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Contents introduction
Contents introduction 4-way handshake
4-way handshake Transmission control block
Transmission control block Tcp (transmission control protocol) to protokół
Tcp (transmission control protocol) to protokół Transmission control protocol
Transmission control protocol Wireless electricity
Wireless electricity Labial mounting method
Labial mounting method Quats cosmetology
Quats cosmetology Chapter 16 infection control and standard precautions
Chapter 16 infection control and standard precautions Chapter 16 infection control and standard precautions
Chapter 16 infection control and standard precautions Chapter 15:3 washing hands
Chapter 15:3 washing hands Chapter 15:3 washing hands
Chapter 15:3 washing hands Certification in infection control cic
Certification in infection control cic Infection control definition
Infection control definition Infection control in dental radiology
Infection control in dental radiology Learning objectives for infection control
Learning objectives for infection control Infection control conclusion
Infection control conclusion Neutropenic precautions
Neutropenic precautions Cbic recertification
Cbic recertification Infection control is everyone's responsibility
Infection control is everyone's responsibility Infection control
Infection control Standard precautions nsw health
Standard precautions nsw health Infection control information
Infection control information Standard 3 infection control
Standard 3 infection control Infection control orientation
Infection control orientation Infection control
Infection control Infection control audits
Infection control audits Environmental controls infection control
Environmental controls infection control Infection control isolation signs
Infection control isolation signs Infection control committee
Infection control committee Conclusion of infection control
Conclusion of infection control 10 principles of infection control
10 principles of infection control Infection control champion
Infection control champion Unit 13 infection control
Unit 13 infection control Infection control surveyor worksheet
Infection control surveyor worksheet Chapter 10 infection control
Chapter 10 infection control Portfolio of evidence example
Portfolio of evidence example Deep perineal pouch contents
Deep perineal pouch contents Febrile non hemolytic transfusion reaction
Febrile non hemolytic transfusion reaction Febrile nonhemolytic transfusion reaction
Febrile nonhemolytic transfusion reaction Mediastinum
Mediastinum Adductor hiatus
Adductor hiatus Middle mediastinum: contents mnemonic
Middle mediastinum: contents mnemonic The immortal life of henrietta lacks table of contents
The immortal life of henrietta lacks table of contents Medial lemniscus
Medial lemniscus The vertebral region is to the scapular region
The vertebral region is to the scapular region Ark of the covenant lampstand
Ark of the covenant lampstand Anatomy of a comic book
Anatomy of a comic book Mesenteric root
Mesenteric root Mla table of contents
Mla table of contents Stylistic synonyms lexicology
Stylistic synonyms lexicology School magazine cover
School magazine cover What is methodology
What is methodology Appendix writing
Appendix writing Fresh frozen plasma contents
Fresh frozen plasma contents Occupied
Occupied Mediastinum
Mediastinum Indicative and informative verbs
Indicative and informative verbs Persepolis table of contents
Persepolis table of contents Persepolis table
Persepolis table Interactive notebook table of contents
Interactive notebook table of contents Cryoprecipitate contains
Cryoprecipitate contains Funiculus separans
Funiculus separans Spermatic cord coverings
Spermatic cord coverings Greater sciatic foramen
Greater sciatic foramen Event management swot analysis
Event management swot analysis Anterior mediastinum contents
Anterior mediastinum contents Superior mediastinum
Superior mediastinum Carotid sheath contents
Carotid sheath contents Triangular space contents
Triangular space contents Contents of curriculum vitae
Contents of curriculum vitae Ctd module 2 table of contents
Ctd module 2 table of contents What is cost audit report
What is cost audit report Interactive notebook table of contents
Interactive notebook table of contents The city of ember poppy
The city of ember poppy Fresh frozen plasma contents
Fresh frozen plasma contents Contents of air pollution
Contents of air pollution Content page magazine
Content page magazine Cubital fossa boundaries
Cubital fossa boundaries Fossa in elbow joint
Fossa in elbow joint Carotid sheath contents
Carotid sheath contents Thigh muscles medial
Thigh muscles medial Cubital fossa boundaries
Cubital fossa boundaries Contents of femoral triangle
Contents of femoral triangle Transverse humeral ligament attachments
Transverse humeral ligament attachments Hamlet table of contents
Hamlet table of contents Optab
Optab