Congenital Heart Disease Congenital heart disease Foetal PVRSVR

































- Slides: 94

Congenital Heart Disease

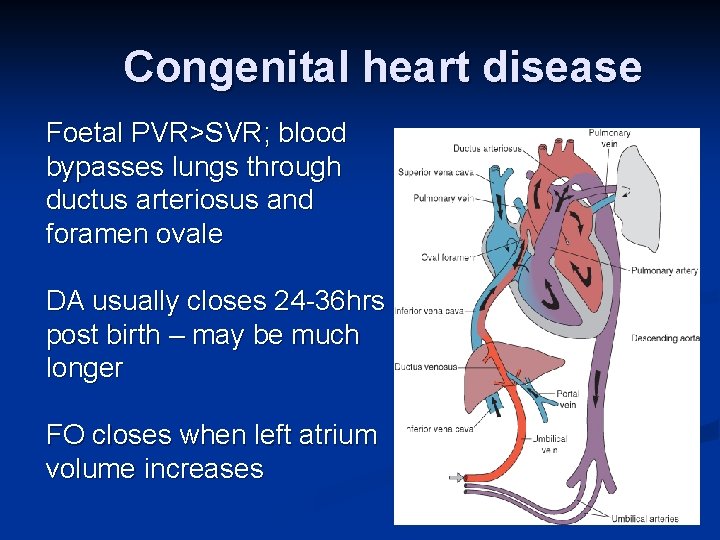
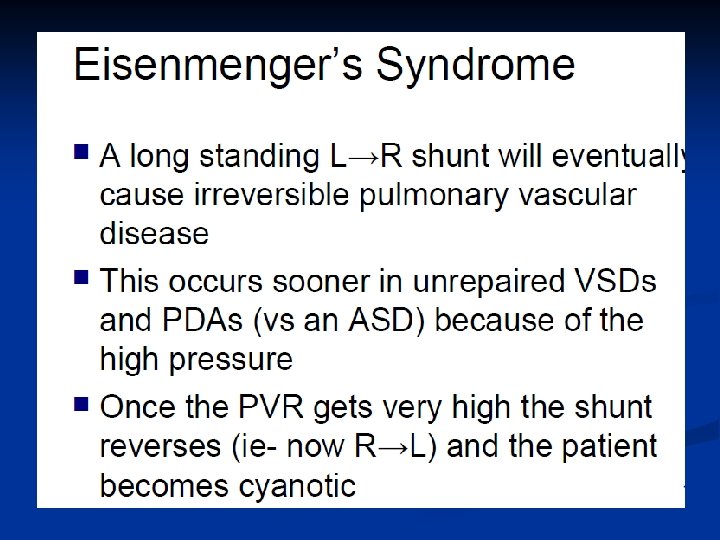
Congenital heart disease Foetal PVR>SVR; blood bypasses lungs through ductus arteriosus and foramen ovale DA usually closes 24 -36 hrs post birth – may be much longer FO closes when left atrium volume increases

Blood circulation after birth: The transformation from fetal to neonatal circulation involves two major changes: 1. A marked increase in systemic resistance. caused by loss of the low-resistance placenta. 2. A marked decrease in pulmonary resistance. caused by pulmonary artery dilation with the neonate’s first breaths.

Blood circulation after birth: n With the first breaths of air the baby takes at birth, the fetal circulation changes. A larger amount of blood is sent to the lungs to pick up oxygen. n Because the ductus arteriosus is no longer needed, it begins to wither and close off. (72 hrs. ) n The circulation in the lungs increases and more blood flows into the left atrium of the heart pressure causes the foramen ovale to close and blood circulates normally

Epidemiology of CHD Incidence - 8/1000 live births -10 -25/100 abortuses n Most congenital defects are well tolerated during fetal life. Etiology - Unknown in most cases 80 -90% (generally considered to be caused by multifactorial inheritance. ) - Genetic factors - single gene defect - Environmental factors - Chromosomal abnormality - Gender differences in type of CHD - occur during the 1 st 8 wks. of fetal development

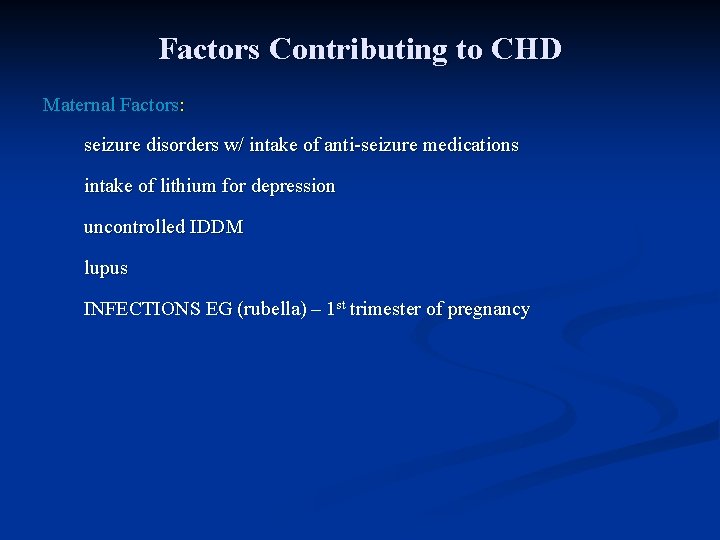
Factors Contributing to CHD Maternal Factors: seizure disorders w/ intake of anti-seizure medications intake of lithium for depression uncontrolled IDDM lupus INFECTIONS EG (rubella) – 1 st trimester of pregnancy

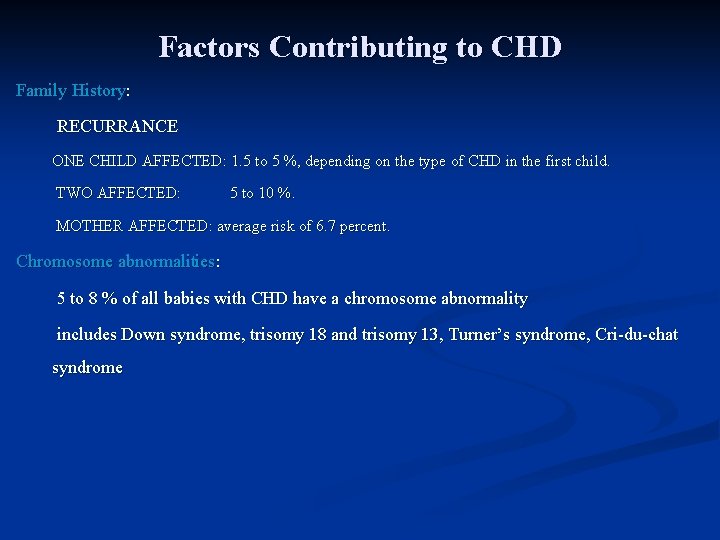
Factors Contributing to CHD Family History: RECURRANCE ONE CHILD AFFECTED: 1. 5 to 5 %, depending on the type of CHD in the first child. TWO AFFECTED: 5 to 10 %. MOTHER AFFECTED: average risk of 6. 7 percent. Chromosome abnormalities: 5 to 8 % of all babies with CHD have a chromosome abnormality includes Down syndrome, trisomy 18 and trisomy 13, Turner’s syndrome, Cri-du-chat syndrome

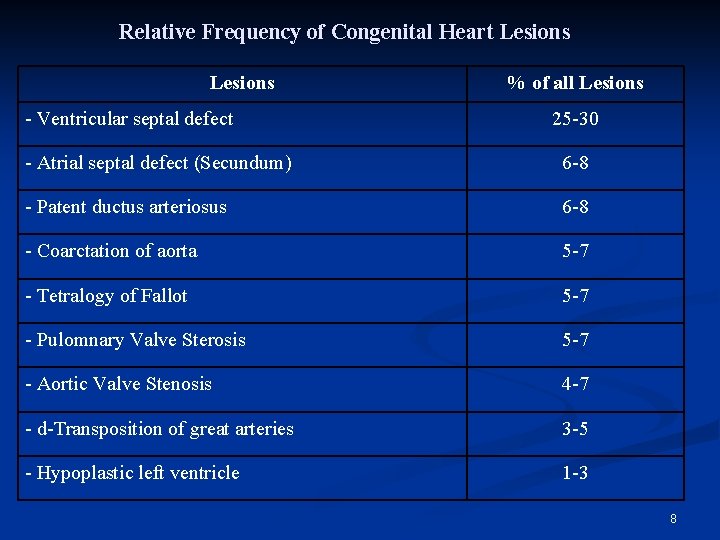
Relative Frequency of Congenital Heart Lesions - Ventricular septal defect % of all Lesions 25 -30 - Atrial septal defect (Secundum) 6 -8 - Patent ductus arteriosus 6 -8 - Coarctation of aorta 5 -7 - Tetralogy of Fallot 5 -7 - Pulomnary Valve Sterosis 5 -7 - Aortic Valve Stenosis 4 -7 - d-Transposition of great arteries 3 -5 - Hypoplastic left ventricle 1 -3 8

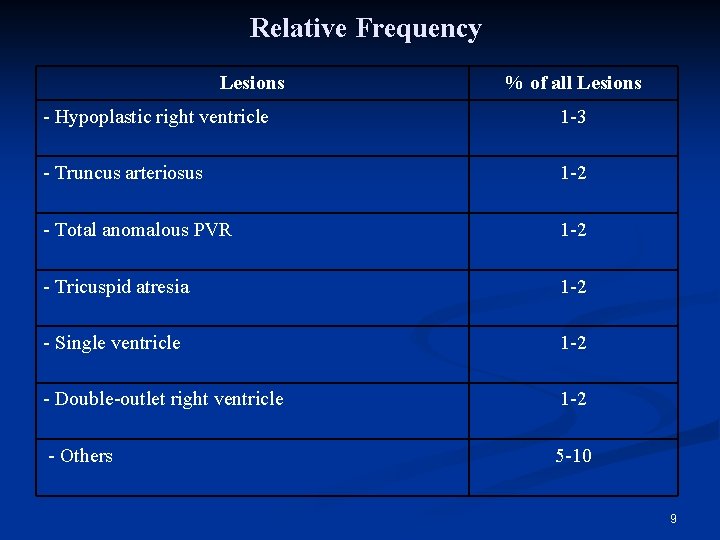
Relative Frequency Lesions % of all Lesions - Hypoplastic right ventricle 1 -3 - Truncus arteriosus 1 -2 - Total anomalous PVR 1 -2 - Tricuspid atresia 1 -2 - Single ventricle 1 -2 - Double-outlet right ventricle 1 -2 - Others 5 -10 9

Presentation Congenital heart disease presents with: • Antenatal cardiac ultrasound diagnosis • Detection of a heart murmur • Heart failure • Shock. • Cyanosis. n

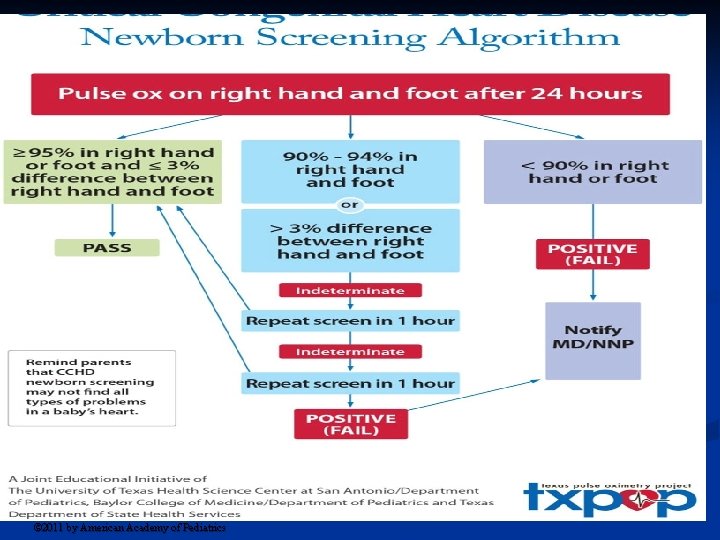
The pulse-oximetry monitoring protocol based on results from the right hand (RH) and either foot (F). Kemper A R et al. Pediatrics 2011; 128: e 1259 -e 1267 © 2011 by American Academy of Pediatrics

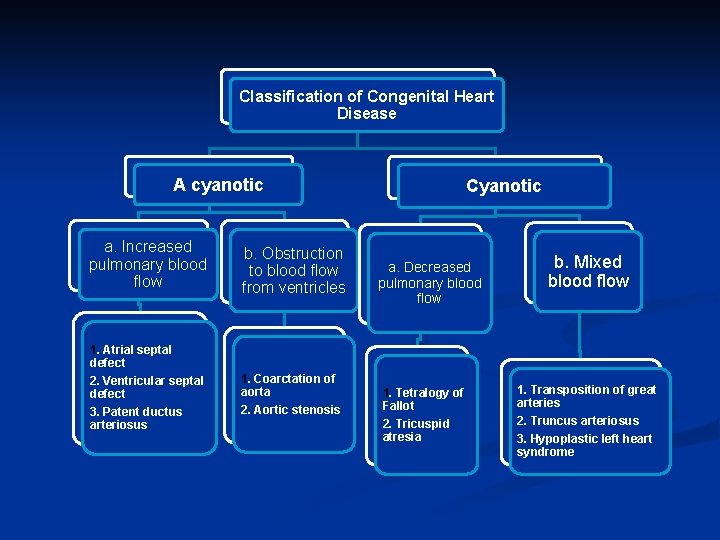
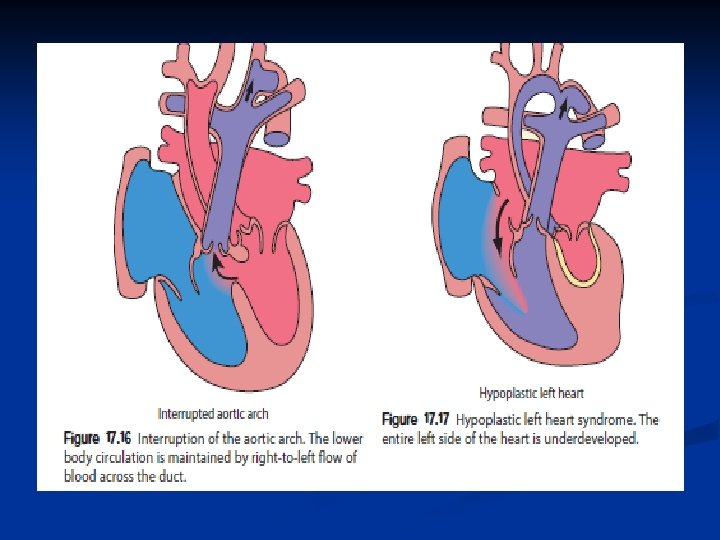
Classification of Congenital Heart Disease A cyanotic a. Increased pulmonary blood flow 1. Atrial septal defect 2. Ventricular septal defect 3. Patent ductus arteriosus b. Obstruction to blood flow from ventricles 1. Coarctation of aorta 2. Aortic stenosis Cyanotic a. Decreased pulmonary blood flow 1. Tetralogy of Fallot 2. Tricuspid atresia b. Mixed blood flow 1. Transposition of great arteries 2. Truncus arteriosus 3. Hypoplastic left heart syndrome



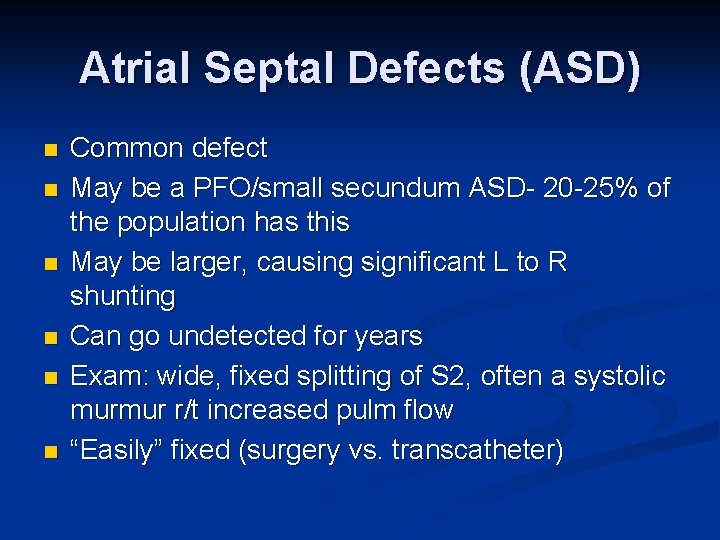
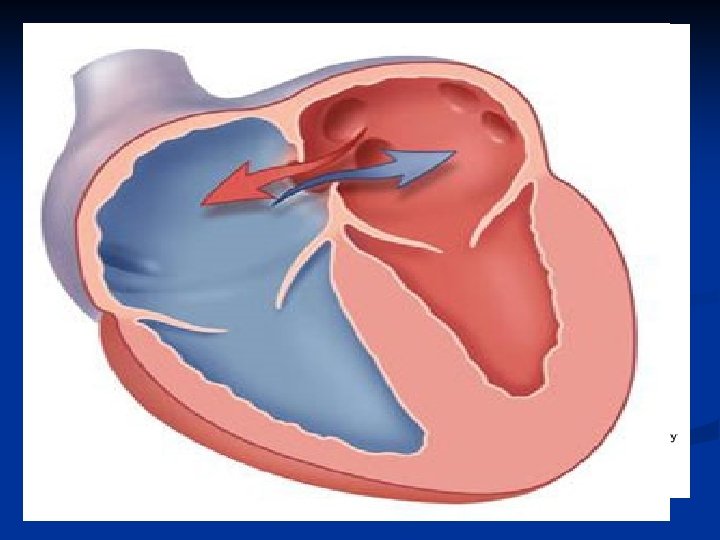
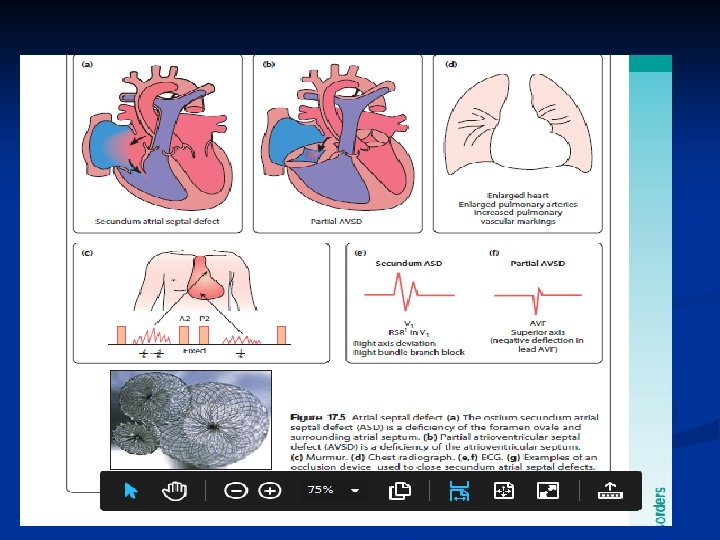
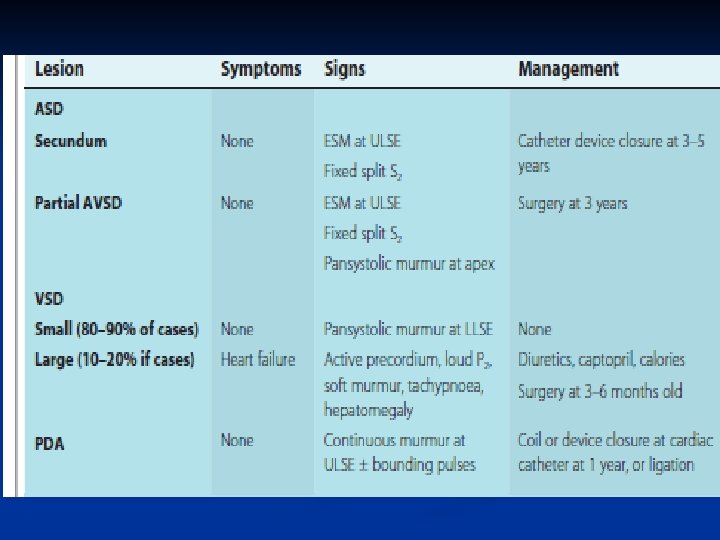
Atrial Septal Defects (ASD) n n n Common defect May be a PFO/small secundum ASD- 20 -25% of the population has this May be larger, causing significant L to R shunting Can go undetected for years Exam: wide, fixed splitting of S 2, often a systolic murmur r/t increased pulm flow “Easily” fixed (surgery vs. transcatheter)




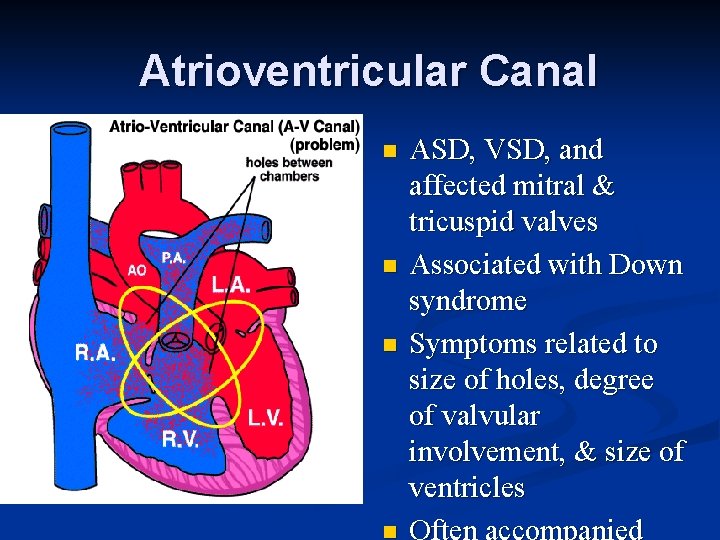
Atrioventricular Canal n n n ASD, VSD, and affected mitral & tricuspid valves Associated with Down syndrome Symptoms related to size of holes, degree of valvular involvement, & size of ventricles

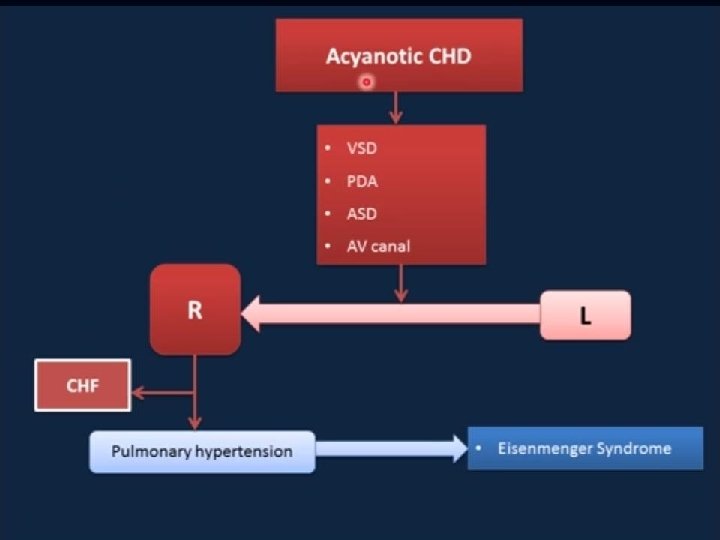
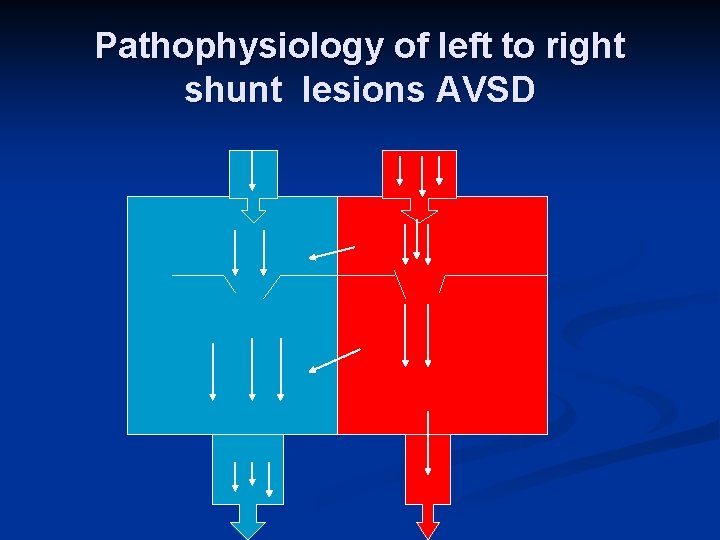
Pathophysiology of left to right shunt lesions AVSD

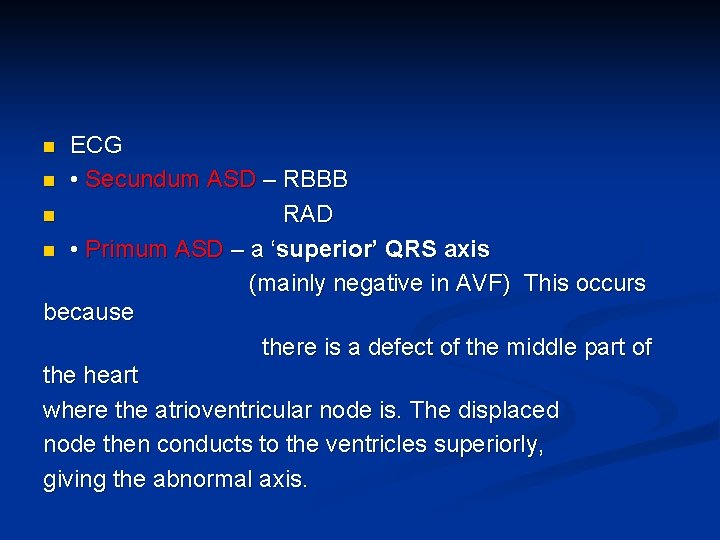
ECG n • Secundum ASD – RBBB n RAD n • Primum ASD – a ‘superior’ QRS axis (mainly negative in AVF) This occurs because there is a defect of the middle part of the heart where the atrioventricular node is. The displaced node then conducts to the ventricles superiorly, giving the abnormal axis. n

VSD for 30% of all CHD n anywhere in the ventricular septum, n perimembranous (adjacent to the tricuspid valve) or n muscular (completely surrounded by muscle). They can. n

Ventricular Septal Defect n n May not present clinically until 1 -2 months of life Often associated with other lesions Isolated VSD’s typically have favorable surgical outcomes Many small and even mod sized VSD’s can close spontaneously for up to 4 years of age

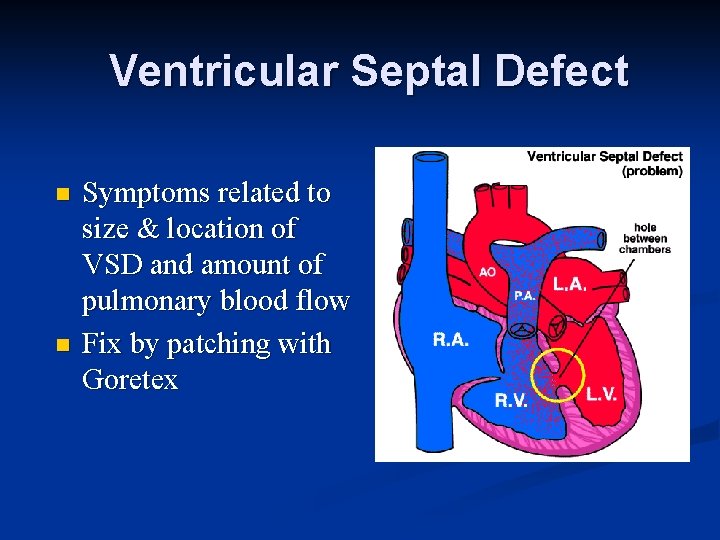
Ventricular Septal Defect n n Symptoms related to size & location of VSD and amount of pulmonary blood flow Fix by patching with Goretex

Small VSDs n n n These are smaller than the aortic valve in diameter, perhaps up to 3 mm. • Asymptomatic. Physical signs • Loud pansystolic murmur at lower left sternal edge (loud murmur implies smaller defect) • Quiet pulmonary second sound (P 2). Investigations CXR • Normal. ECG • Normal.

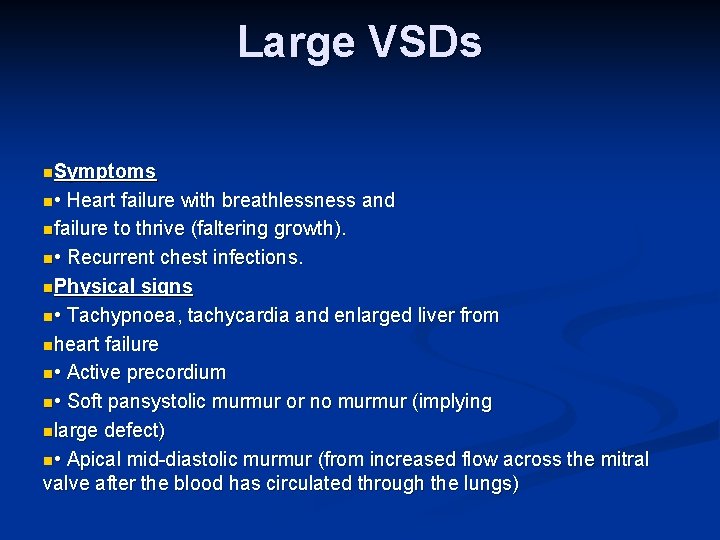
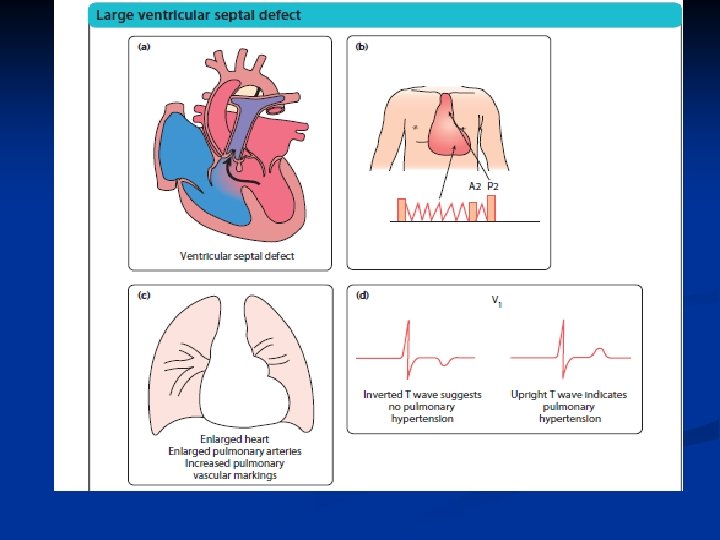
Large VSDs n. Symptoms n • Heart failure with breathlessness and nfailure to thrive (faltering growth). n • Recurrent chest infections. n. Physical signs n • Tachypnoea, tachycardia and enlarged liver from nheart failure n • Active precordium n • Soft pansystolic murmur or no murmur (implying nlarge defect) n • Apical mid-diastolic murmur (from increased flow across the mitral valve after the blood has circulated through the lungs)

Pathophysiology of left to right shunt lesions VSD



Management n AVOID OXYGEN!!!! —use judiciously n n n Especially pre-op Diuretics—furosemide, chlorothiazide, spironolactone Monitor VS, I & 0, daily wt. Encourage rest periods to conserve energy Monitor labs: Hgb, Hct, electrolytes Closely monitor feedings n May need higher calorie feeds

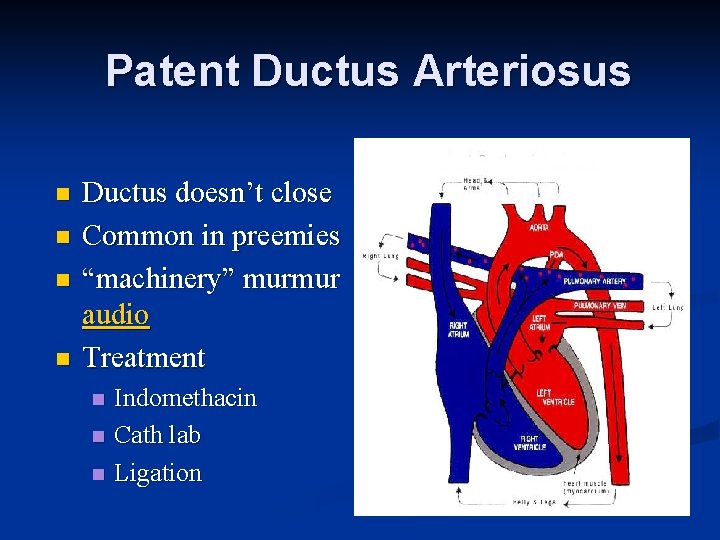
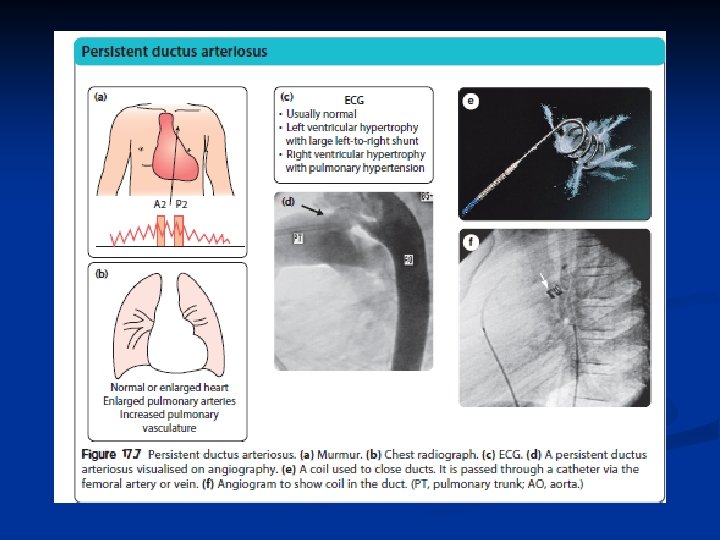
Patent Ductus Arteriosus n n Ductus doesn’t close Common in preemies “machinery” murmur audio Treatment n n n Indomethacin Cath lab Ligation

Pathophysiology of left to right shunt lesions PDA

Medical Management Indomethacin IV (prostaglandin inhibitor) may help close a PDA. - works by stimulating the muscles inside the PDA to constrict, thereby closing the connection Digoxin Diuretics high-calorie formula or breast milk adequate nutrition (premature infants or those infants with a large PDA may become tired when feeding, and are not able to eat enough to gain weight)


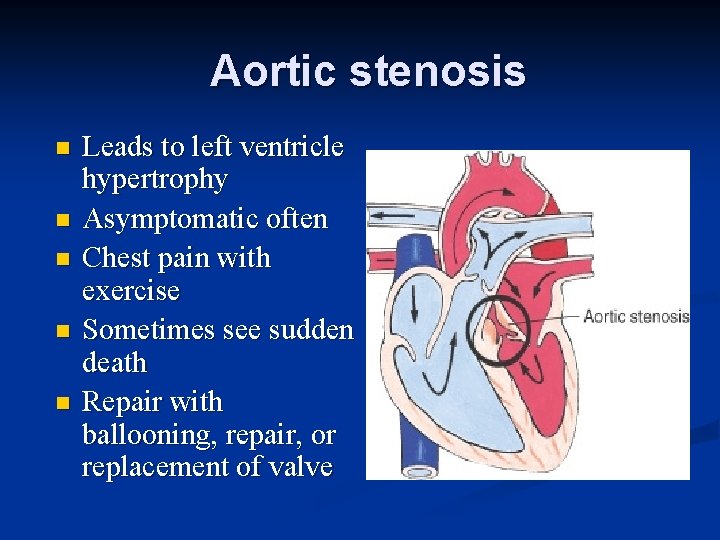
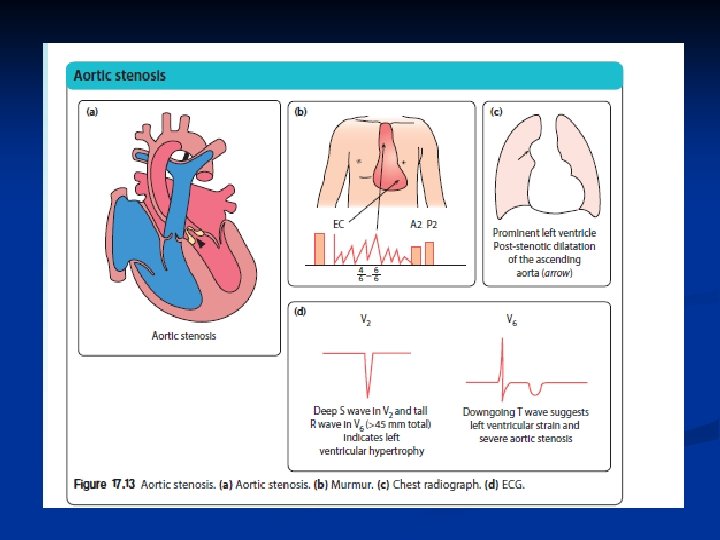
Aortic stenosis n n n • • • The aortic valve leaflets are partly fused together, Clinical features Depends on degree of stenosis. Most - asymptomatic murmur. reduced exercise tolerance, chest pain on exertion. syncope. Physical signs. • Small volume, slow rising pulses • Carotid thrill (always) • Ejection systolic murmur maximal at the upper right sternal edge radiating to the neck • Delayed and soft aortic second sound • Apical ejection click.

Aortic stenosis n n n Leads to left ventricle hypertrophy Asymptomatic often Chest pain with exercise Sometimes see sudden death Repair with ballooning, repair, or replacement of valve


Critical Aortic stenosis n n Critical AS In the neonatal period, those with critical aortic stenosis and a duct-dependent systemic circulation may present with severe heart failure leading to shock.

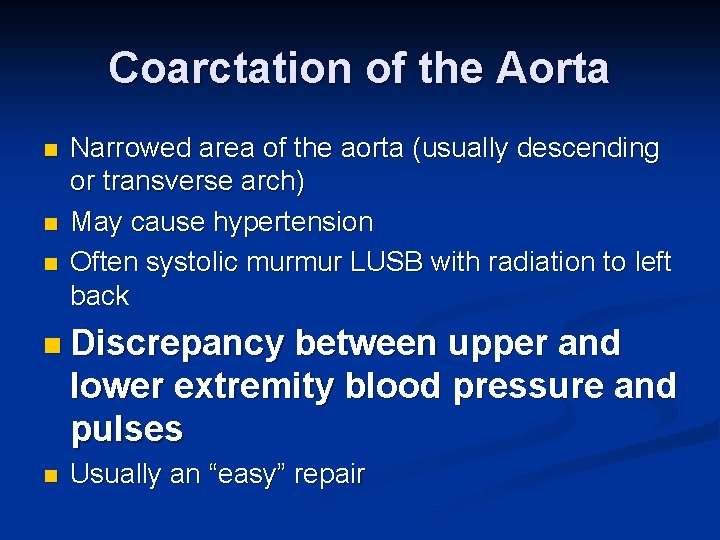
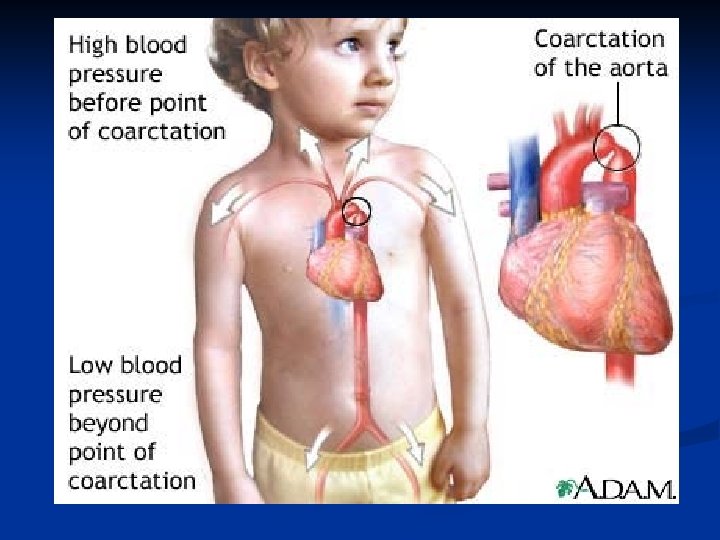
Coarctation of the Aorta n n n Narrowed area of the aorta (usually descending or transverse arch) May cause hypertension Often systolic murmur LUSB with radiation to left back n Discrepancy between upper and lower extremity blood pressure and pulses n Usually an “easy” repair

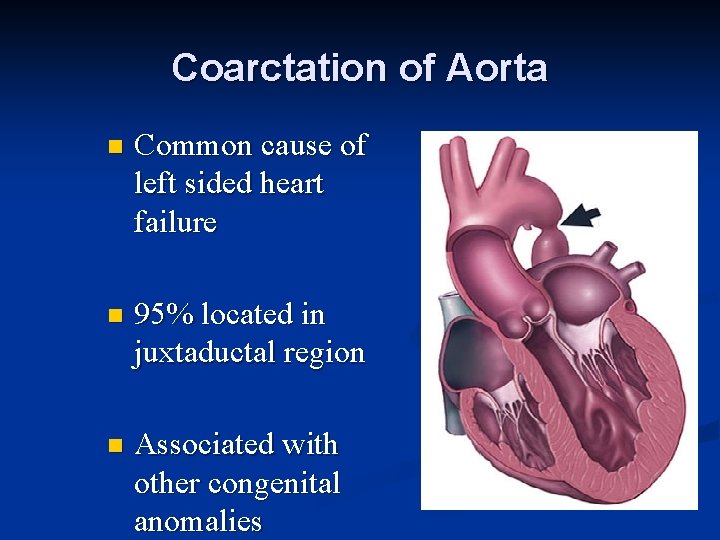
Coarctation of Aorta n Common cause of left sided heart failure n 95% located in juxtaductal region n Associated with other congenital anomalies

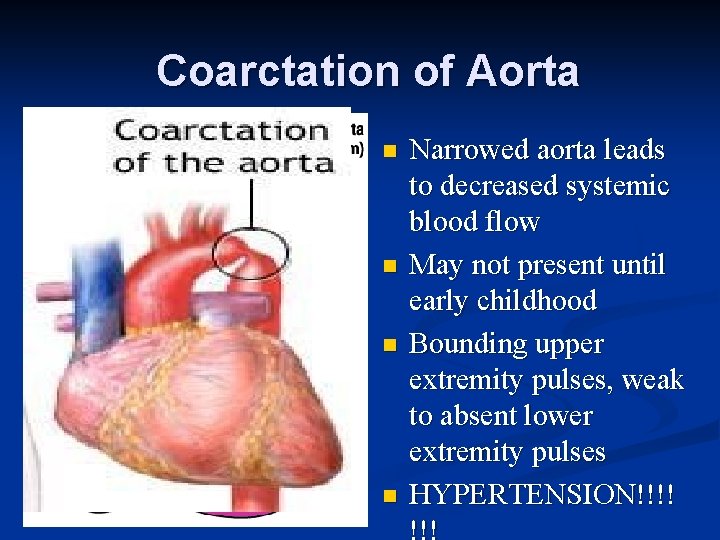
Coarctation of Aorta n n Narrowed aorta leads to decreased systemic blood flow May not present until early childhood Bounding upper extremity pulses, weak to absent lower extremity pulses HYPERTENSION!!!!


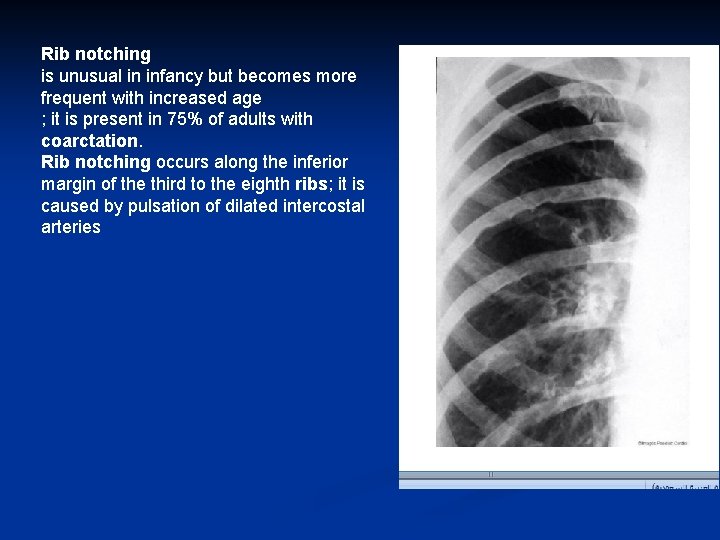
Rib notching is unusual in infancy but becomes more frequent with increased age ; it is present in 75% of adults with coarctation. Rib notching occurs along the inferior margin of the third to the eighth ribs; it is caused by pulsation of dilated intercostal arteries

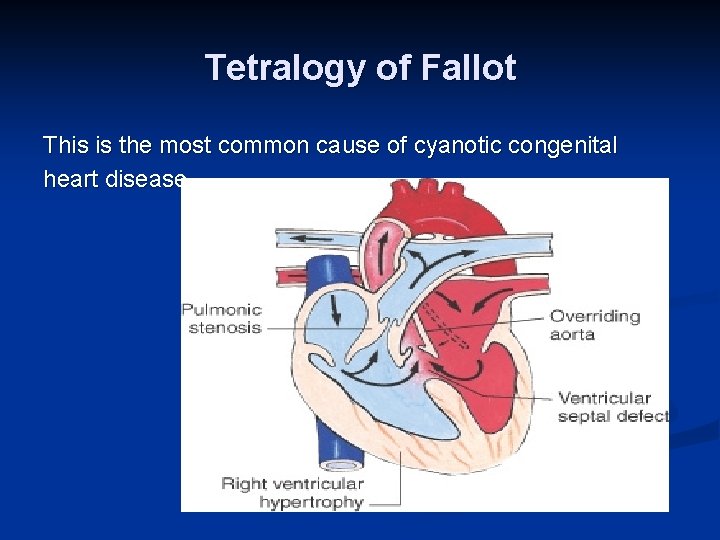
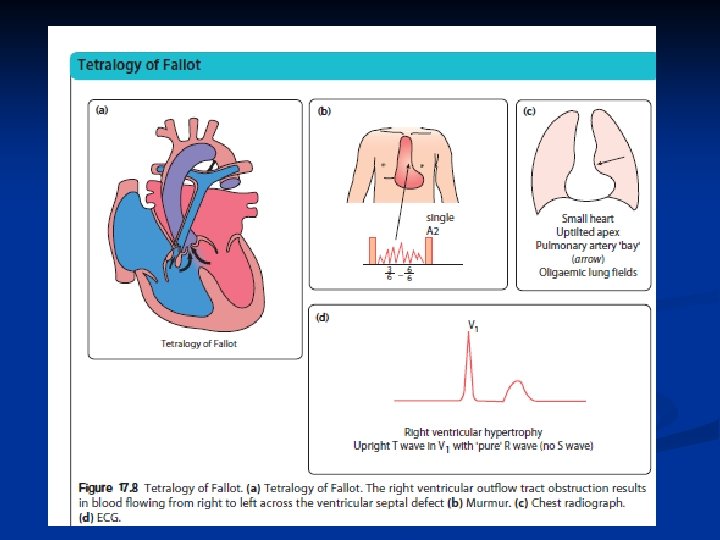
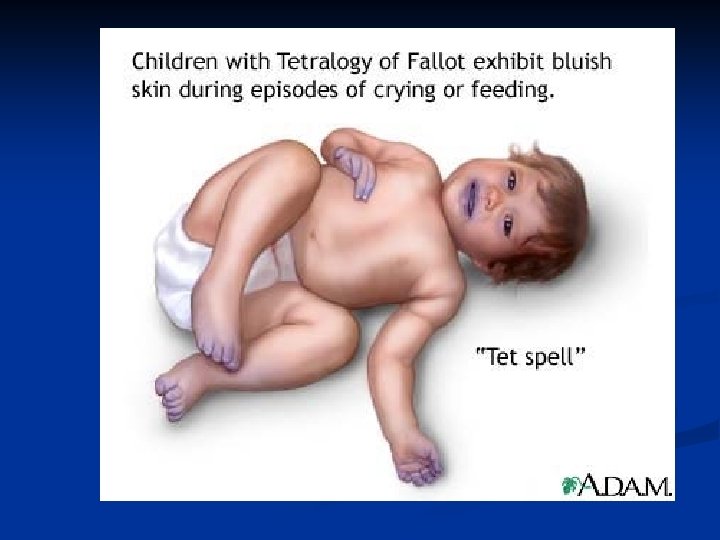
Tetralogy of Fallot This is the most common cause of cyanotic congenital heart disease.

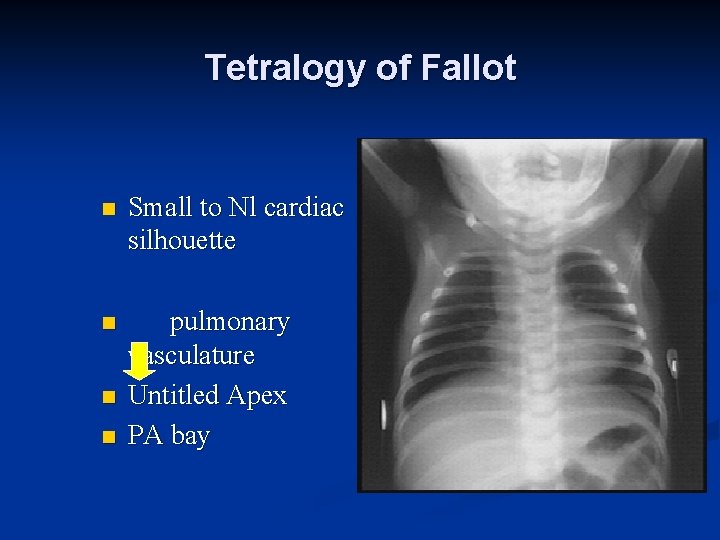
Tetralogy of Fallot n Small to Nl cardiac silhouette n pulmonary vasculature Untitled Apex PA bay n n


Consequences and Complications n n n Polycythemia (erythrocytosis) Clubbing (>6 mos of age) Hypoxic spells CNS n Cyanotic heart disease accounts for 510% of brain abscesses n Cerebral venous thrombosis - <2 yrs, cyanotic and microcytic anemia Dyscrasias

Hypercyanotic “tet spells” Acutely cyanotic n ↓ pulm. blood flow & ↑ right to left shunting n Prompt tx to prevent brain damage &/or death n Calm infant/child n Place in knee chest position n Toddler will get in “squatting” position to compensate for hypoxia n Give oxygen n Morphine/fentanyl/versed given n



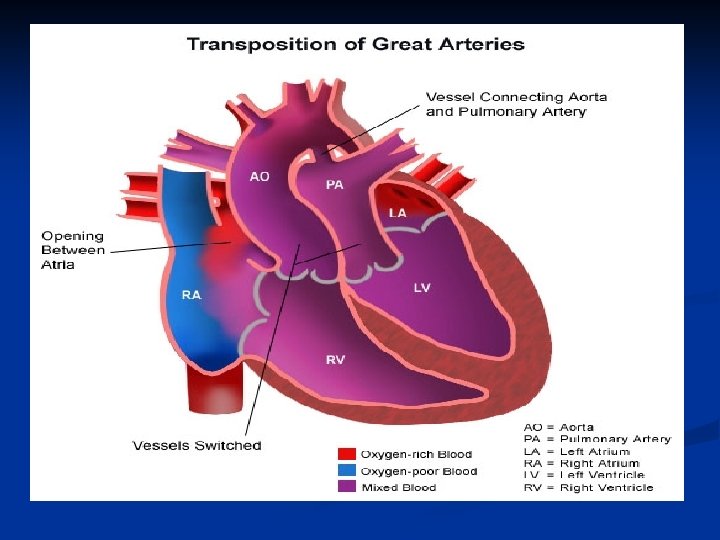
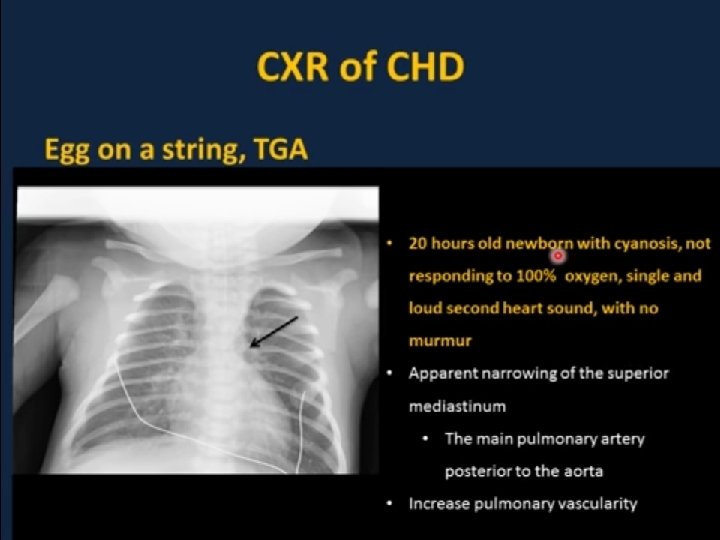
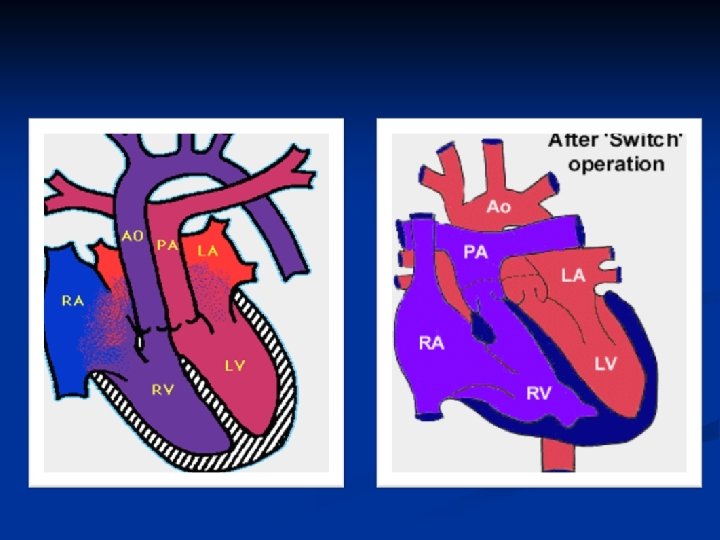
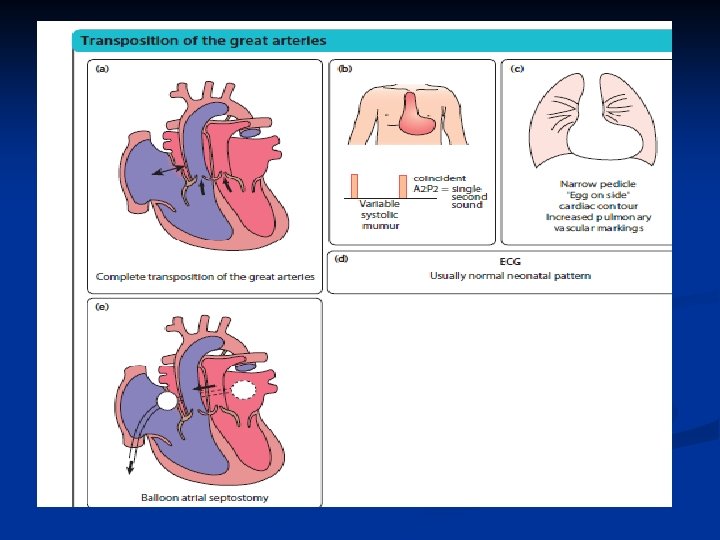
D-transposition of the Great Arteries n Aorta rises from the RV and Pulmonary Artery rises from the LV- complete separation of pulmonary and systemic circulations


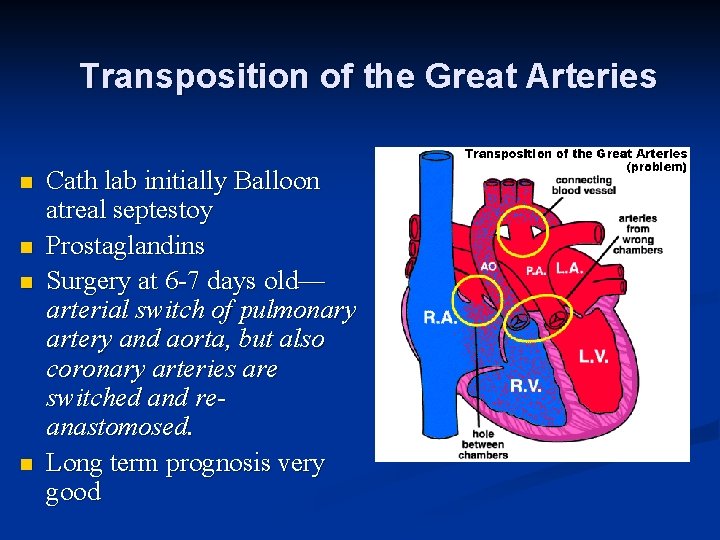
Transposition of the Great Arteries n n Cath lab initially Balloon atreal septestoy Prostaglandins Surgery at 6 -7 days old— arterial switch of pulmonary artery and aorta, but also coronary arteries are switched and reanastomosed. Long term prognosis very good




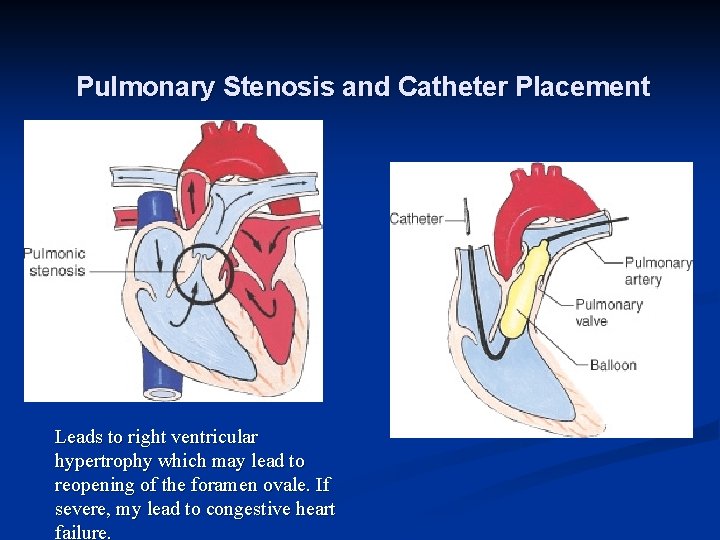
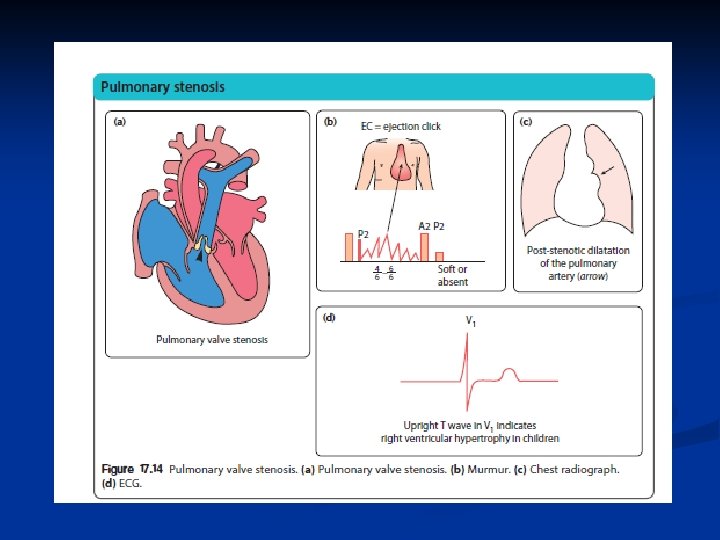
Pulmonary stenosis The pulmonary valve leaflets are partly fused together, n giving a restrictive exit from the right ventricle. n Clinical features n Most are asymptomatic. n A small number of neonates with critical pulmonary stenosis have a duct-dependent pulmonary circulation and present in the first few days of life with n

Pulmonary Stenosis and Catheter Placement Leads to right ventricular hypertrophy which may lead to reopening of the foramen ovale. If severe, my lead to congestive heart failure.

Pulmonary valve is stenosed. Narrowing at entrance to pulmonary artery. >>R Ventricular hypertrophy and decreased pulm blood flow. Extreme form of PS is Pulmonary atresia (total fusion of the commissures and no blood flow to lungs) PS>>R vent hypertrophy, R ventricular failure>> R atrial pressure increases and may reopen foramen ovale. Shunts unoxygenaeted blood to L atrium>>systemic cyanosis. May lead to CHF. Often have PDA as well. Cardiomegaly on CXR; TX: Balloon angioplasty to dilate the valve. SURG TX —Brock procedure (Bypass to do valvotomy). Usually can repair w/ catheterization.


Obstructive Lesions Pulmonic Stenosis - 4 types - Valvular - Infundibular - Supra valvular - Peripheral Clinical Manifestation Pathophysiology - Mild to moderate - Rt outlet obstruction → Pressure work - asymptomatic ↓ - Critical stenosis Rt vent. hyperthropy - Systolic ejection murmur Diagnosis - Heart failure in neonates & infants - Clinical - Rarely cyanosis - CXR - Rt vent. hypertrophy - reduced pulm. blood flow - ECG - Echocardiography Prognosis - good in mild to moderate Complications - CHF in severe Ps - rarely IE Treatment - vavular PS - ballon valvoplasty - surgery


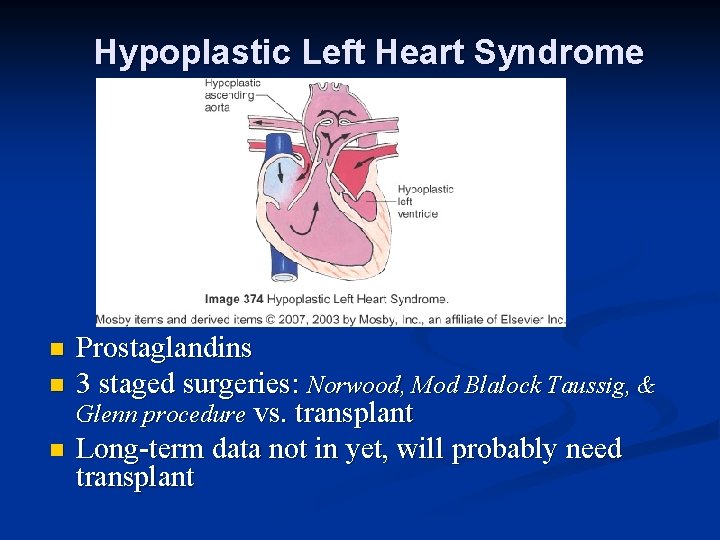
Hypoplastic Left Heart Syndrome n n n Prostaglandins 3 staged surgeries: Norwood, Mod Blalock Taussig, & Glenn procedure vs. transplant Long-term data not in yet, will probably need transplant

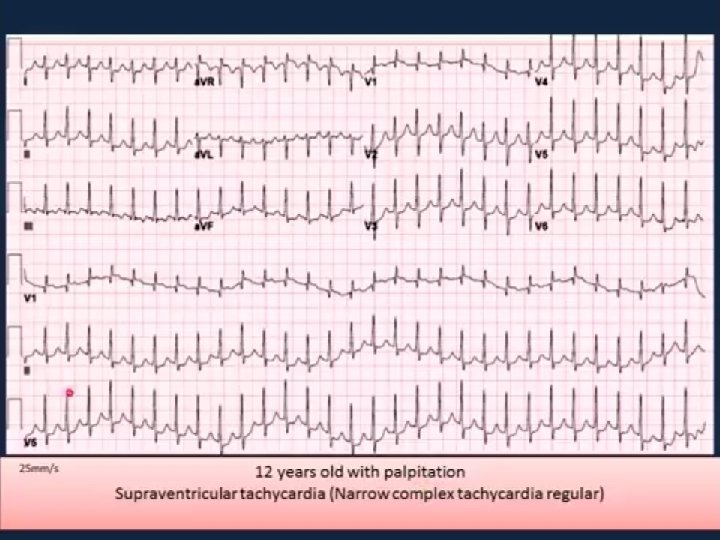
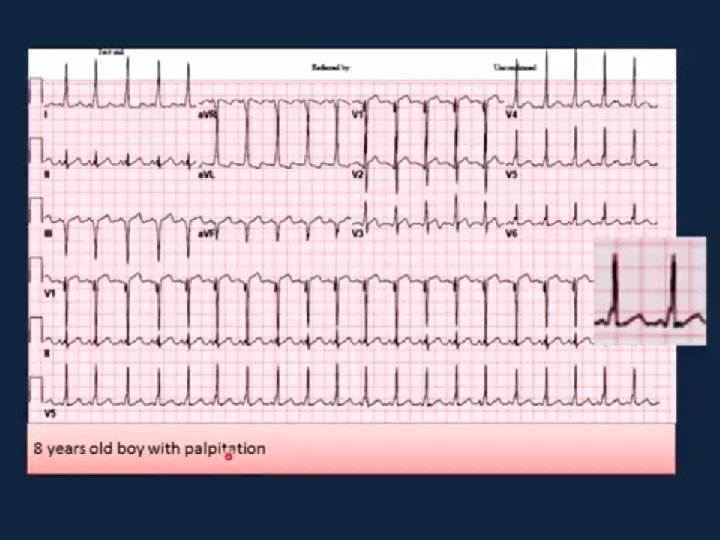
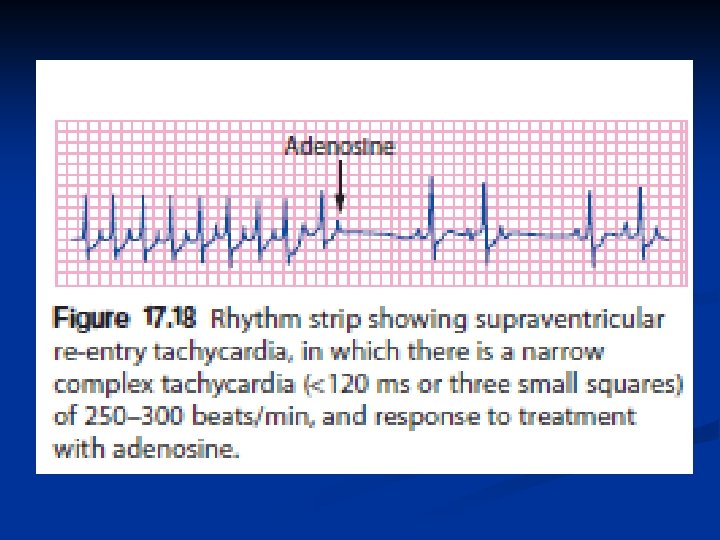
Supraventricular tachycardia n n n This is the most common childhood arrhythmia. HR: between 250 and 300 beats/min. can cause poor cardiac output and pulmonary oedema. symptoms of heart failure in the neonate or young infant. It is a cause of hydrops fetalis and intrauterine death.







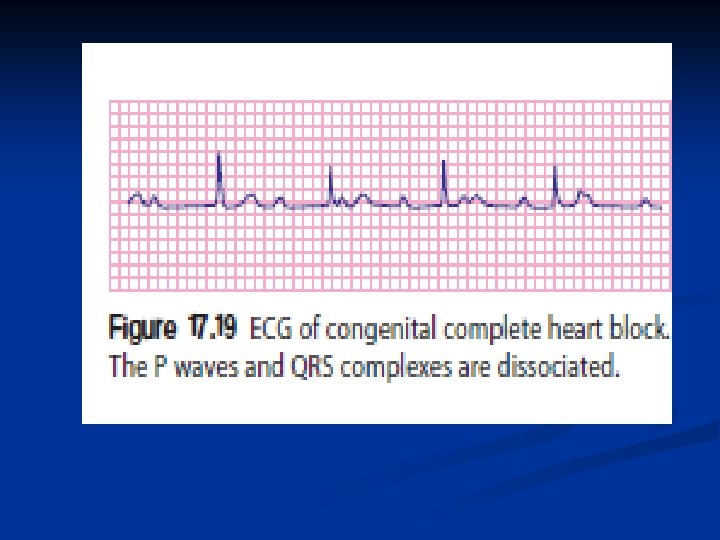
Congenital complete heart block related to the presence of anti-Ro or anti-La antibodies in maternal serum. These mothers will have either manifest or latent connective tissue disorders. Subsequent pregnancies are often affected. This antibody appears to prevent normal development of the electrical n conduction system in the developing heart, with n atrophy and fibrosis of the atrioventricular node. It n may cause fetal hydrops, death in utero and heart n failure in the neonatal period. However, most remain n symptom-free for many years, but a few become n symptomatic with presyncope or syncope. All children n with symptoms require insertion of an endocardial n pacemaker. n


Acquired Heart Disease

Kawasaki Disease (KD) An acute, self-limited vasculitis n Unknown cause, but an infectious cause/virus is suspected n Leading cause of acquired heart disease in US and Japan n Usually seen in children <5 y. o. n First described by Dr. Kawasaki in Japan, 1961 n

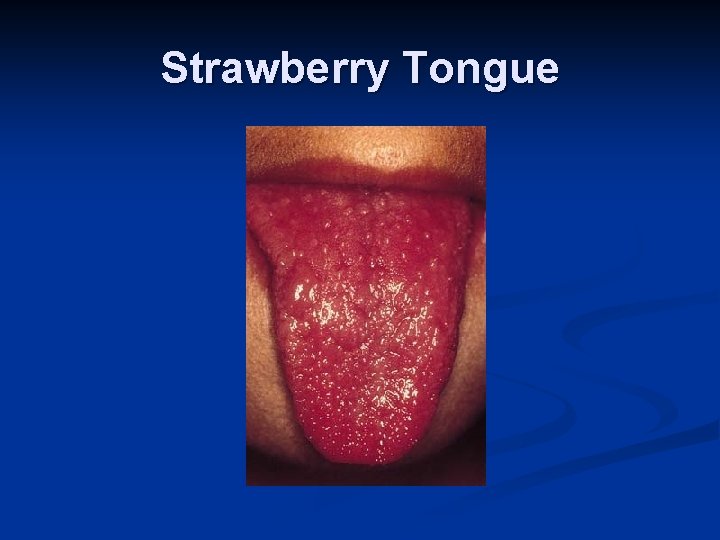
KD- Diagnostic Criteria n n n n Fever >/= 5 days Bilateral conjuctival injection Changes of mucous membranes- injected pharynx, fissured lips, strawberry tongue Changes of peripheral extremities- peripheral edema, peripheral erythema, desquamation of palms Polymorphous rash Cervical adenopathy Diagnosis is presence of fever and 4 of 5 remaining criteria

KD- Cardiovascular concerns Development of coronary artery aneurysms (usually around 2 wks after onset of symptoms) n Evaluated by echocardiogram n Half of patients with aneurysms will remodel vessel wall- never completely normal. Probably at higher risk for future coronary artery disease- long-term antiplatelet therapy generally recommended n

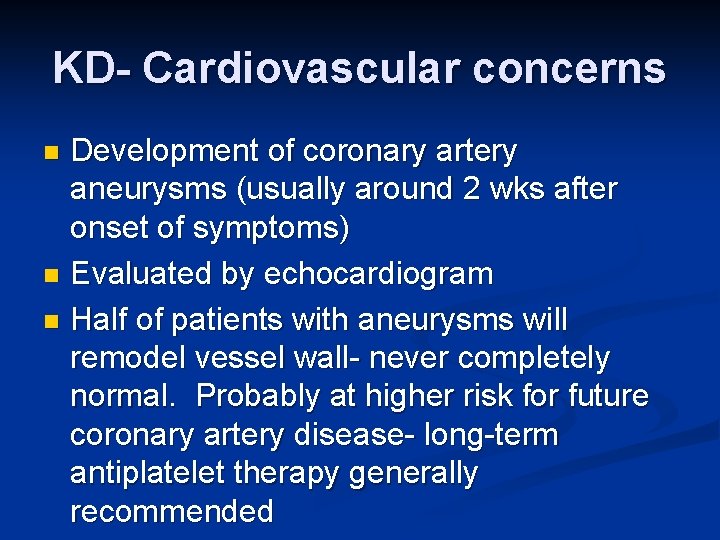
Strawberry Tongue



Treatment Standard treatment is high-dose IVIG (immunoglobulins) in combination with high doses of aspirin n Screening/repeat echocardiograms n Possible cardiac cath with intervention if coronary artery aneurysms causing myocardial dysfunction or further evaluation n

Rheumatic Fever n A systemic illness thought to occur following group A beta hemolytic streptococcal pharyngitis (GABHS) in children, median age 10 years n Increased risk- untreated or delayed treatment of a strep infection


Chronic rheumatic heart disease The most common form of long-term damage from n scarring and fibrosis of the valve tissue of the heart is n

History Sore throat 1 -5 wks prior to onset of RF symptoms n Fever, rash, headache, wt loss, fatigue, diaphoresis, chest pain/pounding, migratory joint pain, skin nodules, motor dysfunction, previous RF (higher risk of recurrence) n Can cause endocarditis, myocarditis, and pericarditis. Usually affecting the mitral and aortic valves resulting in regurgitation n

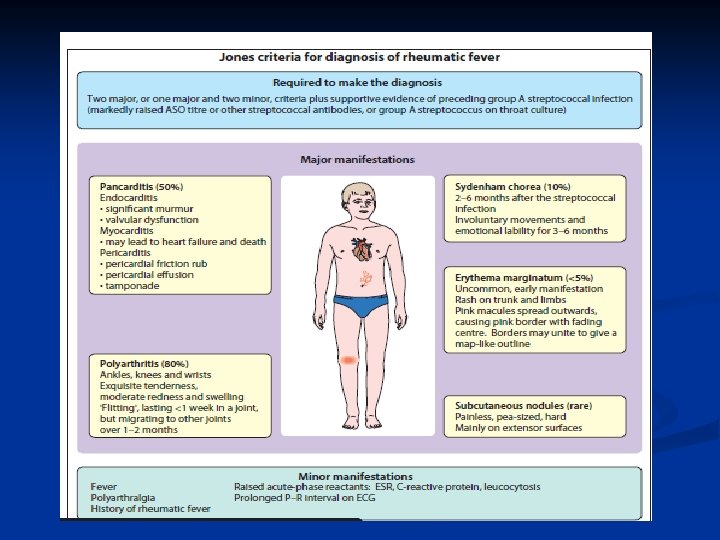
Jones Criteria Requires presence of 2 major, or 1 major and 2 minor criteria. Previous Group A strep is also necessary

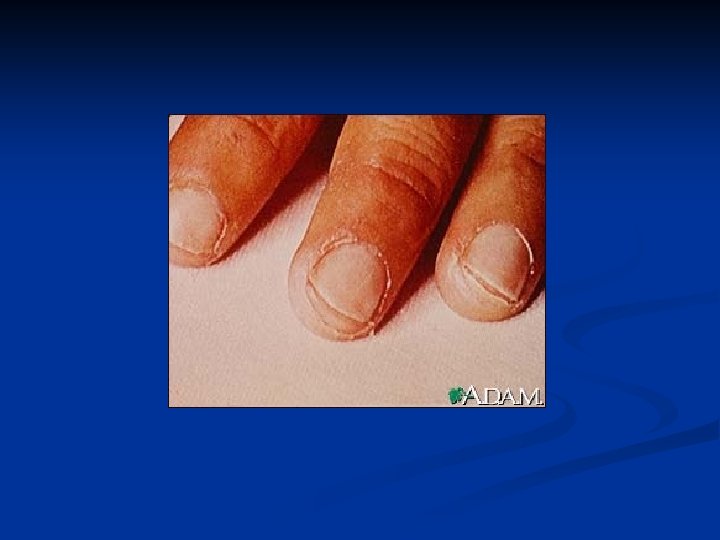
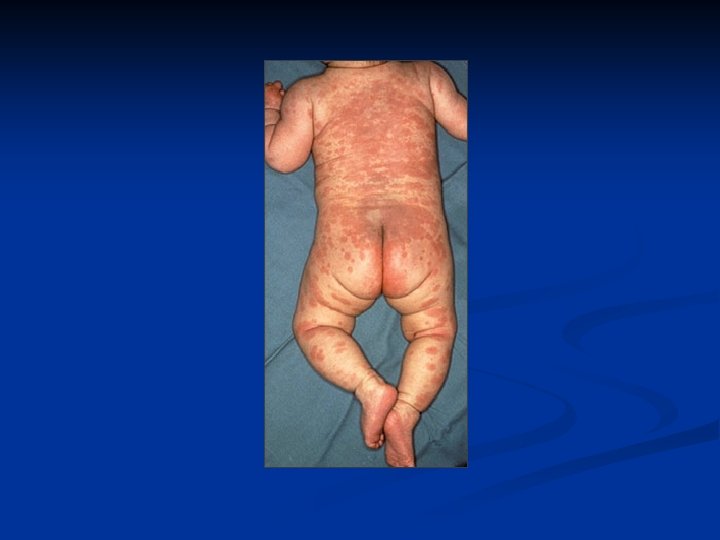
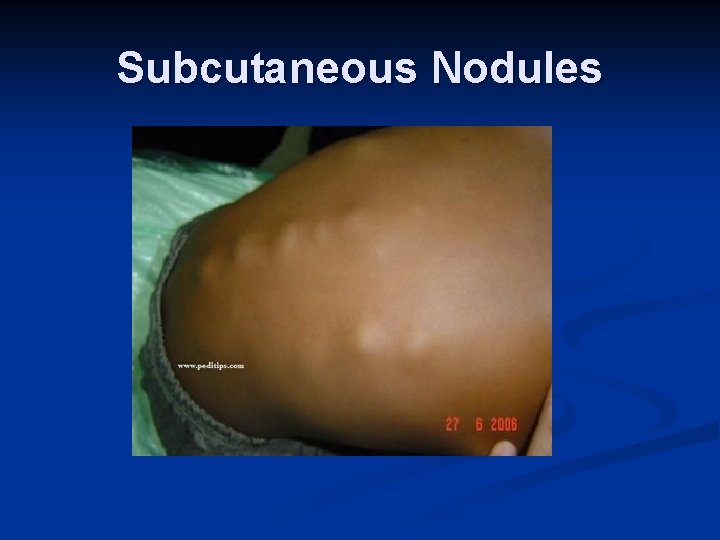
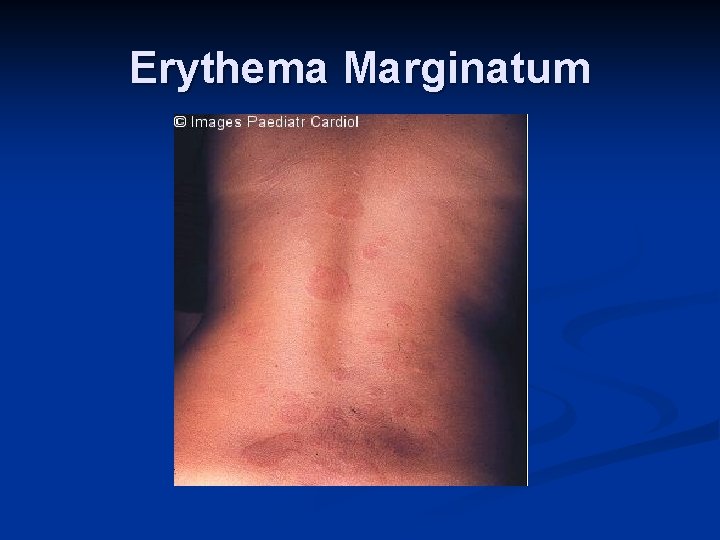
Major Criteria n n n Carditis (EKG, CXR, ECHO, Exam)- new murmur and tachycardia. Wide pulse pressure if severe aortic regurgitation. May also have CHF- JVD, hepatomegaly, gallop rhythm, friction rub, peripheral edema Polyarthritis Chorea- brief, irregular, unpredictable, purposeless movements that flow from one body part to another without a rhythmic pattern Erythema marginatum- 1 to 3 cm pink/red nonpruitic macules or papules on trunk and limbs (not on face) Subcutaneous nodules- infrequent, but occur on surfaces of elbows, knees, ankles, knuckles, and other spinous processes- firm and nontender

Subcutaneous Nodules

Erythema Marginatum

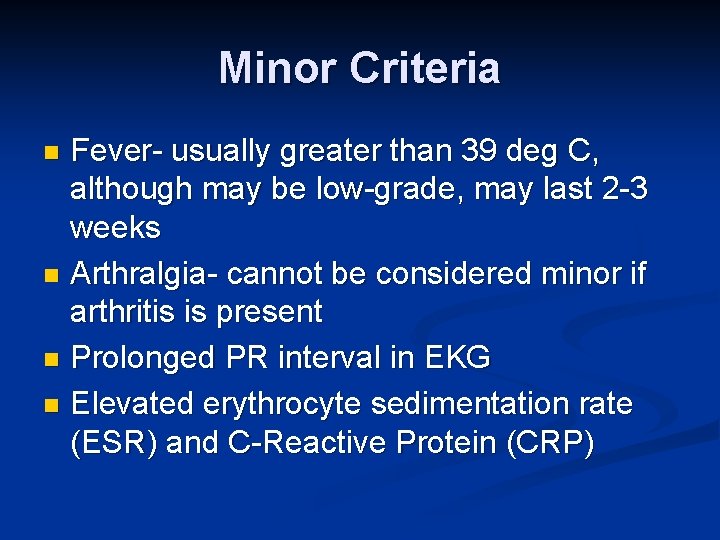
Minor Criteria Fever- usually greater than 39 deg C, although may be low-grade, may last 2 -3 weeks n Arthralgia- cannot be considered minor if arthritis is present n Prolonged PR interval in EKG n Elevated erythrocyte sedimentation rate (ESR) and C-Reactive Protein (CRP) n

Evidence of GAS pharyngitis – one of the following MUST be present Positive throat culture or rapid strep antigen test n Elevated or rising streptococcal antibody titer (such as antistreptolysin O [ASO]) n

Lab Studies Throat culture n Rapid antigen detection test n Antistreptococcal antibodies- usually using antistreptolysin O (ASO) n C-RP, ESR n

Imaging CXR- pulm cong/CHF n Echo- MR, AR, LV dilation n ECG- sinus tachy, 1 st, 2 nd or 3 rd degree AV block (myocarditis), ST segment elevation, atrial arrhythmias n

Treatment n n Antibiotics- Oral penicillin remains drug of choice - alternatives: IM Pen G, E-mycin, 1 st gen Cephalosporin (do not use tetracyclines or sulfonamides) High dose aspirin and steroid to treat inflammation (cont to monitor C-RP and ESR for effectiveness) Digoxin, diuretics, afterload reduction depending on cardiac manifestations After acute phase symptoms have subsided, improved, may undergo cardiac surgery for valve repair/replacement- if AV and/or MV replaced with prosthetic, will require lifetime anticoagulation (Warfarin)

n n n Recognizing and treating streptococcal infections in a timely manner Awareness of potential systemic symptoms related to untreated/delayed treatment of a strep infection- these may not occur until up to several weeks after the sore throat has resolved Only infections of the pharynx initiate RF (in developed countries) Secondary prophylaxis of patients with history of RF- daily PCN or injections q 3 -4 wks SBE prophylaxis with antibiotics prior to dental and other procedures