Transplantation Introduction Transplantation is a widely used treatment





































- Slides: 94

Transplantation

Introduction • Transplantation is a widely used treatment for replacement of nonfunctioning organs and tissues with healthy organs or tissues. • Technically, transplantation is the process of taking cells, tissues, or organs, called a graft, from one individual and placing them into a (usually) different individual. • The individual who provides the graft is called the donor, and the individual who receives the graft is called recepient or host.

Introduction • If the graft is placed into its normal anatomic location, the procedure is called orthotopic transplantation; if the graft is placed in a different site, the procedure is called heterotopic transplantation.

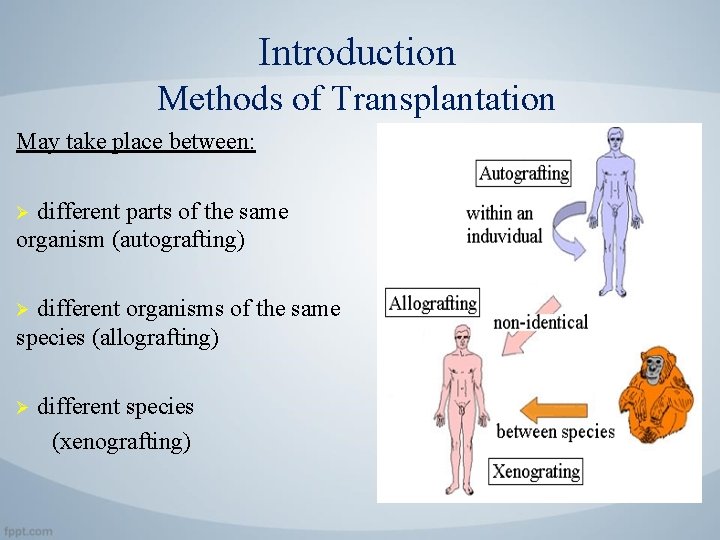
Introduction Methods of Transplantation May take place between: different parts of the same organism (autografting) Ø different organisms of the same species (allografting) Ø Ø different species (xenografting)

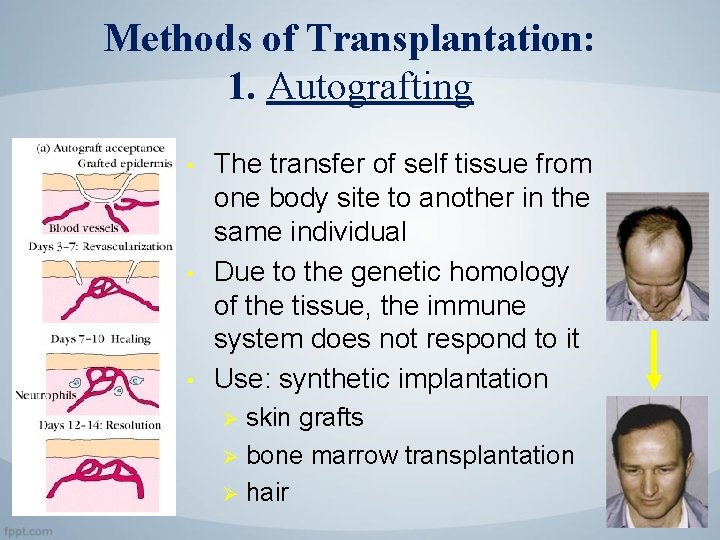
Methods of Transplantation: 1. Autografting • • • The transfer of self tissue from one body site to another in the same individual Due to the genetic homology of the tissue, the immune system does not respond to it Use: synthetic implantation skin grafts Ø bone marrow transplantation Ø hair Ø

Methods of Transplantation: 2. Allografting Definition: The transfer of organs or tissue from human to human. • As there are more and more people every year waiting for donor organs and tissues, allografting transplantation has become quite common. • Allografting transplantation has many applications.

Methods of Transplantation: 3. Xenografting Definition: Xenotransplantation is the transfer of tissue from one species to another Ø Usually refers to the implantation of animal tissue in humans Ø provides a new source of organs for humans Ø many different types of tissue can be transplanted: e. g. heart, kidney, liver or lung

Xenogeneic Transplantation • The use of solid organ transplantation as a clinical therapy is greatly limited by the lack of availability of donor organs. • For this reason, the possibility of transplantation of organs from other mammals, such as pigs, into human recipients has kindled great interest.

Xenogeneic Transplantation Ø A major immunologic barrier to xenogeneic transplantation is the presence of natural antibodies that cause hyperacute rejection. • More than 9 5 % of primates have natural Ig. M antibodies that are reactive with carbohydrate determinants expressed by cells of species that are evolutionarily distant, such as the pig. • The majority of human anti-pig natural antibodies are directed at one particular carbohydrate determinant formed by α-galactosyltransferase enzyme. • This enzyme places an a-linked galactose moiety on the same substrate that in human and other primate cells is fucosylated to form the blood group H antigen.

Introduction Transplantation Terminology • A graft transplanted from one individual to the same individual is called an autologous graft. • A graft transplanted between two genetically identical or syngeneic individuals is called a syngeneic graft. • A graft transplanted between two genetically different individuals of the same species is called an allogeneic graft (or allograft). • A graft transplanted between individuals of different species is called a xenogeneic graft (or xenograft).

Introduction Transplantation Terminology • The molecules that are recognized as foreign on allografts are called alloantigens, and those on xenografts are called xenoantigens. • The lymphocytes and antibodies that react with alloantigens or xenoantigens are described as being alloreactive or xenoreactive, respectively.

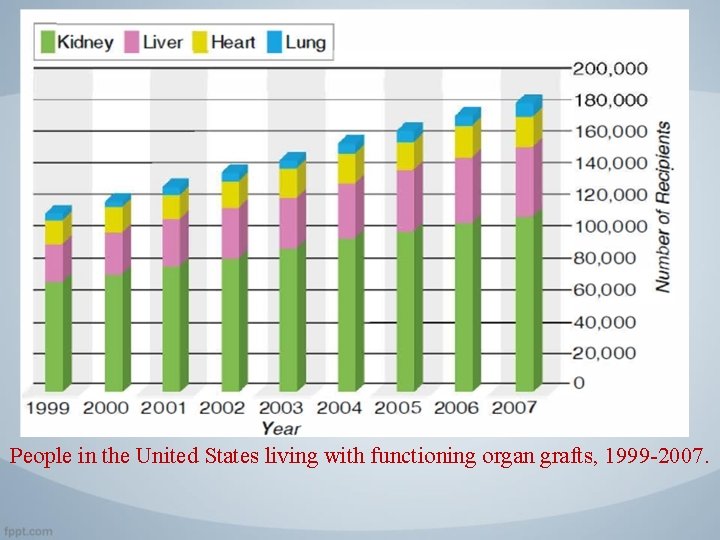
Introduction • Clinical transplantation to treat human diseases has increased steadily during the past 45 years, and transplantation of kidneys, hearts, lungs, livers, pancreata, and bone marrow is widely used today. • More than 30, 000 kidney, heart, lung, liver, and pancreas transplantations are currently performed in the United States each year. In addition, transplantation of many other organs or cells, including stem cells, is now being attempted.

People in the United States living with functioning organ grafts, 1999 -2007.

Transplantation: an overview v Transplantation of cells or tissues from one individual to a genetically non identical individual invariably leads to rejection of the transplant due to an adaptive immune response. • This problem was first appreciated when attempts to replace damaged skin on burn patients with skin from unrelated donors proved to be uniformly unsuccessful. • During a matter of 1 to 2 weeks, the transplanted skin would undergo necrosis and fall off.

Transplantation: an overview • The failure of the grafts led Peter Medawar and many other investigators to study skin transplantation in animal models. These experiments established that the failure of skin grafting was caused by an inflammatory reaction called rejection. • Graft rejection is the result of an adaptive immune response demonstrate that the process had characteristics of memory and specificity and was mediated by lymphocytes.


Transplantation: an overview • Second set rejection is usually faster that first set rejection. • This implying that the recipient developed memory for the grafted tissue. • Individuals who have rejected a graft from one donor show accelerated rejection of another graft from the same donor but not from a different donor, demonstrating that the rejection process is immunologically specific.

The Immunology of Transplantation is Important for Several Reasons • First, immunologic rejection remains one of the major problems in clinical transplantation. • Second, the immune response to allogeneic molecules has been a useful model for studying the mechanisms of lymphocyte activation. • Third, many immunosuppressive therapies that have proved to be useful for a variety of immunologic and inflammatory diseases were first tested and shown to be effective for treatment of graft rejection.

Immune Responses to Allografts

1. Recognition of Alloantigens • Recognition of transplanted cells as self or foreign is determined by polymorphic genes, called MHC genes, which differ among different members of a species. • This conclusion is based on the results of experimental transplantation between inbred strains of mice, and in some cases, the results have been confirmed in human transplantation ( as shown in the figure, next slide).


1. Recognition of Alloantigens Ø The basic rules of transplantation immunology, which are derived from such animal experiments, are the following: 1. Cells or organs transplanted between genetically identical individuals (identical twins or members of the same inbred strain of animals) are never rejected. 2. Cells or organs transplanted between genetically non-identical people or members of two different inbred strains of a species are almost always rejected. 3. The offspring of a mating between two different inbred strains of animal will typically not reject grafts from either parent. In other words, an (A x B)F 1 animal will not reject grafts from an A or B strain animal. (This rule is violated by bone marrow transplantation). 4. A graft derived from the offspring of a mating between two different inbred strains will almost always be rejected by either parent. In other words, a graft from an (A x B)F 1 animal will be rejected by either an A or a B strain animal.

2. Alloantigent MHC Presentation • Allogeneic MHC molecules of a graft may be presented for recognition by the T cells of the recipient in two fundamentally different ways, called direct and indirect. • When the recipient T cells recognize intact, unprocessed MHC molecules in the graft, and this is called direct presentation of alloantigens. • When the recipient T cells recognize graft MHC molecules only in the context of the recipient's MHC molecules, implying that the recipient's MHC molecules must be presenting allogenic graft MHC proteins to recipient T cells. This process is called indirect presentation, and it is essentially the same as the presentation of any foreign (e. g. , microbial) protein antigen.

Direct Presentation of MHC Alloantigens: keynotes Ø In direct presentation, an intact MHC molecule is displayed by donor antigen-presenting cells (APCs) in the graft and recognized by recipient T cells without a need for host APCs.

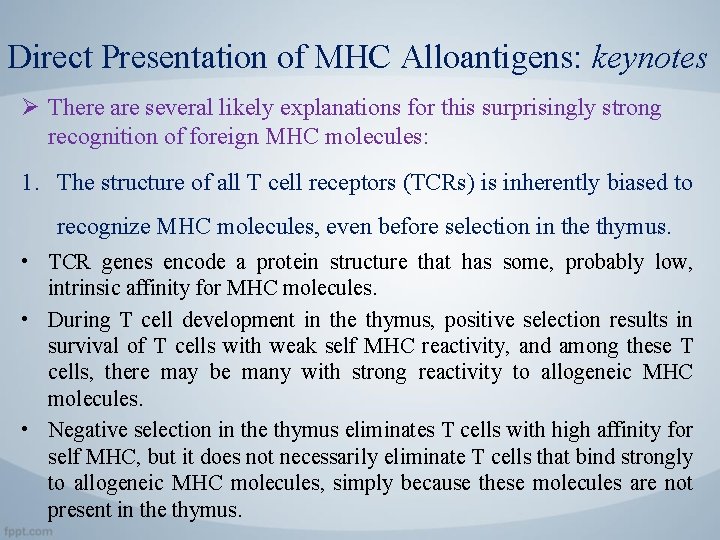
Direct Presentation of MHC Alloantigens: keynotes Ø There are several likely explanations for this surprisingly strong recognition of foreign MHC molecules: 1. The structure of all T cell receptors (TCRs) is inherently biased to recognize MHC molecules, even before selection in the thymus. • TCR genes encode a protein structure that has some, probably low, intrinsic affinity for MHC molecules. • During T cell development in the thymus, positive selection results in survival of T cells with weak self MHC reactivity, and among these T cells, there may be many with strong reactivity to allogeneic MHC molecules. • Negative selection in the thymus eliminates T cells with high affinity for self MHC, but it does not necessarily eliminate T cells that bind strongly to allogeneic MHC molecules, simply because these molecules are not present in the thymus.

The result is that the mature repertoire has an intrinsic weak affinity for self MHC molecules and includes many T cells that bind allogeneic MHC molecules with high affinity.

Direct Presentation of MHC Alloantigens: keynotes 2. The structure of an allogeneic MHC molecule is similar enough to self MHC that many self MHC-restricted T cells recognize the foreign MHC molecule. • Allogeneic MHC molecule with a bound peptide can mimic the determinant formed by a self MHC molecule plus a particular foreign peptide.

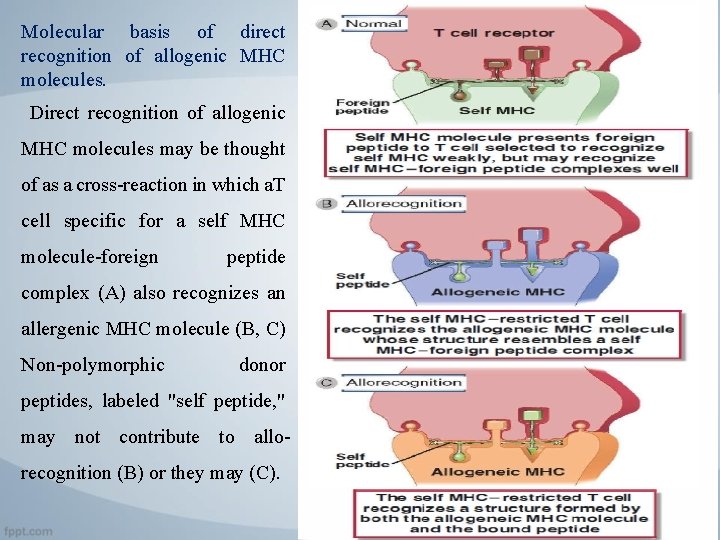
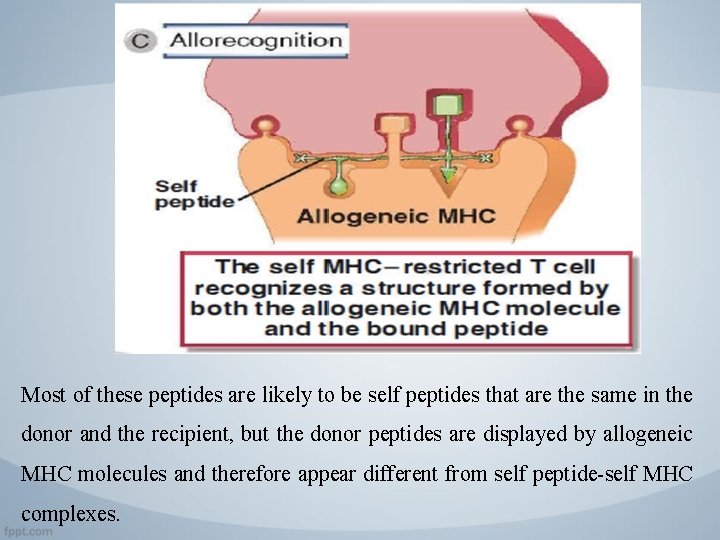
Molecular basis of direct recognition of allogenic MHC molecules. Direct recognition of allogenic MHC molecules may be thought of as a cross-reaction in which a. T cell specific for a self MHC molecule-foreign peptide complex (A) also recognizes an allergenic MHC molecule (B, C) Non-polymorphic donor peptides, labeled "self peptide, " may not contribute to allorecognition (B) or they may (C).

Direct Presentation of MHC Alloantigens: keynotes 2. Many peptides may combine with a single MHC molecule and further expand the number of T cells that can recognize these combinations. • MHC molecules that are expressed on cell surfaces normally contain bound peptides, and the peptides form part of the structure recognized by the alloreactive T cell, exactly like the role of peptides in the normal recognition of foreign antigens by self MHC-restricted T cells.

Most of these peptides are likely to be self peptides that are the same in the donor and the recipient, but the donor peptides are displayed by allogeneic MHC molecules and therefore appear different from self peptide-self MHC complexes.

Direct Presentation of MHC Alloantigens: keynotes 3. All the MHC molecules on a donor APC will be foreign and will be recognized by alloreactive T cells; in contrast, in the case of an infection, less than 1 % (and perhaps as few as 0. 1 % ) of the MHC molecules on an APC normally present microbial peptides at any time and are recognized by T cells. 4. Direct allorecognition can generate both CD 4+ and CD 8+ T cells that recognize graft antigens and contribute to rejection.

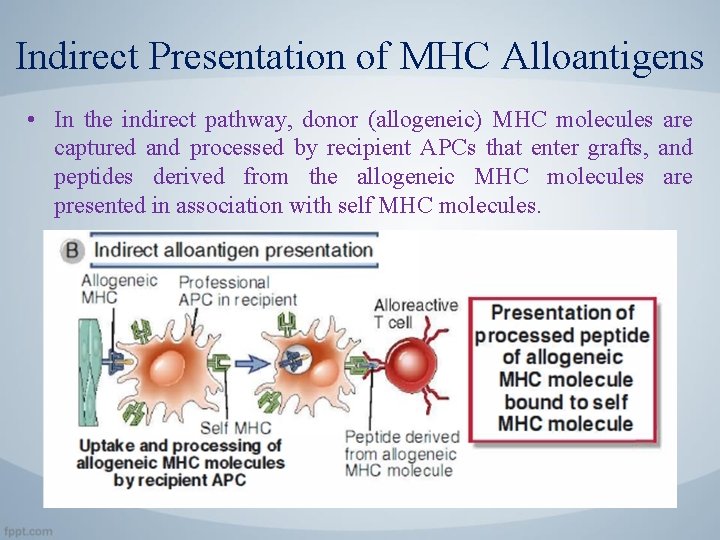
Indirect Presentation of MHC Alloantigens • In the indirect pathway, donor (allogeneic) MHC molecules are captured and processed by recipient APCs that enter grafts, and peptides derived from the allogeneic MHC molecules are presented in association with self MHC molecules.

Indirect Presentation of MHC Alloantigens • Because allogeneic MHC molecules have amino acid sequences different from those of the host, they can generate foreign peptides associated with self MHC molecules on the surface of host APCs. • Indirect presentation may result in allorecognition by CD 4+ T cells because alloantigen is acquired by host APCs primarily through the endosomal vesicular pathway (i. e. , as a consequence of phagocyto· sis) and is therefore presented by class II MHC molecules. • Some antigens of phagocytosed graft cells appear to enter the class. I MHC pathway of antigen presentation and are indirectly recognized by C D 8+ T cells.

Indirect Presentation of MHC Alloantigens • In the setting of any transplant between genetically nonidentical donor and recipient, there will be polymorphic antigens other than MHC molecules against which the recipient may mount an immune response. • These antigens typically induce weak or slower (more gradual) rejection reactions than do MHC molecules and are therefore called minor histocompatibility antigens. • Most minor histocompatibility antigens are proteins that are processed and presented to host T cells in association with self MHC molecules on host APCs (i. e. , by the indirect pathway).

3. Activation of Alloreactive Lymphocytes 3. 1 T cell Recognition of Alloantigens: • The T cell response to an organ graft may be initiated in the lymph nodes that drain the graft. • Most organs contain resident APCs such as dendritic cells. • Transplantation of these organs into an allogeneic recipient provides APCs that express donor MHC molecules as well as costimulators. • It is believed that these donor APCs migrate to regional lymph nodes and present, on their surface, unprocessed allogeneic MHC molecules to the recipient's T cells (the direct pathway of allorecognition).

3. Activation of Alloreactive Lymphocytes 3. 1 T cell Recognition of Alloantigens: • Host dendritic cells from the recipient may also migrate into the graft, pick up graft alloantigens, and transport these back to the draining lymph nodes, where they are displayed (the indirect pathway). • Naive lymphocytes that normally traffic through the lymph node encounter these alloantigens and are induced to proliferate and differentiate into effector cells. This process is sometimes called sensitization to alloantigens. Effector T cells migrate back into the graft and mediate rejection.


3. Activation of Alloreactive Lymphocytes 3. 1 T cell Recognition of Alloantigens: • As many as 1 % to 2 % of an individual's T cells are capable of recognizing and responding to a single foreign MHC molecule, and this high frequency of T cells reactive with allogeneic MHC molecules is one reason that allografts elicit strong immune responses. • Many of the T cells that respond to an allogeneic MHC molecule, even on first exposure, are memory T cells.

3. Activation of Alloreactive Lymphocytes 3. 2 Role of Costmiulation in T Cell Responses to Alloantigens • In addition to recognition of alloantigen, costimulation of T cells primarily by B 7 molecules on APCs is important for activating alloreactive T cells.

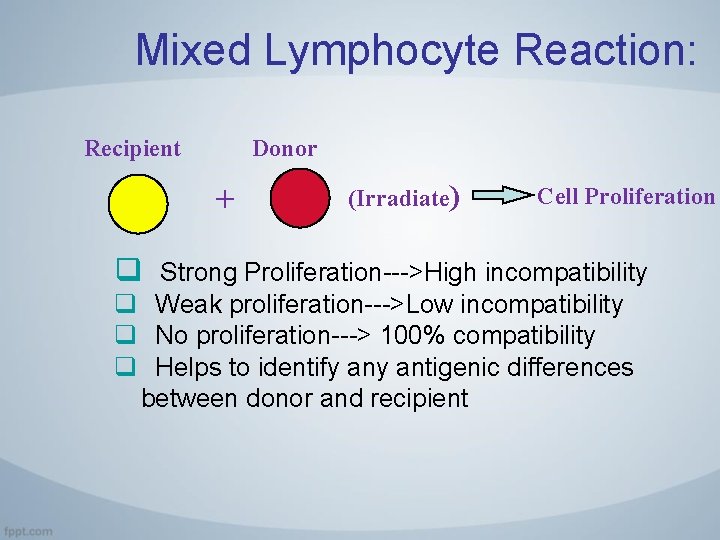
3. Activation of Alloreactive Lymphocytes 3. 3 The Mixed Lymphocyte Reaction • The response of alloreactive T cells to foreign MHC molecules can be analyzed in an in vitro reaction called the mixed lymphocyte reaction (MLR). • The MLR is used as a predictive test of T cell-mediated graft rejection. • Studies of the MLR were among the first to establish the role of class I and class II MHC molecules in activating distinct populations of T cells (CDS+ and CD 4+, respectively).

3. Activation of Alloreactive Lymphocytes 3. 3 The Mixed Lymphocyte Reaction • The MLR is induced by culturing mononuclear leukocytes (which include T cells, B cells, natural killer [NK] cells, mononuclear phagocytes, and dendritic cells) from one individual with mononuclear leukocytes derived from another individual. • In clinical practice, these cells are typically isolated from peripheral blood; in mouse or rat experiments, mononuclear leukocytes are usually purified from the spleen or lymph nodes.

3. Activation of Alloreactive Lymphocytes 3. 3 The Mixed Lymphocyte Reaction • If the two individuals have differences in the alleles of the MHC genes, a large proportion of the mononuclear cells will proliferate during a period of 4 to 7 days. • This proliferative response is called the allogeneic MLR • If cells from two MHC-disparate individuals are mixed, each can react against the other and both will proliferate, thus resulting in a two-way MLR.


Mixed Lymphocyte Reaction: Recipient Donor + q (Irradiate) Cell Proliferation Strong Proliferation--->High incompatibility q Weak proliferation--->Low incompatibility q No proliferation---> 100% compatibility q Helps to identify antigenic differences between donor and recipient

4. Effector Functions of Alloreactive T Cells Ø Effector Functions of Alloreactive T Cells Alloreactive CD 4+ and CD 8+ T cells that are activated by graft alloantigens cause rejection by distinct mechanisms. • The CD 4+ helper T cells differentiate into cytokine producing effector cells that damage grafts in a way similar to delayed type hypersensitivity. While alloreactive CD 8+ cells differentiate into cytotoxic T lymphocytes, which kill graft cells that possess MHCI. • CTLs also secrete inflammatory cytokines, which can contribute to graft damage.

4. Effector Functions of Alloreactive T Cells Ø Only CTLs that are generated by direct allogeneic MHC recognition can kill graft cells, whereas CTLs or helper T cells generated by either direct or indirect alloantigen recognition cause cytokine-mediated damage to grafts.

Activation of Alloreactive B Cells and Production of Alloantibodies • Most high-affinity alloantibodies are produced by helper T celldependent activation of alloreactive B cells, much like antibodies against other protein antigens. • The antigens most frequently recognized by alloantibodies in graft rejection are donor HLA molecules, including both class I and class II MHC proteins.

Activation of Alloreactive B Cells and Production of Alloantibodies • The likely sequence of events leading to the generation of these alloantibody-producing cells is that: 1. Naive B lymphocytes recognize foreign MHC molecules, 2. Internalize and process these proteins, 3. and present peptides derived from them to helper T cells that were previously • Thus activation of alloreactive B cells is an example of indirect presentation of alloantigens. • Anti-HLA antibodies contribute significantly to allograft rejection.

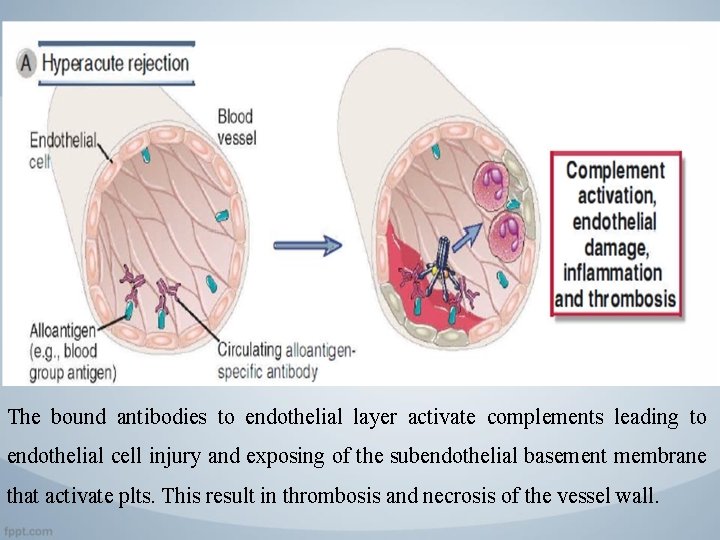
Patterns and Mechanisms of Allograft Rejection 1. Hyperacute Rejection • Hyperacute rejection is characterized by thrombotic occlusion of the graft vasculature that begins within minutes to hours after host blood vessels are anastomosed to graft vessels and is mediated by preexisting antibodies in the host circulation that bind to donor endothelial antigens.

The bound antibodies to endothelial layer activate complements leading to endothelial cell injury and exposing of the subendothelial basement membrane that activate plts. This result in thrombosis and necrosis of the vessel wall.

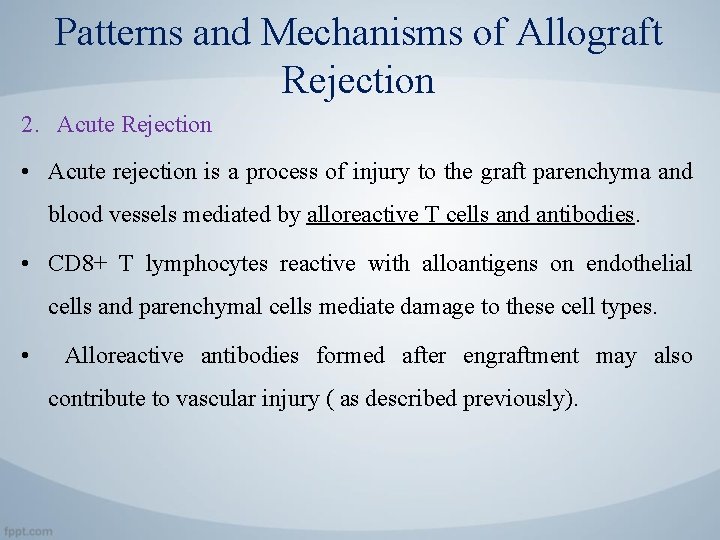
Patterns and Mechanisms of Allograft Rejection 2. Acute Rejection • Acute rejection is a process of injury to the graft parenchyma and blood vessels mediated by alloreactive T cells and antibodies. • CD 8+ T lymphocytes reactive with alloantigens on endothelial cells and parenchymal cells mediate damage to these cell types. • Alloreactive antibodies formed after engraftment may also contribute to vascular injury ( as described previously).

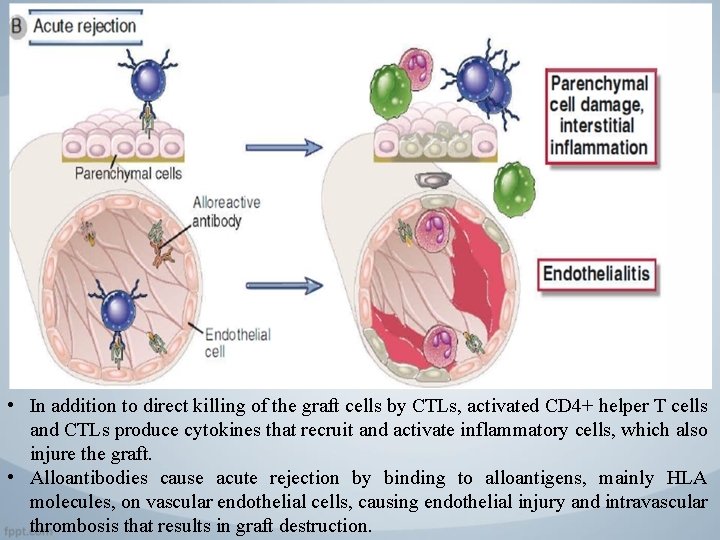
• In addition to direct killing of the graft cells by CTLs, activated CD 4+ helper T cells and CTLs produce cytokines that recruit and activate inflammatory cells, which also injure the graft. • Alloantibodies cause acute rejection by binding to alloantigens, mainly HLA molecules, on vascular endothelial cells, causing endothelial injury and intravascular thrombosis that results in graft destruction.

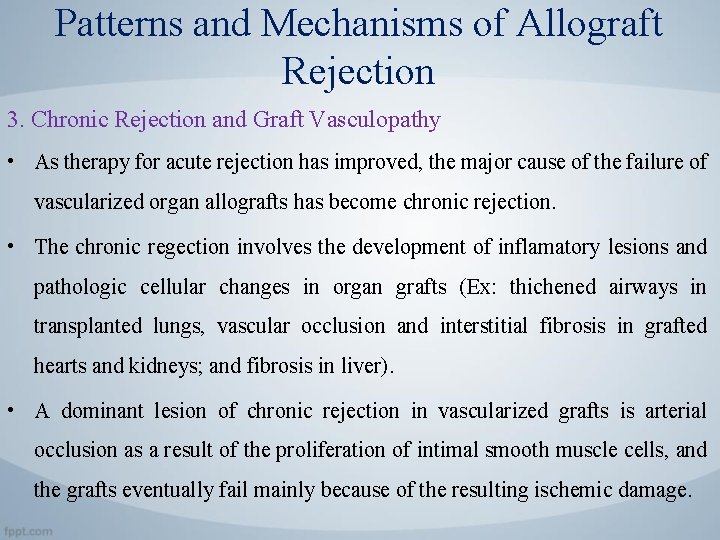
Patterns and Mechanisms of Allograft Rejection 3. Chronic Rejection and Graft Vasculopathy • As therapy for acute rejection has improved, the major cause of the failure of vascularized organ allografts has become chronic rejection. • The chronic regection involves the development of inflamatory lesions and pathologic cellular changes in organ grafts (Ex: thichened airways in transplanted lungs, vascular occlusion and interstitial fibrosis in grafted hearts and kidneys; and fibrosis in liver). • A dominant lesion of chronic rejection in vascularized grafts is arterial occlusion as a result of the proliferation of intimal smooth muscle cells, and the grafts eventually fail mainly because of the resulting ischemic damage.

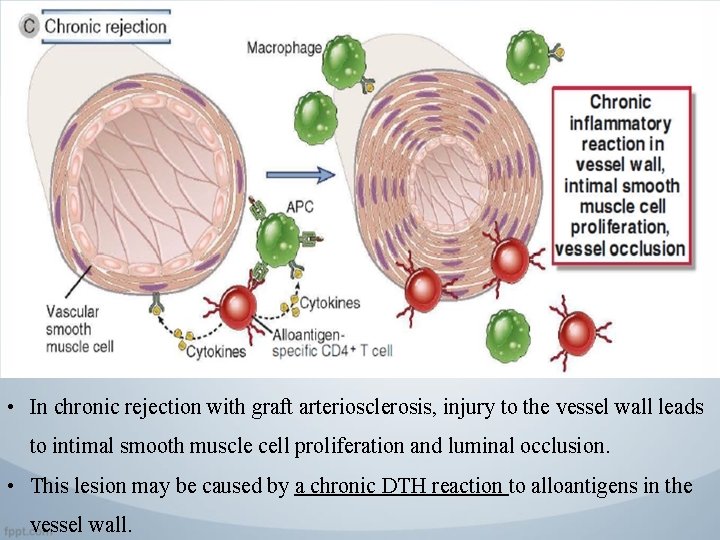
• In chronic rejection with graft arteriosclerosis, injury to the vessel wall leads to intimal smooth muscle cell proliferation and luminal occlusion. • This lesion may be caused by a chronic DTH reaction to alloantigens in the vessel wall.

Prevention and Treatment of Allograft Rejection

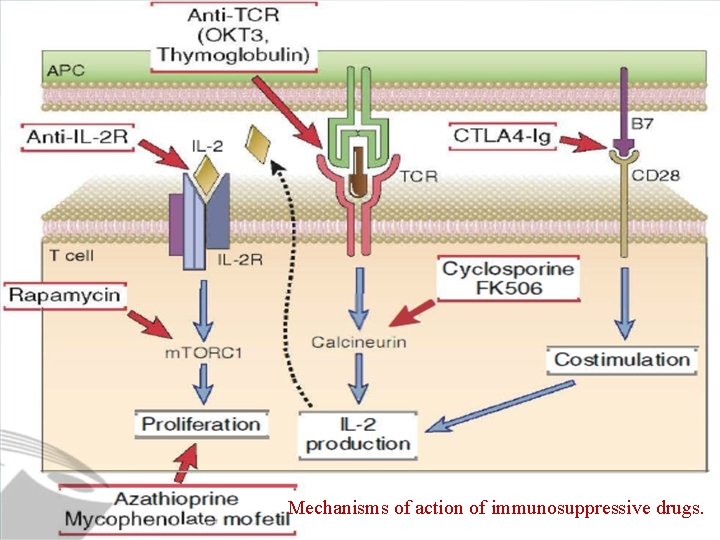
Immunosuppression to Prevent or to Treat Allograft Rejection • Immunosuppressive drugs that inhibit or kill T lymphocytes are the principal agents used to treat or prevent graft rejection. • Several methods of immunosuppression are commonly used: 1. Inhibitors of T Cell Signaling Pathways • The calcineurin inhibitors cyclosporine and FK 506 (tacrolimus) inhibit transcription of certain genes in T cells, most notably those encoding cytokines such as IL-2.

Immunosuppression to Prevent or to Treat Allograft Rejection 2. Antimetabolites • Metabolic toxins that kill proliferating T cells are used in combination with other drugs to treat graft rejection. These agents inhibit the proliferation of lymphocyte precursors during their maturation and also kill proliferating mature T cells that have been stimulated by alloantigens. • The first such drug to be developed for the prevention and treatment of rejection was azathioprine. • The most widely used drug in this class is mycophenolate mofetil (MMF). MMF is metabolized to mycophenolic acid, which blocks a lymphocytespecific isoform of inosine monophosphate dehydrogenase, an enzyme required for de novo synthesis of guanine nucleotides.

Immunosuppression to Prevent or to Treat Allograft Rejection 3. Function-Blocking or Depleting Antilymphocyte • Antibodies that react with T cell surface structures and deplete or inhibit T cells used to treat acute rejection. • One widely used antibody is a mouse monoclonal antibody called OKT 3 that is specific for human CD 3. These anti-T cell antibodies deplete circulating T cells either by activating the complement system to eliminate T cells or by opsonizing them for phagocytosis. • Monoclonal antibodies are now in clinical use that are specific for CD 25, the a subunit of the IL-2 receptor. These reagents presumably prevent T cell activation by blocking IL-2 binding to activated T cells and IL-2 signaling.

Immunosuppression to Prevent or to Treat Allograft Rejection 4. Costimulatory Blockade • Drugs that block T cell costimulatory pathways reduce acute allograft rejection. • A soluble high-affinity form of CTLA-4 fused to an Ig. G Fc domain binds to B 7 molecules on APCs and prevents them from interacting with T cell CD 28 and is near approval for use in allograft recipients. • Clinical studies have shown that CTLA-4 -Ig can be as effective as cyclosporine in preventing acute rejection.

Immunosuppression to Prevent or to Treat Allograft Rejection 5. Drugs Targeting Alloantibodies and Alloreactive 8 Cells. • A monoclonal antibody specific for the B cell surface protein CD 20 very effectively depletes mature B cells from the circulation and secondary lymphoid organs. • Anti-CD 20 has been used for treatment of B cell lymphomas and for autoimmune diseases and is now in clinical trials for treatment of antibody-mediated allograft rejection. • These antibody and B cell targeted therapies have been used in combination to effectively treat antibody-mediated rejection.

Immunosuppression to Prevent or to Treat Allograft Rejection 6. Anti-inflammatory Drugs. • Anti-inflammatory agents, specifically corticosteroids, are frequently used to reduce the inflammatory reaction to organ allografts. • The proposed mechanism of action of these natural hormones and their synthetic analogues is to block the synthesis and secretion of cytokines, including TNF and IL-l, and other mediators, such as prostaglandins, reactive oxygen species, and nitric oxide, produced by macrophages and other inflammatory cells. • The net result is reduced leukocyte recruitment, inflammation, and graft damage. • Very high doses of corticosteroids may inhibit T cell secretion of cytokines or even kill some T cells. • Newer anti-inflammatory agents are in clinical trials, including soluble cytokine receptors and anticytokine antibodies.

Immunosuppression to Prevent or to Treat Allograft Rejection 7. Inhibitors of Leukocyte Migration. • A new therapeutic agent, called fingolimod (FTY 720), works by binding to and blocking sphingosine l –phosphate (Sl. P) receptors on lymphocytes. Sl. P is required for the egress of lymphocytes from lymphoid organs, and blocking its action leads to the sequestration of lymphocytes in lymph nodes. • Anti-integrin antibodies have proved to be effective treatments for some autoimmune diseases because they block leukocyte recruitment from the circulation into inflamed tissues.

Mechanisms of action of immunosuppressive drugs.

Methods to Reduce the lmmunogenicity of Allografts Ø In human transplantation, the major strategy to reduce graft immunogenicity has been to minimize alloantigenic differences between the donor and recipient. • These include ABO blood typing; the determination of HLA alleles expressed on donor and recipient cells, called tissue typing; the detection of preformed antibodies in the recipient that recognize HLA and other antigens representative of the donor population; and the detection of preformed antibodies in the recipient that bind to antigens of an identified donor's leukocytes, called crossmatching.

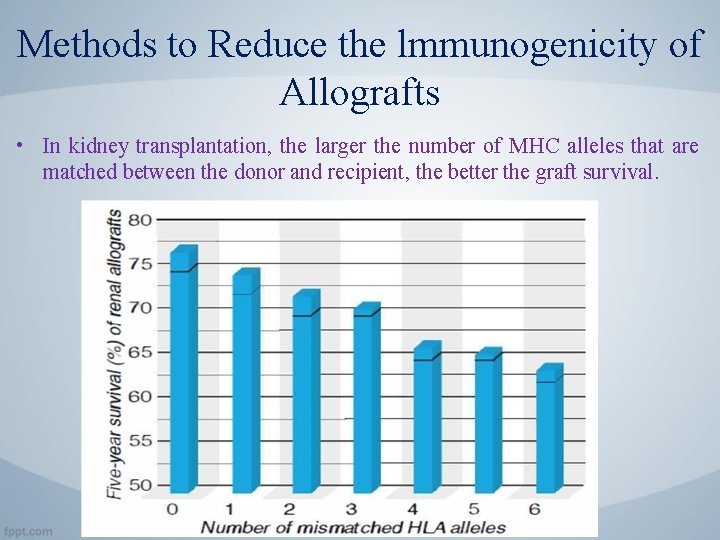
Methods to Reduce the lmmunogenicity of Allografts • In kidney transplantation, the larger the number of MHC alleles that are matched between the donor and recipient, the better the graft survival.

Methods to Reduce the lmmunogenicity of Allografts Ø To avoid hyperacute rejection, the ABO blood group antigens of the graft donor are selected to be identical to those of the recipient. • if there are ABO incompatibilities between the donor and recipient. Natural Ig. M antibodies specific for allogeneic ABO blood group antigens (discussed earlier) will cause hyperacute rejection.

Methods to Reduce the lmmunogenicity of Allografts Ø To avoid hyperacute rejection, the ABO blood group antigens of the graft donor are selected to be identical to those of the recipient. • if there are ABO incompatibilities between the donor and recipient. Natural Ig. M antibodies specific for allogeneic ABO blood group antigens (discussed earlier) will cause hyperacute rejection.

Methods to Reduce the lmmunogenicity of Allografts • Patients in need of allografts are also tested for the presence of preformed antibodies against donor MHC molecules or other cell surface antigens. Two types of tests are done to detect these antibodies. • The panel reactive antibody test, patients waiting for organ transplants are screened for the presence of preformed antibodies reactive with allogeneic HLA molecules prevalent in the population. These antibodies, which may be produced as a result of previous pregnancies, transfusions, or transplantation, can identify risk for hyperacute or acute vascular rejection. • The cross matching test will determine whether the patient has antibodies that react specifically with that donor's cells. The test is performed by mixing the recipient's serum with the donor's blood lymphocytes.

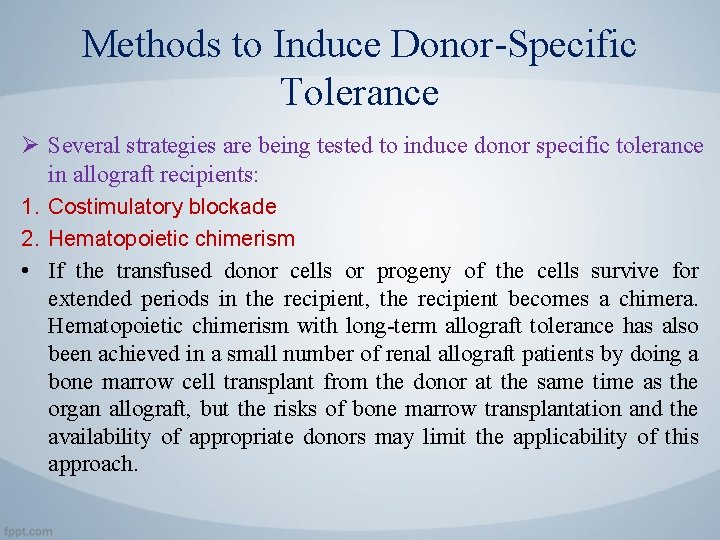
Methods to Induce Donor-Specific Tolerance Ø Several strategies are being tested to induce donor specific tolerance in allograft recipients: 1. Costimulatory blockade 2. Hematopoietic chimerism • If the transfused donor cells or progeny of the cells survive for extended periods in the recipient, the recipient becomes a chimera. Hematopoietic chimerism with long-term allograft tolerance has also been achieved in a small number of renal allograft patients by doing a bone marrow cell transplant from the donor at the same time as the organ allograft, but the risks of bone marrow transplantation and the availability of appropriate donors may limit the applicability of this approach.

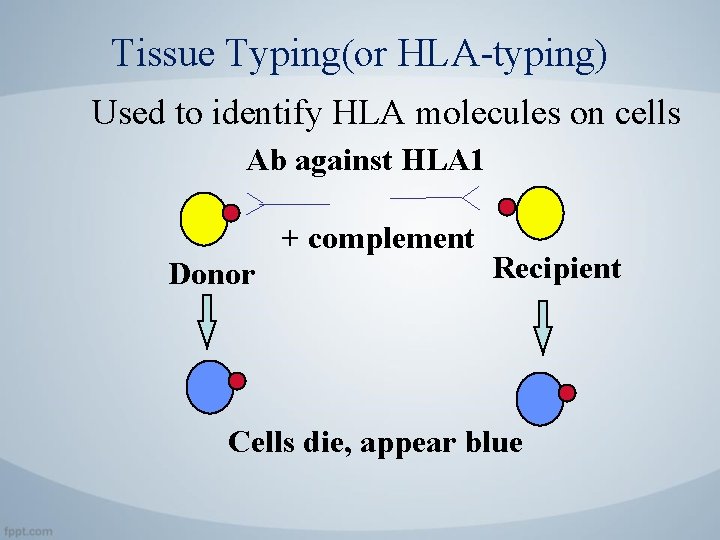
Tissue Typing(or HLA-typing) Used to identify HLA molecules on cells Ab against HLA 1 + complement Donor Recipient Cells die, appear blue

Methods to Induce Donor-Specific Tolerance Ø Several strategies are being tested to induce donor specific tolerance in allograft recipients: 3. Transfer or induction of regulatory T cells. • Attempts to generate donor-specific regulatory T cells in culture and to transfer these into graft recipients are ongoing. 4. Other approaches • Include the administration of soluble MHC proteins or peptides under conditions predicted to induce tolerance.

Blood Transfusion and the ABO AND Rh Blood Group Antigens

Blood Transfusion • Blood transfusion is a form of transplantation in which whole blood or blood cells from one or more individuals are transferred intravenously into the circulation of a host. • It often performed to replace blood lost by hemorrhage or to correct defects caused by inadequate production of blood cells. • The most important alloantigen system in blood transfusion is the ABO system.

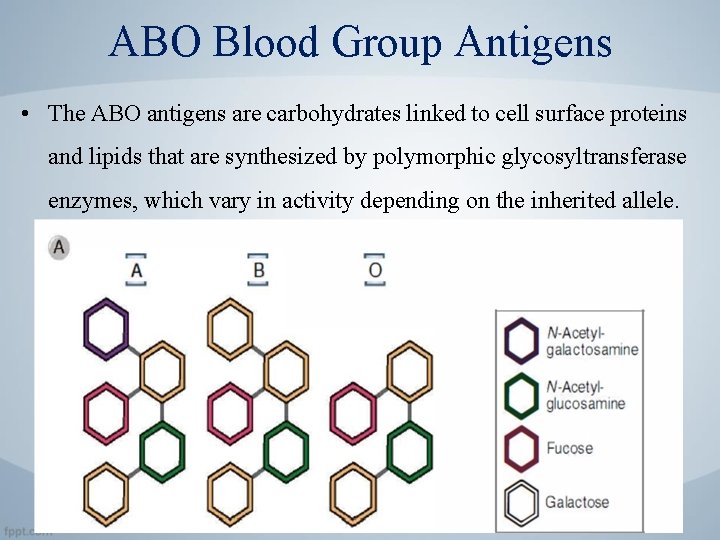
ABO Blood Group Antigens • The ABO antigens are carbohydrates linked to cell surface proteins and lipids that are synthesized by polymorphic glycosyltransferase enzymes, which vary in activity depending on the inherited allele.

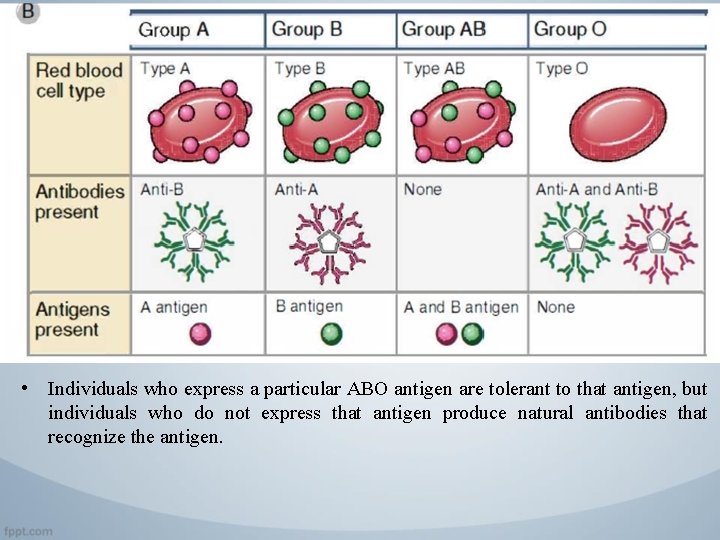
• Individuals who express a particular ABO antigen are tolerant to that antigen, but individuals who do not express that antigen produce natural antibodies that recognize the antigen.

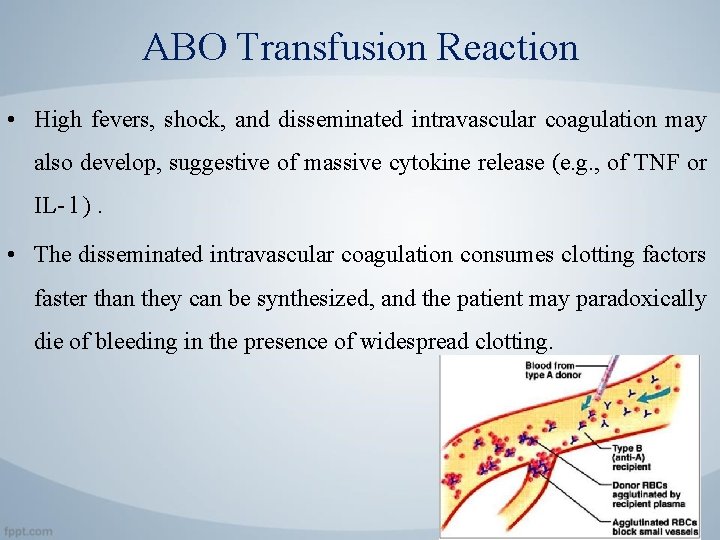
ABO Transfusion Reaction • Transfusion across an ABO barrier may trigger an immediate hemolytic reaction, resulting in both intravascular lysis of red blood cells, probably mediated by the complement system, and extensive phagocytosis of antibody- and complement-coated erythrocytes by macrophages of the liver and spleen. • Hemoglobin is liberated from the lysed red cells in quantities that may be toxic for kidney cells, causing acute renal tubular cell necrosis and kidney failure.

ABO Transfusion Reaction • High fevers, shock, and disseminated intravascular coagulation may also develop, suggestive of massive cytokine release (e. g. , of TNF or IL- l ). • The disseminated intravascular coagulation consumes clotting factors faster than they can be synthesized, and the patient may paradoxically die of bleeding in the presence of widespread clotting.


Hematopoietic Stem Cell Transplantation • Hematopoietic stem cells can be purified from the blood of donors after treatment with colony-stimulating factors, which mobilize stem cells from the bone marrow. • The recipient is treated before transplantation to deplete bone marrow cells to free up niches for the transferred stem cells. • After transplantation, stem cells repopulate the recipient's bone marrow and differentiate into all the hematopoietic lineages.




Hematopoietic Stem Cell Transplantation • Allogeneic hematopoietic stem cells are rejected by even a minimally immunocompetent host, and therefore the donor and recipient must be carefully matched at all MHC loci. • The mechanisms of rejection of bone marrow cells are not completely known, but in addition to adaptive immune mechanisms, hematopoietic stem cells may be rejected by NK cells.

Graft-Versus-Host Disease • Graft-versus-host disease (GVHD) is caused by the reaction of grafted mature T cells in the marrow inoculum with alloantigens of the host. • It occurs when the host is immunocompromised and therefore unable to reject the allogeneic cells in the graft. • the reaction is directed against MHC antigens of the host because bone marrow transplantation is not performed when the donor and recipient have differences in MHC molecules. • GVHD may also develop when solid organs that contain significant numbers of T cells are transplanted, such as the small bowel, lung, or liver.

Graft-Versus-Host Disease • Although GVHD is initiated by grafted T cells recognizing host alloantigens, the effector cells that cause epithelial cell injury are less well defined. • On histologic examination, NK cells are often attached to the dying epithelial cells, suggesting that NK cells are important effector cells of acute GVHD. • CD 8+ CTLs and cytokines also appear to be involved in tissue injury in acute GVHD.


Graft-Versus-Host Disease • GVHD may be classified on the basis of histologic patterns into acute and chronic forms: • Acute GVHD is characterized by epithelial cell death in the skin, liver (mainly the biliary epithelium), and gastrointestinal tract. It is manifested clinically by rash, jaundice, diarrhea, and gastrointestinal hemorrhage. • When the epithelial cell death is extensive, the skin or lining of the gut may slough off. In this circumstance, acute GVHD may be fatal.

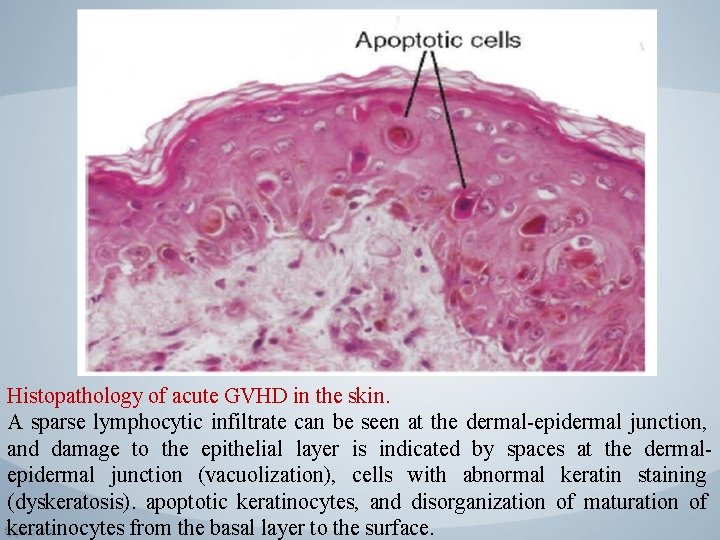
Histopathology of acute GVHD in the skin. A sparse lymphocytic infiltrate can be seen at the dermal-epidermal junction, and damage to the epithelial layer is indicated by spaces at the dermalepidermal junction (vacuolization), cells with abnormal keratin staining (dyskeratosis). apoptotic keratinocytes, and disorganization of maturation of keratinocytes from the basal layer to the surface.

Graft-Versus-Host Disease • Chronic GVHD is characterized by fibrosis and atrophy of one or more of the same organs, without evidence of acute cell death. Chronic GVHD may also involve the lungs and produce obliteration of small airways. • When it is severe, chronic GVHD leads to complete dysfunction of the affected organ.

Graft-Versus-Host Disease • Both acute and chronic GVHD are commonly treated with intense immunosuppression. • Chronic GVHD may represent the fibrosis of wound healing secondary to loss of epithelial cells. • However, chronic GVHD can arise without evidence of prior acute GVHD. An alternative explanation is that chronic GVHD represents a response to ischemia caused by vascular injury.

Immunodeficiency after Bone Marrow Transplantation • Bone marrow transplantation is often accompanied by clinical immunodeficiency. • Several factors may contribute to defective immune responses in recipients: 1. Bone marrow transplant recipients may be unable to regenerate a complete new lymphocyte repertoire. 2. Radiation therapy and chemotherapy used to prepare recipients for transplantation are likely to deplete the patient's memory cells and long-lived plasma cells, 3. and it can take a long time to regenerate these populations.

Immunodeficiency after Bone Marrow Transplantation • The consequence of immunodeficiency is that bone marrow transplant recipients are susceptible to viral infections, especially cytomegalovirus infection, and to many bacterial and fungal infections. • They are also susceptible to Epstein-Barr virus-provoked B cell lymphomas. • The immune deficiencies of bone marrow transplant recipients can be more severe than those of conventionally Immunosuppressed patients.

Immunodeficiency after Bone Marrow Transplantation • Bone Marrow transplant recipients commonly receive prophylactic antibiotics and anti-cytomegalovirus therapy and are often actively immunized against capsular bacteria such as pneumococcus before transplantation.

The End
 Biomedical therapies are provided by
Biomedical therapies are provided by What are the 3 most widely used self-secured joints?
What are the 3 most widely used self-secured joints? The most widely used agile process originally proposed by
The most widely used agile process originally proposed by What is the sequence of installations on rhipe
What is the sequence of installations on rhipe Distillation theory
Distillation theory The most widely used encryption standard is
The most widely used encryption standard is Bone marrow transplantation sri lanka
Bone marrow transplantation sri lanka Law of transplantation
Law of transplantation Patrick evrard transplantation
Patrick evrard transplantation Cultural transplantation examples
Cultural transplantation examples Types of transplant
Types of transplant Stem cell or bone marrow transplantation bangkok
Stem cell or bone marrow transplantation bangkok Most widely practiced religion
Most widely practiced religion Culture and the workplace
Culture and the workplace Rapid diffusion of popular culture
Rapid diffusion of popular culture Religion of africa
Religion of africa In a premix burner used in fes the fuel used is
In a premix burner used in fes the fuel used is In a premix burner used in fes the fuel used is
In a premix burner used in fes the fuel used is Conclusion paragraph format
Conclusion paragraph format Water treatment fundamentals
Water treatment fundamentals Water pollution paragraph for class 8
Water pollution paragraph for class 8 Purpose of wastewater treatment
Purpose of wastewater treatment Aquaculture characteristics
Aquaculture characteristics Horizontal flow grit chamber
Horizontal flow grit chamber Wast water treatment
Wast water treatment Wastewater treatment process primary secondary tertiary
Wastewater treatment process primary secondary tertiary Objective of water treatment
Objective of water treatment Bv vs trich
Bv vs trich Treatment levels statistics
Treatment levels statistics Astelazine
Astelazine Treatment for prion disease
Treatment for prion disease Matrs
Matrs Phases of dental treatment planning
Phases of dental treatment planning Treatment of herpetic gingivostomatitis
Treatment of herpetic gingivostomatitis Religious ocd islam
Religious ocd islam Cas treatment approaches
Cas treatment approaches Trauma awareness and treatment center utah
Trauma awareness and treatment center utah Agranulocytosis
Agranulocytosis Complications of hypothyroidism
Complications of hypothyroidism Uterine packing
Uterine packing The challenge the odyssey
The challenge the odyssey Preliminary treatment adalah
Preliminary treatment adalah Julie woodside
Julie woodside Social work treatment plan example
Social work treatment plan example Orthopaedic services east london
Orthopaedic services east london Fluid in cul de sac treatment
Fluid in cul de sac treatment Intensive phase of tb treatment
Intensive phase of tb treatment First-line treatment for schizophrenia
First-line treatment for schizophrenia Excess of minimum rent over royalty is called
Excess of minimum rent over royalty is called Water treatment rockingham county
Water treatment rockingham county Elga veolia lc241 pre-treatment pack
Elga veolia lc241 pre-treatment pack Prp in islamabad
Prp in islamabad Cyclophotocoagulation
Cyclophotocoagulation Hemolysis treatment
Hemolysis treatment Ttkg
Ttkg Uterine atony
Uterine atony Pph management
Pph management Uterine atony treatment
Uterine atony treatment Phototherapy principles
Phototherapy principles Bronchospasm treatment
Bronchospasm treatment Pyloromyotomy surgery
Pyloromyotomy surgery Tet spell treatment
Tet spell treatment Asthma treatment
Asthma treatment Ascites treatment
Ascites treatment Charting exercise chapter 28
Charting exercise chapter 28 Open fracture treatment
Open fracture treatment Communicating hydrocephalus
Communicating hydrocephalus Official disability guidelines texas
Official disability guidelines texas Blood stagnation treatment
Blood stagnation treatment Causes of metabolic acidosis
Causes of metabolic acidosis Treatment of herpetic gingivostomatitis
Treatment of herpetic gingivostomatitis Dr shahzad ahmad
Dr shahzad ahmad Causes of acute abdominal pain
Causes of acute abdominal pain Scout and aunt alexandra communicate very poorly
Scout and aunt alexandra communicate very poorly Ground water treatment process
Ground water treatment process Copyright ppt
Copyright ppt Maple syrup urine disease treatment
Maple syrup urine disease treatment Accelerated idioventricular rhythm
Accelerated idioventricular rhythm Malabsorption syndrome
Malabsorption syndrome The next treatment
The next treatment Phases of dental treatment planning
Phases of dental treatment planning Laryngeal nerve damage treatment
Laryngeal nerve damage treatment Impulse control disorder treatment
Impulse control disorder treatment Hypothyroidism treatment in pregnancy
Hypothyroidism treatment in pregnancy Dr sofi
Dr sofi Familial hypertriglyceridemia
Familial hypertriglyceridemia Kaexalate
Kaexalate Parasite life cycle
Parasite life cycle Hymenolepis
Hymenolepis How to write a movie treatment
How to write a movie treatment Ann arbor staging
Ann arbor staging Hemolysis treatment
Hemolysis treatment Wholesale hardening and tempering
Wholesale hardening and tempering Heat treatment normalizing
Heat treatment normalizing Normalizing heat treatment
Normalizing heat treatment