PAPER CHROMATOGRAPHY INTRODUCTION Chromatography is a widely used


- Slides: 8

PAPER CHROMATOGRAPHY

INTRODUCTION • Chromatography is a widely used method of separating mixtures into their component parts. • Paper chromatography separates substances using two things (called phases) • The first thing is chromatography paper; also called the stationary phase • Next we have a solvent – called the mobile phase

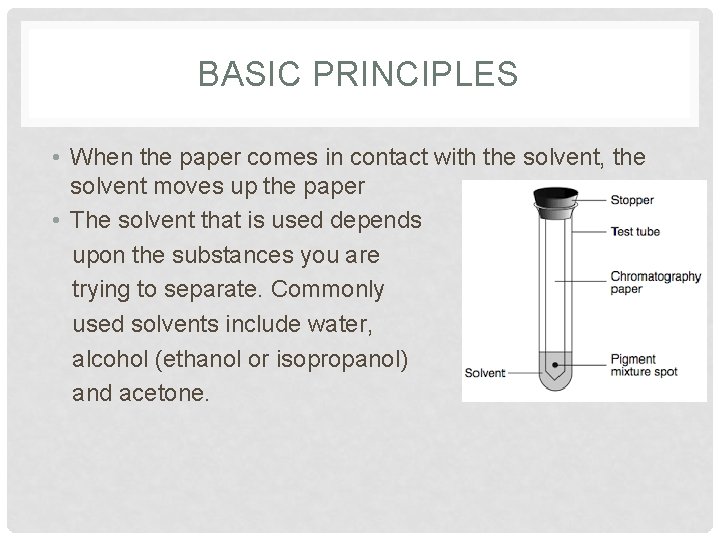
BASIC PRINCIPLES • When the paper comes in contact with the solvent, the solvent moves up the paper • The solvent that is used depends upon the substances you are trying to separate. Commonly used solvents include water, alcohol (ethanol or isopropanol) and acetone.

SEPARATION • the different components of a mixture will travel different lengths along a piece of paper when a solvent is allowed to travel up it • The length that a specific component will travel along the paper is dependent upon several forces.

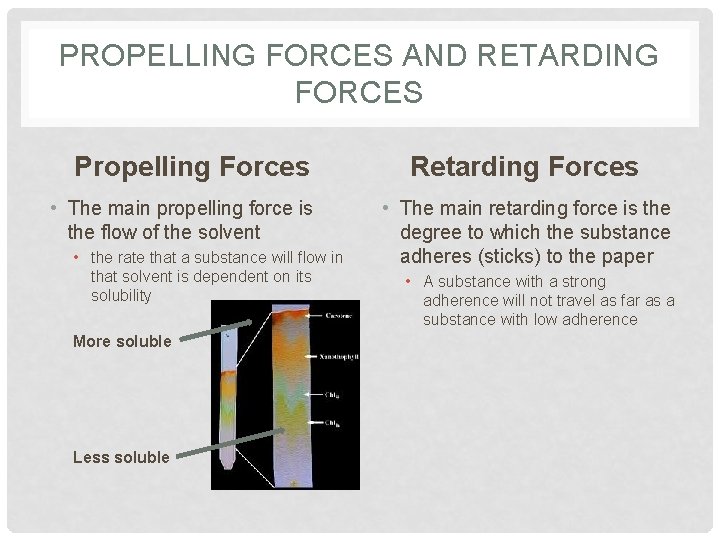
PROPELLING FORCES AND RETARDING FORCES Propelling Forces • The main propelling force is the flow of the solvent • the rate that a substance will flow in that solvent is dependent on its solubility More soluble Less soluble Retarding Forces • The main retarding force is the degree to which the substance adheres (sticks) to the paper • A substance with a strong adherence will not travel as far as a substance with low adherence

SOLUBILITY • If a component of a mixture is completely soluble in a solvent then it will travel farther along the paper than a component that is not soluble or only partially soluble. • The degree to which a substance is soluble depends on the solvent • Generally speaking, a polar substance will have a greater solubility in a polar solvent (like dissolves like) and non polar substances will be more soluble in a nonpolar solvent

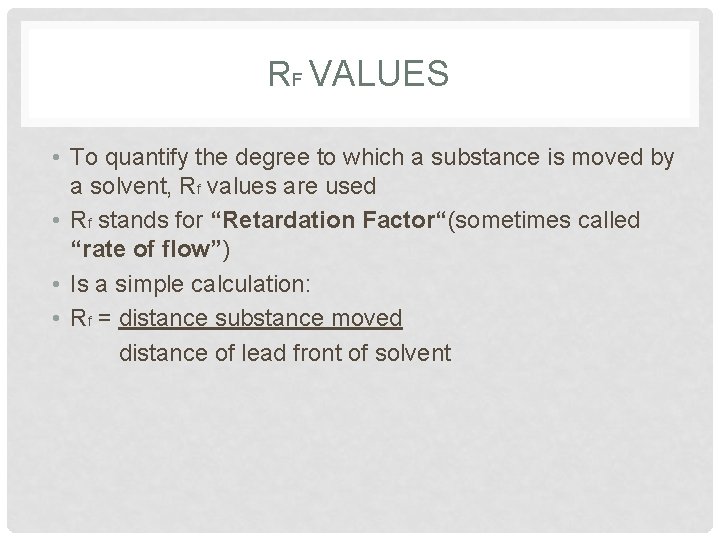
RF VALUES • To quantify the degree to which a substance is moved by a solvent, Rf values are used • Rf stands for “Retardation Factor“(sometimes called “rate of flow”) • Is a simple calculation: • Rf = distance substance moved distance of lead front of solvent

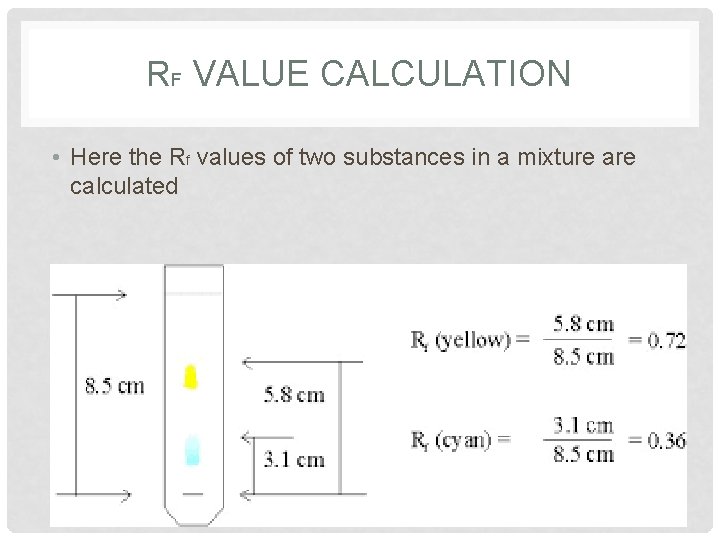
RF VALUE CALCULATION • Here the Rf values of two substances in a mixture are calculated