Chapter Chromatography 1 Introduction to Chromatography Definition Chromatography






- Slides: 31

Chapter: Chromatography 1

Introduction to Chromatography Definition Chromatography is a separation technique based on the different interactions of compounds with two phases, a mobile phase and a stationary phase, as the compounds travel through a supporting medium. Components: mobile phase: a solvent that flows through the supporting medium stationary phase: a layer or coating on the supporting medium that interacts with the analytes supporting medium: a solid surface on which the stationary phase is bound or coated 2

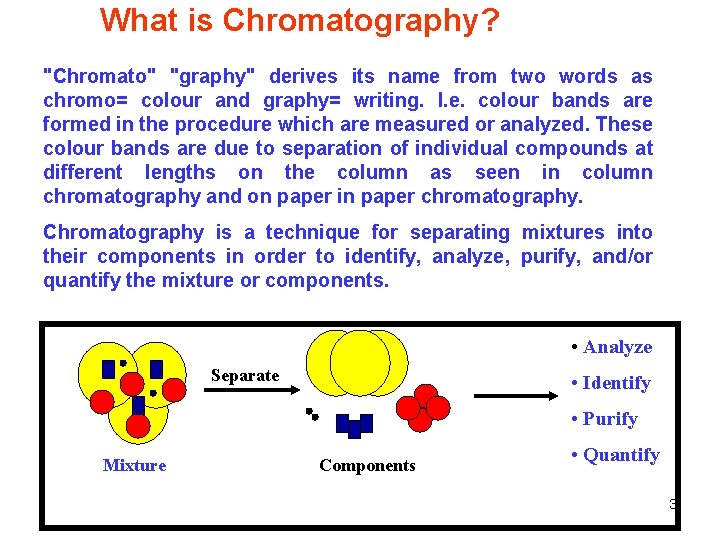
What is Chromatography? "Chromato" "graphy" derives its name from two words as chromo= colour and graphy= writing. I. e. colour bands are formed in the procedure which are measured or analyzed. These colour bands are due to separation of individual compounds at different lengths on the column as seen in column chromatography and on paper in paper chromatography. Chromatography is a technique for separating mixtures into their components in order to identify, analyze, purify, and/or quantify the mixture or components. • Analyze Separate • Identify • Purify Mixture Components • Quantify 3

Uses for Chromatography is used by scientists to: • Analyze – examine a mixture, its components, their relations to one another and • Identify – determine the identity of a mixture or components based on known components • Purify – separate components in order to isolate one of interest for further study • Quantify – determine the amount of the a mixture and/or the components present in the sample 4

Uses for Chromatography Real-life examples of uses for chromatography: • Pharmaceutical Company – determine amount of each chemical found in new product • Hospital – detect blood or alcohol levels in a patient’s blood stream • Environmental Agency – determine the level of pollutants in the water supply • Manufacturing Plant – to purify a chemical needed to make a product 5

Definition of Chromatography Detailed Definition: Chromatography is a laboratory technique that separates components within a mixture by using the differential affinities of the components for a mobile medium and for a stationary adsorbing medium through which they pass. Terminology: • Differential – showing a difference, distinctive • Affinity – natural attraction or force between things • Mobile Medium – gas or liquid that carries the components (mobile phase) • Stationary Medium – the part of the apparatus that does not move with the sample (stationary phase). 6

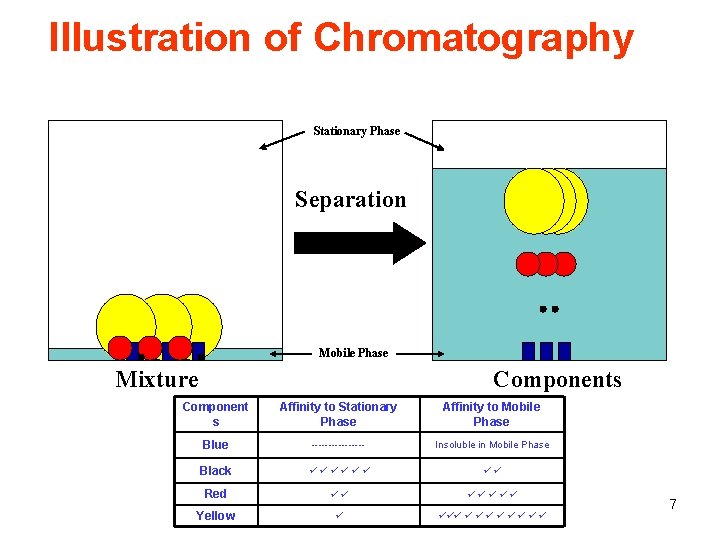
Illustration of Chromatography Stationary Phase Separation Mobile Phase Mixture Components Component s Affinity to Stationary Phase Affinity to Mobile Phase Blue -------- Insoluble in Mobile Phase Black Red Yellow 7

8

Types of Chromatography 1. ) The primary division of chromatographic techniques is based on the type of mobile phase used in the system: Type of Chromatography Gas chromatography (GC) Liquid chromatograph (LC) Type of Mobile Phase gas liquid 2. ) Further divisions can be made based on the type of stationary phase used in the system: 9

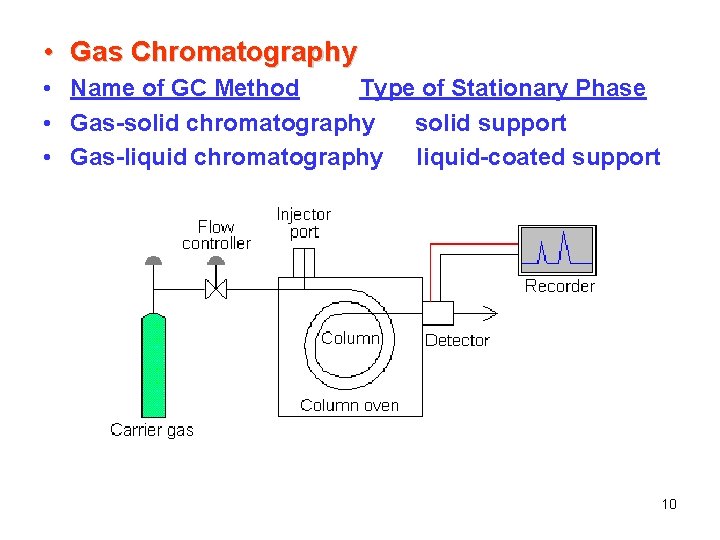
• Gas Chromatography • Name of GC Method Type of Stationary Phase • Gas-solid chromatography solid support • Gas-liquid chromatography liquid-coated support 10

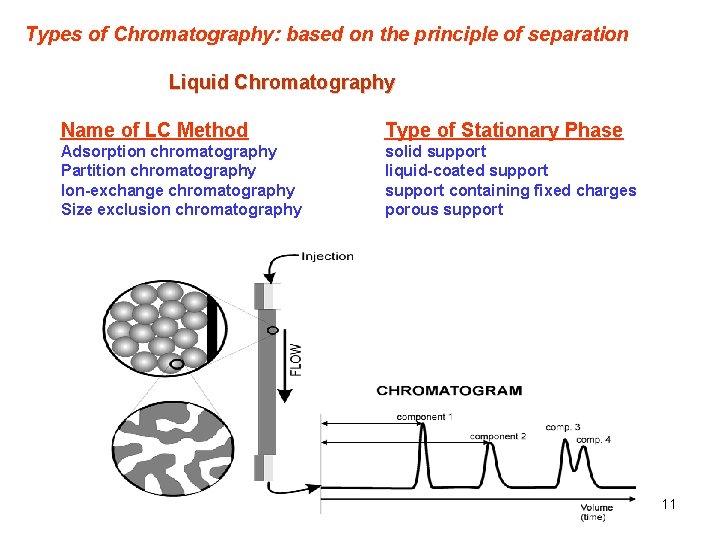
Types of Chromatography: based on the principle of separation Liquid Chromatography Name of LC Method Type of Stationary Phase Adsorption chromatography Partition chromatography Ion-exchange chromatography Size exclusion chromatography solid support liquid-coated support containing fixed charges porous support 11

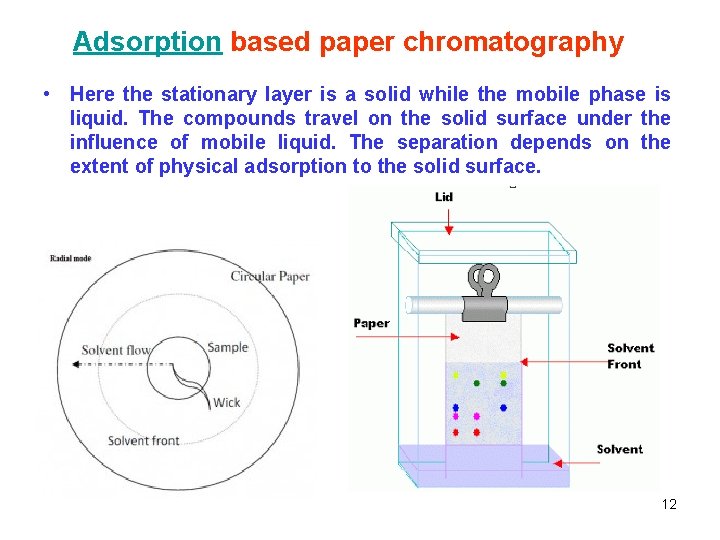
Adsorption based paper chromatography • Here the stationary layer is a solid while the mobile phase is liquid. The compounds travel on the solid surface under the influence of mobile liquid. The separation depends on the extent of physical adsorption to the solid surface. 12

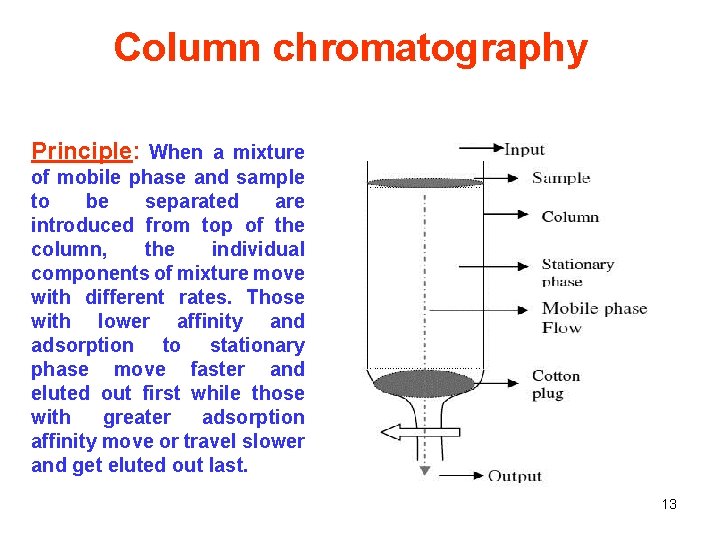
Column chromatography Principle: When a mixture of mobile phase and sample to be separated are introduced from top of the column, the individual components of mixture move with different rates. Those with lower affinity and adsorption to stationary phase move faster and eluted out first while those with greater adsorption affinity move or travel slower and get eluted out last. 13

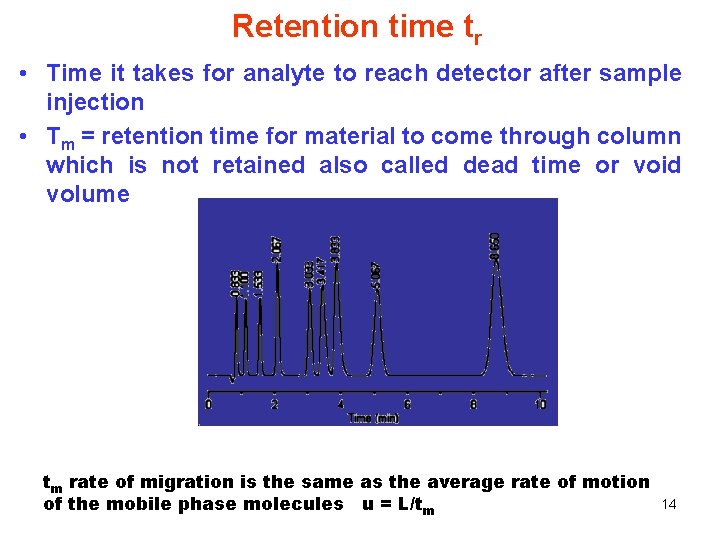
Retention time tr • Time it takes for analyte to reach detector after sample injection • Tm = retention time for material to come through column which is not retained also called dead time or void volume tm rate of migration is the same as the average rate of motion of the mobile phase molecules u = L/tm 14

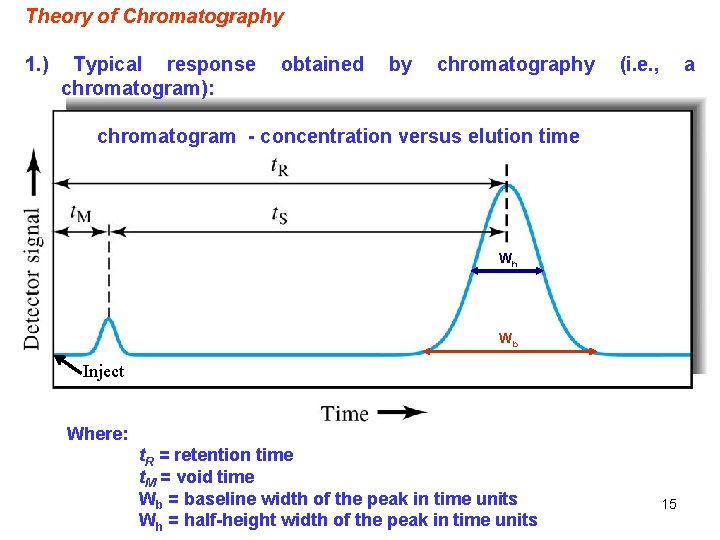
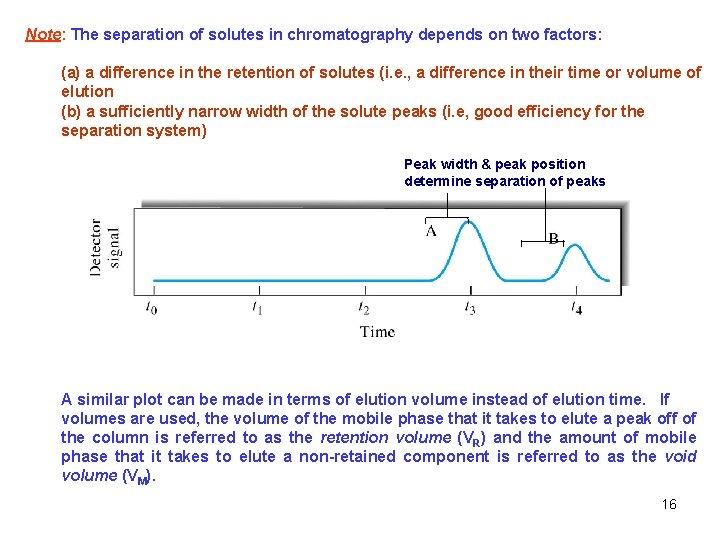
Theory of Chromatography 1. ) Typical response chromatogram): obtained by chromatography (i. e. , a chromatogram - concentration versus elution time Wh Wb Inject Where: t. R = retention time t. M = void time Wb = baseline width of the peak in time units Wh = half-height width of the peak in time units 15

Note: The separation of solutes in chromatography depends on two factors: (a) a difference in the retention of solutes (i. e. , a difference in their time or volume of elution (b) a sufficiently narrow width of the solute peaks (i. e, good efficiency for the separation system) Peak width & peak position determine separation of peaks A similar plot can be made in terms of elution volume instead of elution time. If volumes are used, the volume of the mobile phase that it takes to elute a peak off of the column is referred to as the retention volume (VR) and the amount of mobile phase that it takes to elute a non-retained component is referred to as the void volume (VM). 16

Types of Chromatography • Liquid Chromatography – separates liquid samples with a liquid solvent (mobile phase) and a column solid beads (stationary phase) composed of • Gas Chromatography – separates vaporized samples with a carrier gas (mobile phase) and a column composed of a liquid or of solid beads (stationary phase) • Paper Chromatography – separates dried liquid samples with a liquid solvent (mobile phase) and a strip (stationary phase) paper • Thin-Layer Chromatography – separates dried liquid samples with a liquid solvent (mobile phase) and a glass plate covered with a thin layer of alumina or silica gel (stationary phase) 17

Principles of Paper Chromatography • Capillary Action – the movement of liquid within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. The liquid is able to move up the filter paper because its attraction to itself is stronger than the force of gravity. • Solubility – the degree to which a material (solute) dissolves into a solvent. Solutes dissolve into solvents that have similar properties. (Like dissolves like) This allows different solutes to be separated by different combinations of solvents. Separation of components depends on both their solubility in the mobile phase and their differential affinity to the mobile phase and the stationary phase. 18

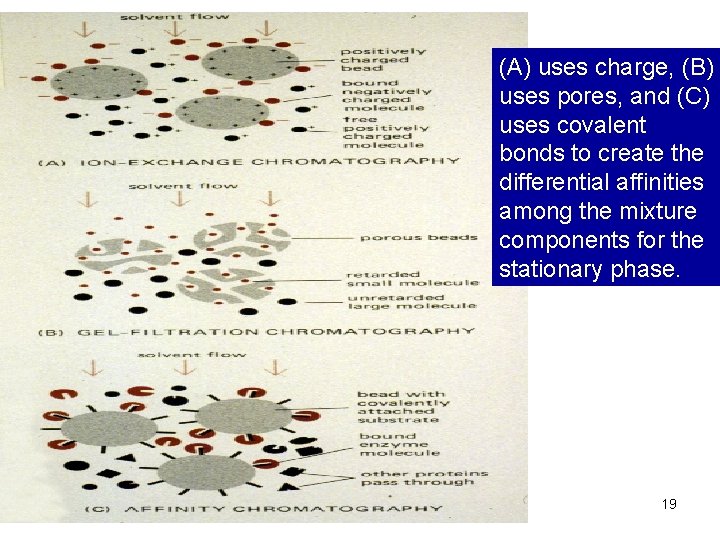
(A) uses charge, (B) uses pores, and (C) uses covalent bonds to create the differential affinities among the mixture components for the stationary phase. 19

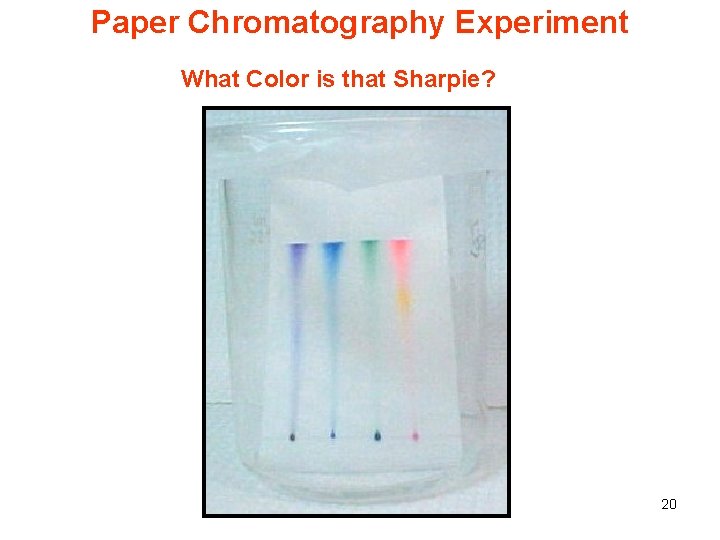
Paper Chromatography Experiment What Color is that Sharpie? 20

Overview of the Experiment Purpose: To introduce students to the principles and terminology of chromatography and demonstrate separation of the dyes in Sharpie Pens with paper chromatography. Time Required: Prep. time: 10 minutes Experiment time: 45 minutes Costs: Less than $10 21

Preparing the Chromatography Strips • Cut 6 strips of filter paper • Draw a line 1 cm above the bottom edge of the strip with the pencil • Label each strip with its corresponding solution • Place a spot from each pen on your starting line 22

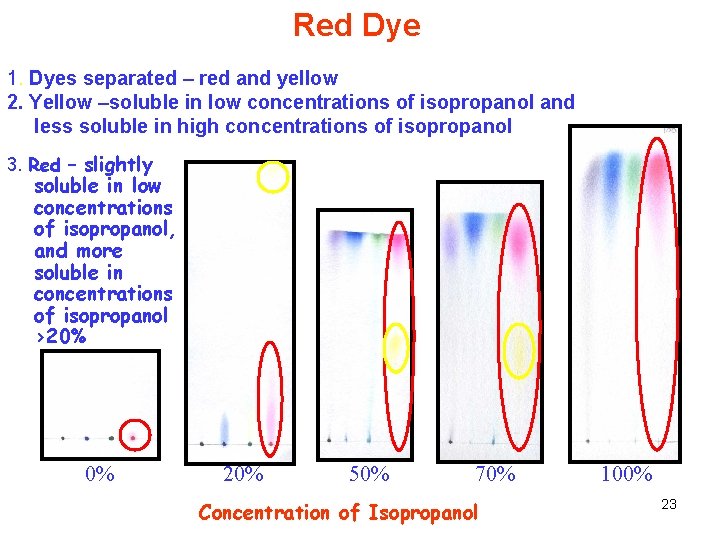
Red Dye 1. Dyes separated – red and yellow 2. Yellow –soluble in low concentrations of isopropanol and less soluble in high concentrations of isopropanol 3. Red – slightly soluble in low concentrations of isopropanol, and more soluble in concentrations of isopropanol >20% 0% 20% 50% 70% Concentration of Isopropanol 100% 23

Alternative Experiments 24

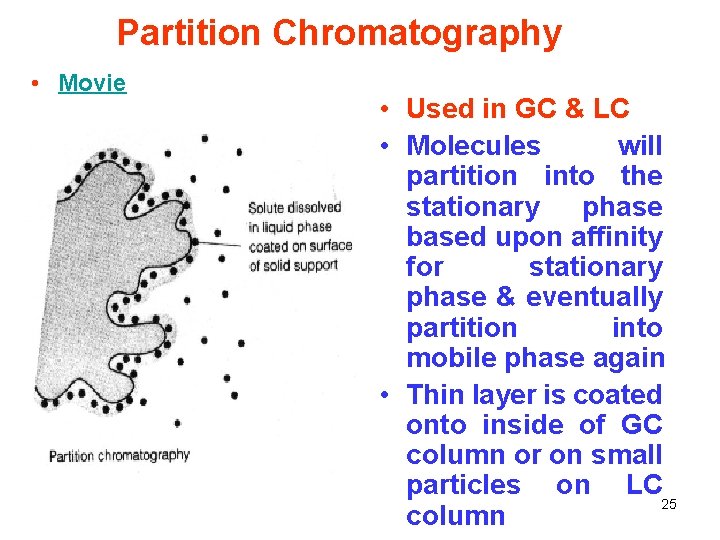
Partition Chromatography • Movie • Used in GC & LC • Molecules will partition into the stationary phase based upon affinity for stationary phase & eventually partition into mobile phase again • Thin layer is coated onto inside of GC column or on small particles on LC 25 column

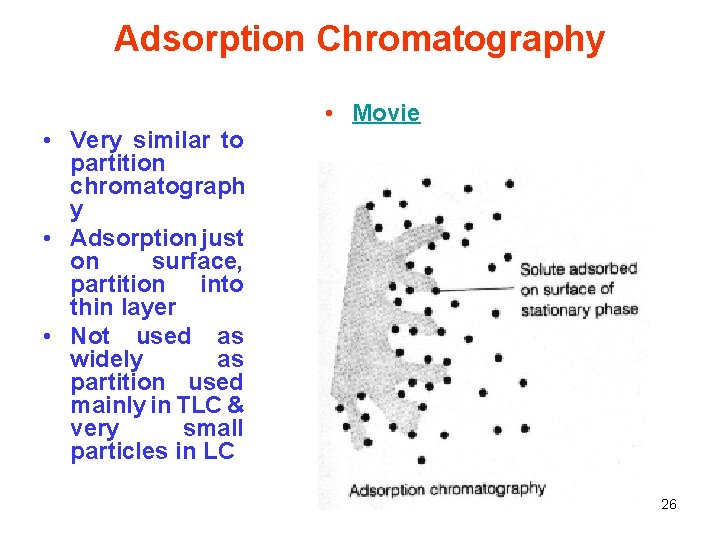
Adsorption Chromatography • Movie • Very similar to partition chromatograph y • Adsorption just on surface, partition into thin layer • Not used as widely as partition used mainly in TLC & very small particles in LC 26

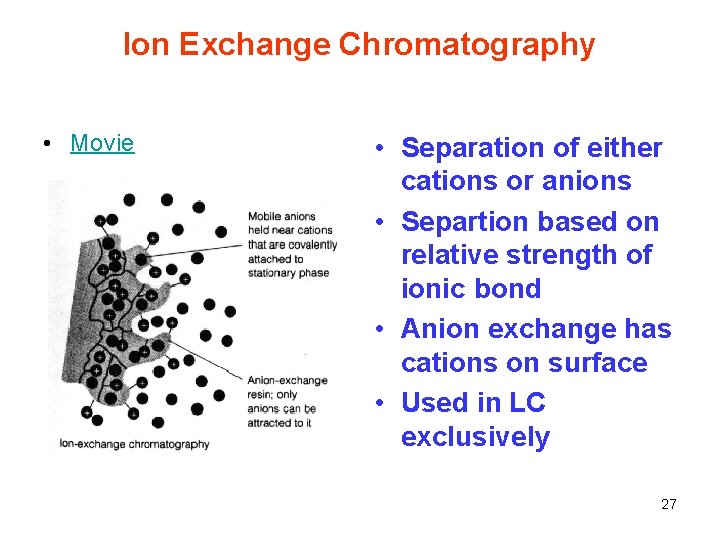
Ion Exchange Chromatography • Movie • Separation of either cations or anions • Separtion based on relative strength of ionic bond • Anion exchange has cations on surface • Used in LC exclusively 27

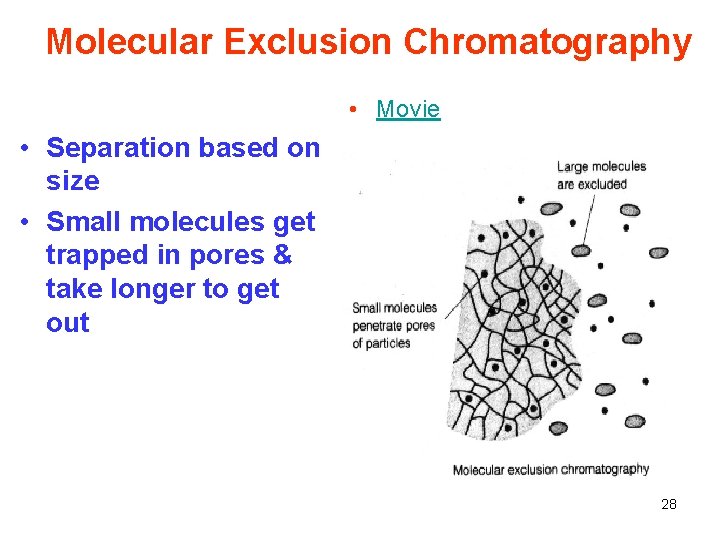
Molecular Exclusion Chromatography • Movie • Separation based on size • Small molecules get trapped in pores & take longer to get out 28

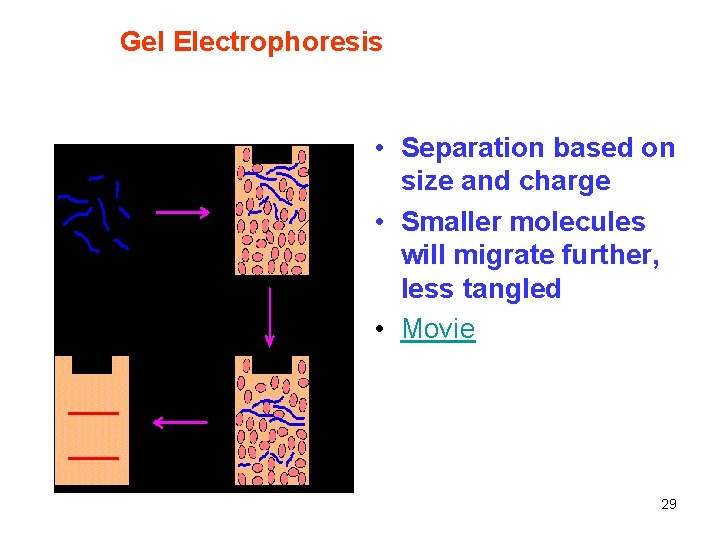
Gel Electrophoresis • Separation based on size and charge • Smaller molecules will migrate further, less tangled • Movie 29

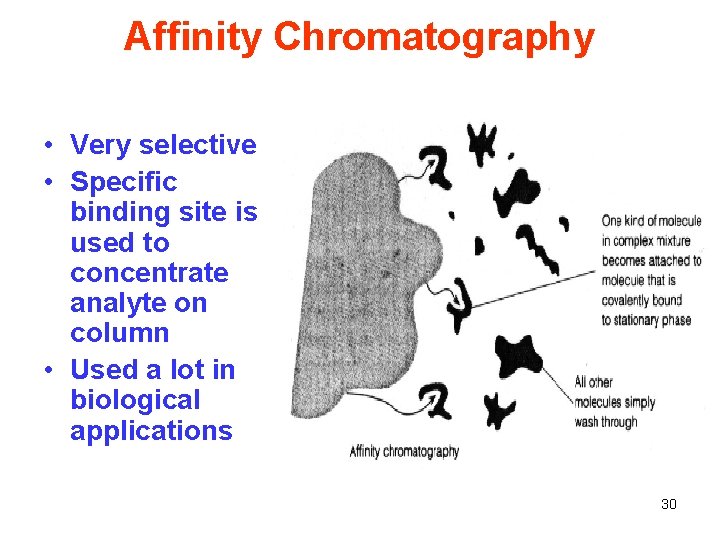
Affinity Chromatography • Very selective • Specific binding site is used to concentrate analyte on column • Used a lot in biological applications 30

THE END 31