PAPER CHROMATOGRAPHY CHROMATOGRAPHY Chromatography is a family of



















- Slides: 31

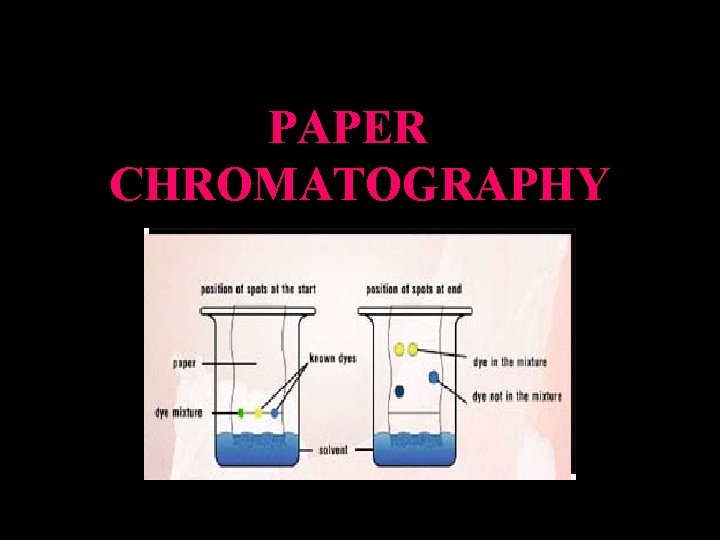
PAPER CHROMATOGRAPHY

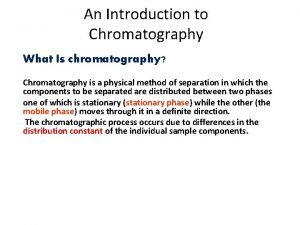
CHROMATOGRAPHY • Chromatography is a family of analytical chemistry techniques for the separation of mixtures. • It was the Russian botanist Mikhail Tsvet (Mikhail Semyonovich Tsvet) who invented the first chromatography technique in 1901.

CHROMATOGRAPHY • The separation of molecules depends on differences of 1 - size 2 - shape 3 - mass 4 - charges 5 - solubility 6 - adsorption.

■ Types of Chromatography: 1. Adsorption chromatography. 2. Partition chromatography e. g. paper chromatography 3. Gel-filtration chromatography.

■ Uses of Chromatography: * Government laboratories used to check • for approved dyes in food • that vegetables contained tiny amounts of pesticides and herbicides

■All types of chromatography involving interaction between: 1. The mixture to be separated. 2. The stationary phase. 3. The mobile phase.


Principle of Paper Chromatography: • Method of separating and identifying both colored and colorless mixtures. • Mixtures can be solids, liquids or gases but their components must be able to dissolve in the same solvent to different extents. * Generally involves 2 phases: 1. stationary phase solid support e. g. water on paper 2. mobile phase solvent or a gas

Principle of Paper Chromatography: • Test mixture is applied onto the chromatography paper and a solvent is then allowed to pass over the paper. As the solvent does so, the components of the mixture travel along with it. • The stationary phase retards the passage of the components of the sample. When components pass through the system at different rates they become separated in time. • The solvent used depends on the substance to be separated

The components will travel at different rates over paper depending on: 1. their solubility in the solvent 2. how well the dyes adsorb on the chromatography paper Generally, the more soluble the component is in the solvent and the less it adsorb onto the chromatography paper, the faster it would move with the solvent on the paper and hence the spot appears further up the paper

• Result of chromatography is known as the Chromatogram.

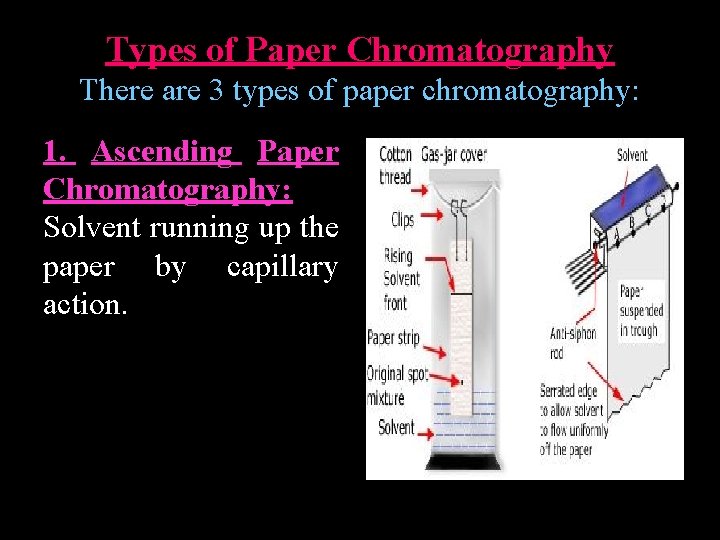
Types of Paper Chromatography There are 3 types of paper chromatography: 1. Ascending Paper Chromatography: Solvent running up the paper by capillary action.

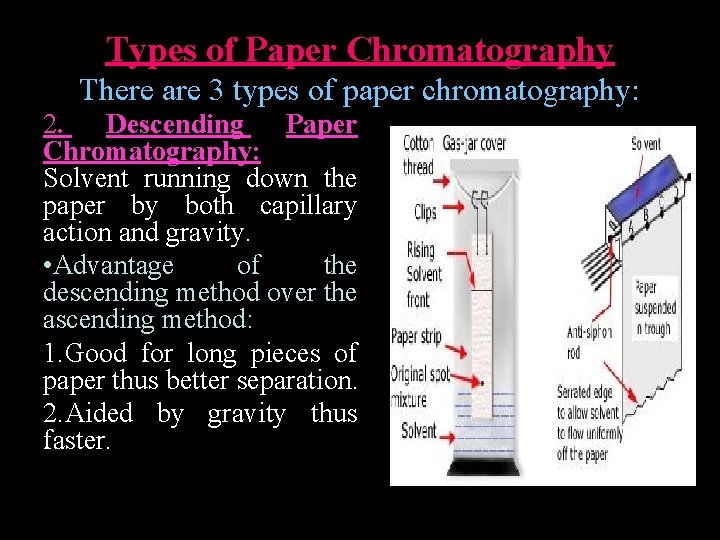
Types of Paper Chromatography There are 3 types of paper chromatography: 2. Descending Paper Chromatography: Solvent running down the paper by both capillary action and gravity. • Advantage of the descending method over the ascending method: 1. Good for long pieces of paper thus better separation. 2. Aided by gravity thus faster.

■ Stationary Phase • In paper chromatography, cellulose in the form of paper sheets makes an identical support medium. WHY? Because it has the ability to adsorb water molecules between cellulose fibers and forms a stationary hydrophilic phase. • Paper: Watman No. 1 of high quality is the paper most frequently used for analytical purposes.

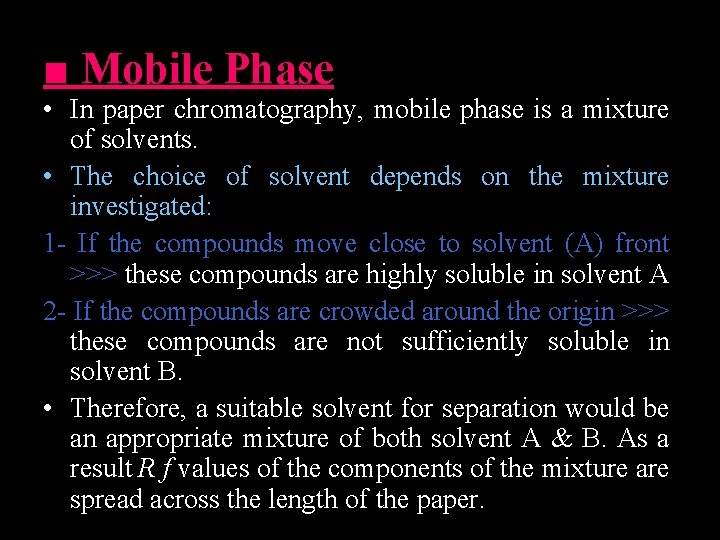
■ Mobile Phase • In paper chromatography, mobile phase is a mixture of solvents. • The choice of solvent depends on the mixture investigated: 1 - If the compounds move close to solvent (A) front >>> these compounds are highly soluble in solvent A 2 - If the compounds are crowded around the origin >>> these compounds are not sufficiently soluble in solvent B. • Therefore, a suitable solvent for separation would be an appropriate mixture of both solvent A & B. As a result R f values of the components of the mixture are spread across the length of the paper.



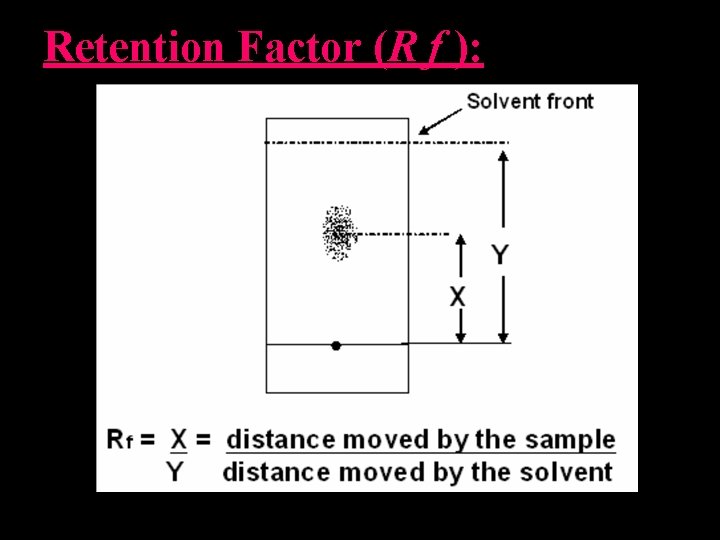
Retention Factor (R f ): • The retention is measured as the retention factor Rf, the run length of the compound divided by the run length of the solvent front: • Unknown substances could be identified by the Rf values Rf = distance moved by the substance distance moved by the solvent

Retention Factor(R f ): • The Rf of a compound often differs considerably between experiments and laboratories due to variations of - the solvent, - the stationary phase, - temperature, and - the setup. • It is therefore important to compare the retention of the test compound to that of one or more standard compounds under absolutely identical conditions.

Retention Factor (R f ):

Detection of Spots: • After development, the spots corresponding to different compounds may be located by their color • However, most compounds are colorless and are visualized by: 1 - Spraying the paper with specific reagents. 2 - Dipping the paper in a solution of the reagent in a volatile solvent. 3 - Fluorescent substances can be visualized by ultraviolet (UV) light.

SEPARATION OF AMINO ACIDS BY PAPER CHROMATOGRAPHY

SEPARATION OF AMINO ACIDS BY PAPER CHROMATOGRAPHY • Separation and identification of amino acids are operations that must be performed frequently by biochemists. • The 20 amino acids present in proteins have similar structures. However, each amino acid is unique in polarity and ionic characteristics. • In this experiment, we will use paper chromatography to separate and identify the components of an unknown amino acid mixture.

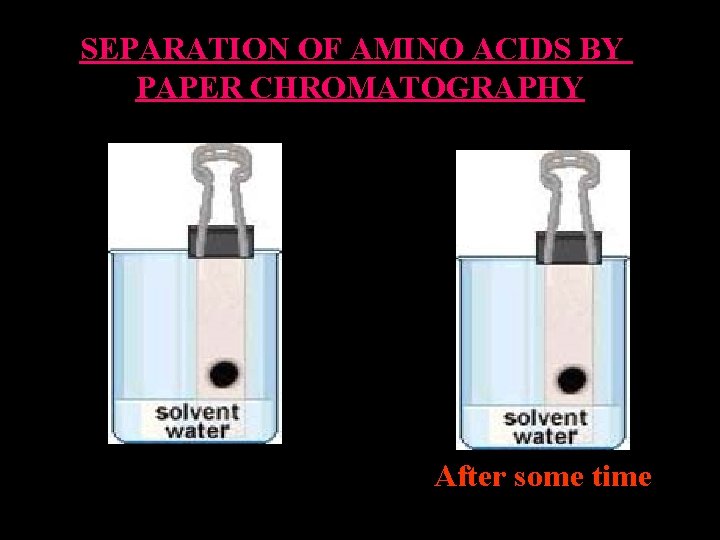
SEPARATION OF AMINO ACIDS BY PAPER CHROMATOGRAPHY • The solvent mixture contains several components, - one of which is usually water and - another of which is a more non-polar solvent. • As the solvent mixture moves up the paper by capillary action, the water in the mixture binds to the hydrophilic paper (cellulose) and creates a liquid stationary phase of many small water droplets. • The non-polar solvent continues to move up the paper forming a liquid mobile phase.

SEPARATION OF AMINO ACIDS BY PAPER CHROMATOGRAPHY • Since amino acids have different R-groups, they also have different degrees of solubility in water vs. the non-polar solvent.

SEPARATION OF AMINO ACIDS BY PAPER CHROMATOGRAPHY • An amino acid with a polar R-group will be more soluble in water than in the non-polar solvent, so it will dissolve more in the stationary water phase and will move up the paper only slightly. • An amino acid with a hydrophobic R-group will be more soluble in the mobile non-polar solvent than in water, so it will continue to move up the paper. • Different amino acids will move different distances up the paper depending upon their relative solubilities in the two solvents, allowing for separation of amino acid mixtures.

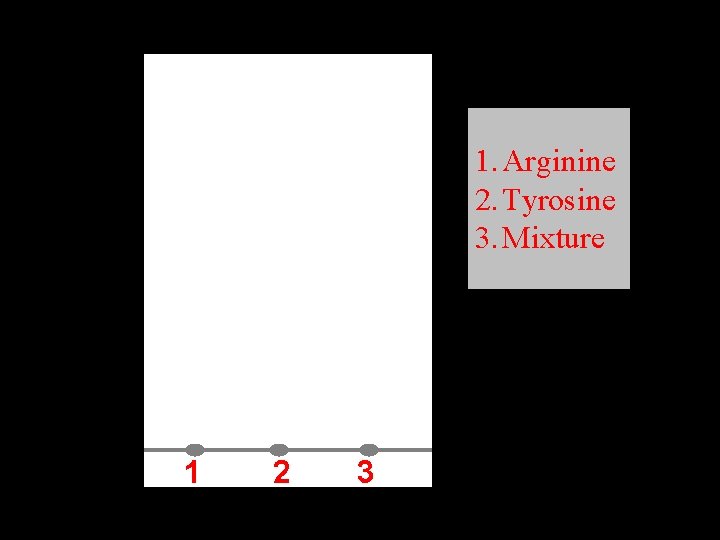
Applications • Materials: 1 - Filter paper: Watman No. 1. 2 - Solvent system: Butanol: glacial acetic acid: water. 3 - Ninhydrine reagent. 4 - Standard amino acids and mixture of unknown.

Applications • Procedure: 1 - Draw a light pencil line 1 -2 cm from the bottom of the paper. 2 - Place a single drop of compound at intervals 2 cm. 3 - Dry with hair dryer. 4 - Dip the paper in the jar with one of the edges of the paper to which the sample of the spot is adjacent into the solvent. 5 - Allow to run. 6 - Remove the paper. 7 - Determine the solvent front. 8 - Dry. 9 - Spray the paper with ninhydrin. 10 - Dry the paper.

1. Arginine 2. Tyrosine 3. Mixture 1 2 3

Applications • Procedure: 1 - Draw a light pencil line 1 -2 cm from the bottom of the paper. 2 - Place a single drop of compound at intervals 2 cm. 3 - Dry with hair dryer. 4 - Dip the paper in the jar with one of the edges of the paper to which the sample of the spot is adjacent into the solvent. 5 - Allow to run. 6 - Remove the paper. 7 - Determine the solvent front. 8 - Dry. 9 - Spray the paper with ninhydrin. 10 - Dry the paper.

SEPARATION OF AMINO ACIDS BY PAPER CHROMATOGRAPHY After some time
 Advantages of paper chromatography
Advantages of paper chromatography Chromatography
Chromatography Paper chromatography separation of cations and dyes
Paper chromatography separation of cations and dyes Principle of paper chromatography
Principle of paper chromatography Youtube
Youtube Relationships and biodiversity lab teacher guide
Relationships and biodiversity lab teacher guide Paper chromatography food coloring
Paper chromatography food coloring Paper chromatography diagram
Paper chromatography diagram Paper chromatography principle
Paper chromatography principle Chromatography
Chromatography Paper chromatography
Paper chromatography Polarity colors
Polarity colors Paper chromatography formula
Paper chromatography formula Rf value in paper chromatography
Rf value in paper chromatography Paper chromatography is a method used in regents
Paper chromatography is a method used in regents Application of paper chromatography
Application of paper chromatography Paper 2 aice general paper
Paper 2 aice general paper General paper essay questions
General paper essay questions Periodic table staircase
Periodic table staircase Binuclear family vs blended family
Binuclear family vs blended family Difference between nuclear family and joint family
Difference between nuclear family and joint family Adsorption chromatography
Adsorption chromatography Advantages of gel filtration chromatography
Advantages of gel filtration chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Mobile phase in affinity chromatography
Mobile phase in affinity chromatography Chromatography is a technique used to separate
Chromatography is a technique used to separate Chromatography definition
Chromatography definition Mobile phase vs stationary phase
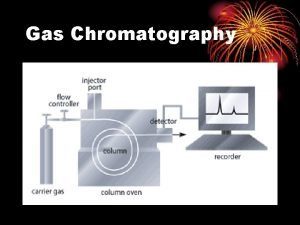
Mobile phase vs stationary phase Gas chromatography
Gas chromatography Dry column vacuum chromatography
Dry column vacuum chromatography Hplc principle
Hplc principle Principles of gc
Principles of gc