Gas Chromatography Gas Chromatography Basics Gas Liquid Chromatography



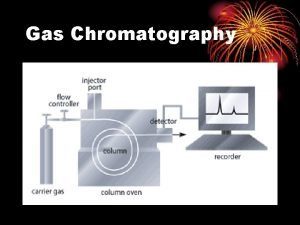
Gas Chromatography
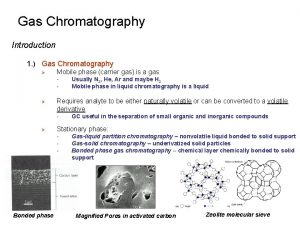
Gas Chromatography Basics Gas Liquid Chromatography (GLC) Gas Solid Chromatography (GSC) Mobile phase does not interact with analyte Separation occurs by interaction of analyte differentially w/liquid stationary phase and temperature GC preferred method, only applicable to volatile substances derivitiazation
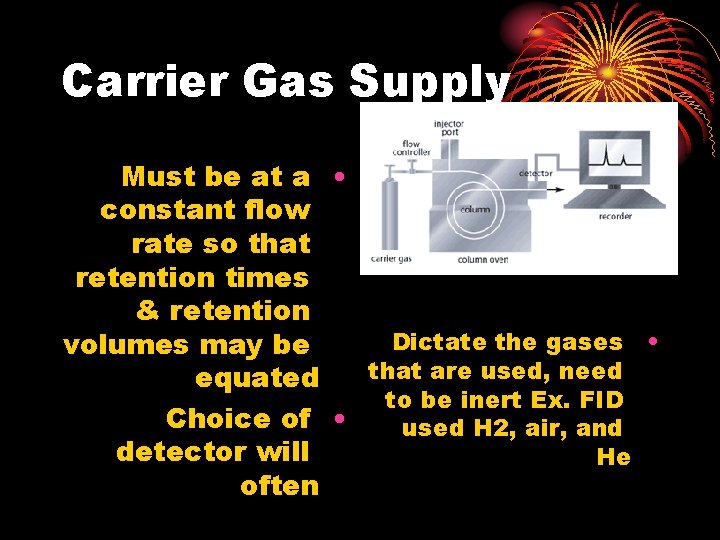
Carrier Gas Supply Must be at a • constant flow rate so that retention times & retention Dictate the gases • volumes may be that are used, need equated to be inert Ex. FID Choice of • used H 2, air, and detector will He often
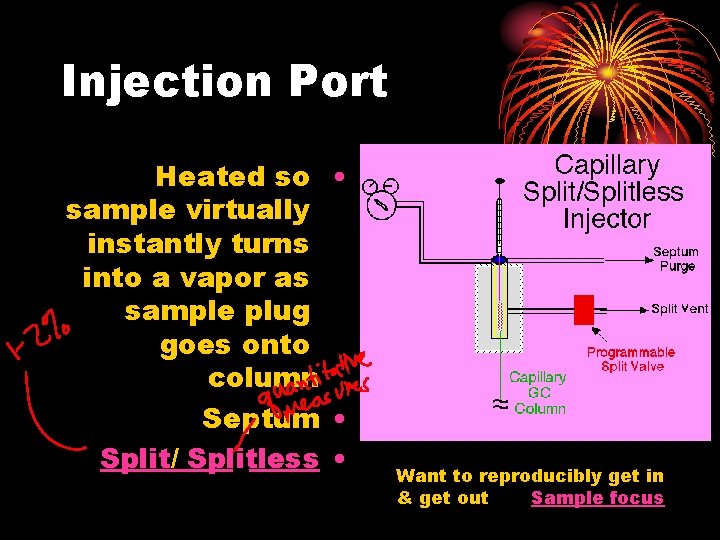
Injection Port Heated so • sample virtually instantly turns into a vapor as sample plug goes onto column Septum • Split/ Splitless • Want to reproducibly get in & get out Sample focus

Flow rate Measurement Why is a known • flow rate critical?

GC Columns Capillary: - have i. d. <1 mm - have wide unrestricted flow through center & inner Variety of • surface is functional groups coated with have been blended into polysiloxane liquid stationary chain to provide phase different polarity & selectivity

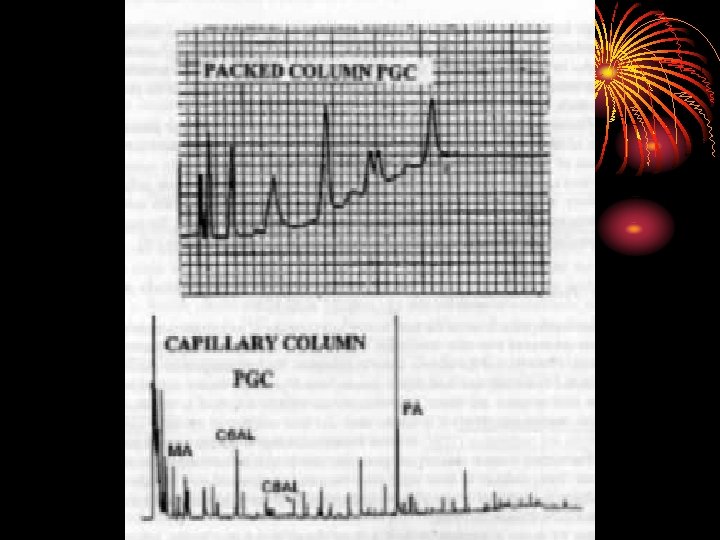
Capillary columns Advantages Disadvantages
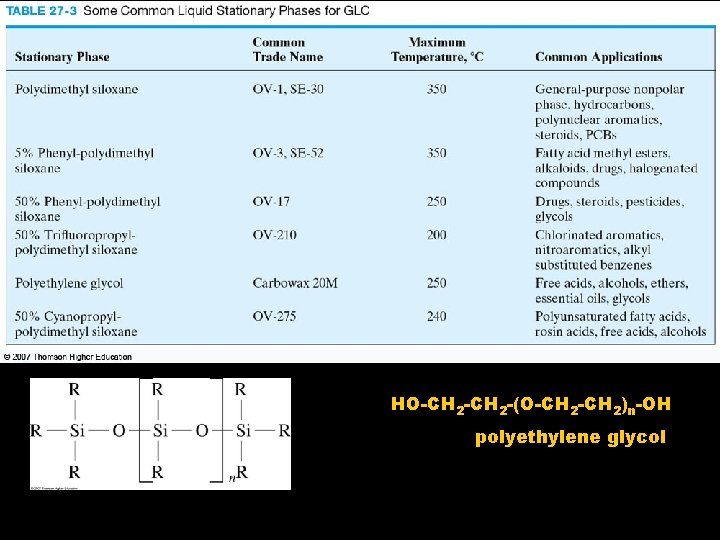
HO-CH 2 -(O-CH 2)n-OH polyethylene glycol
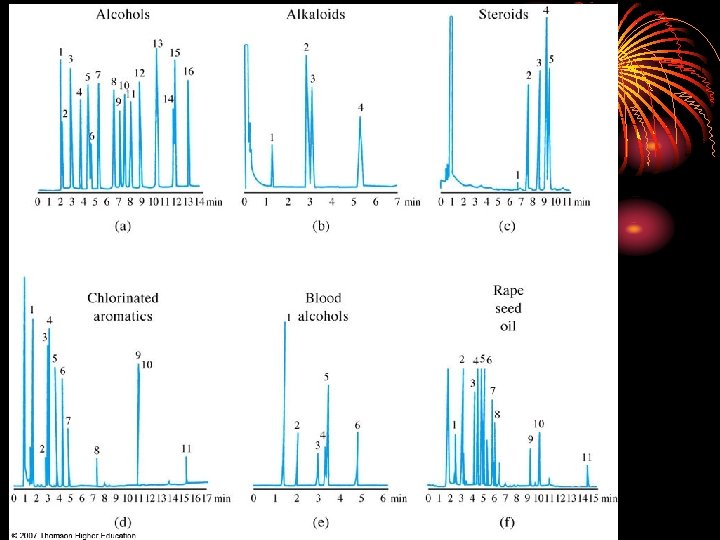
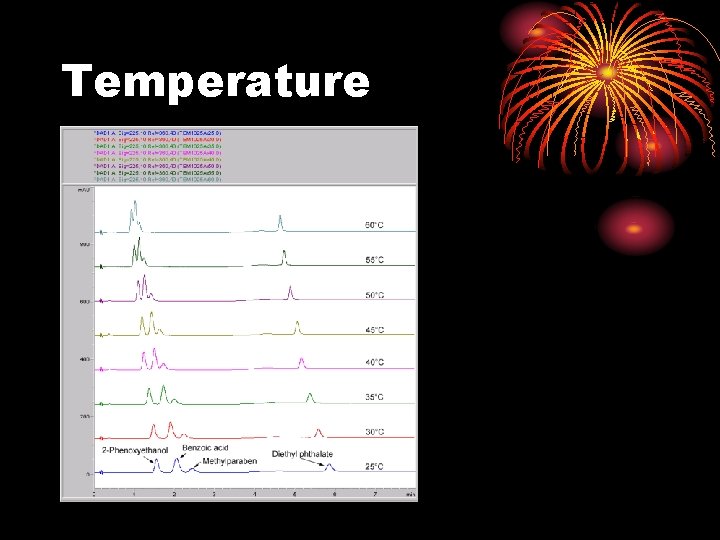
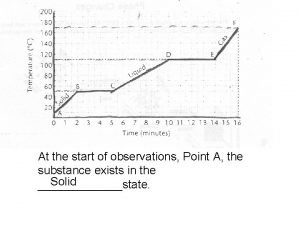
Temperature

Chromatography Catalog Exercise Determine the best type of column to achieve separation for drugs of abuse. Draw the chemical structures of the stationary phase as best you can. Indicate what chromatographic conditions you would do your separation under. Why do you think the catalog recommended the conditions they did? Compare with someone else who used a different catalog, explain any differences. • • •

Detectors in GC The ideal detector should:
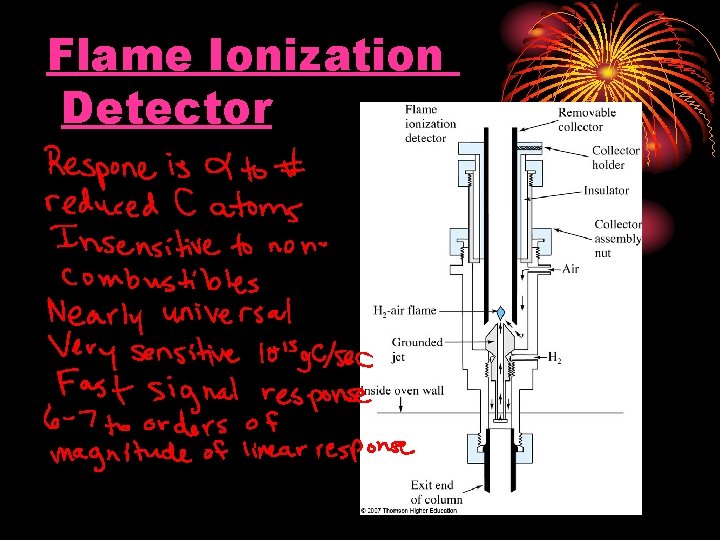
Flame Ionization Detector
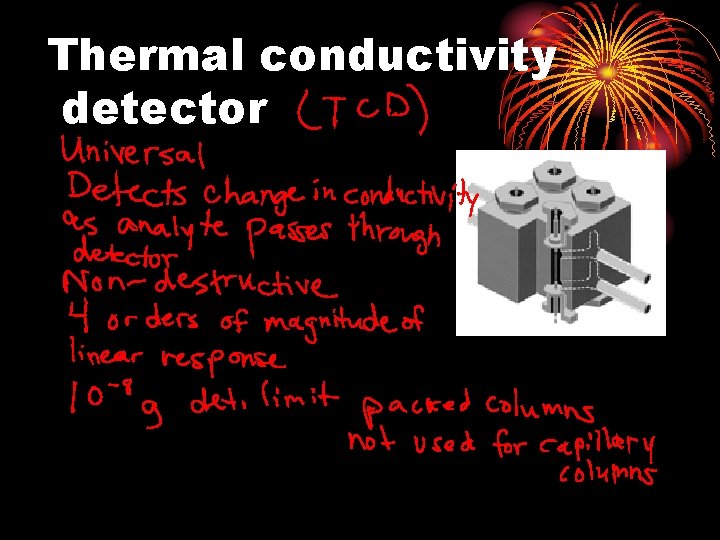
Thermal conductivity detector
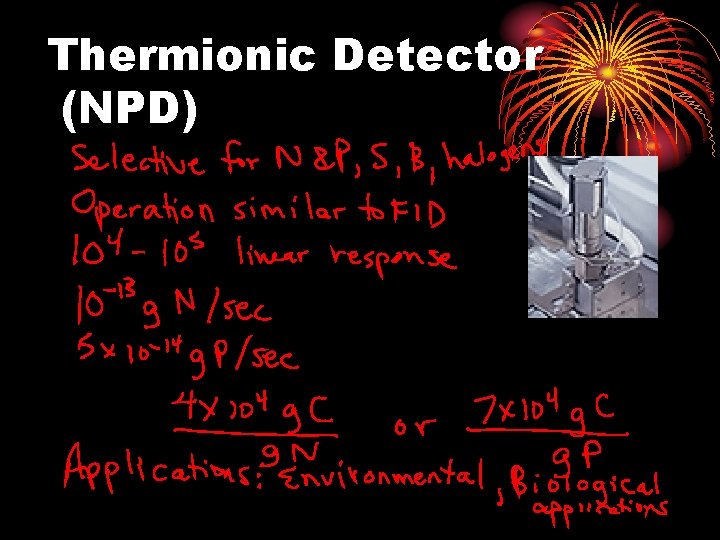
Thermionic Detector (NPD)
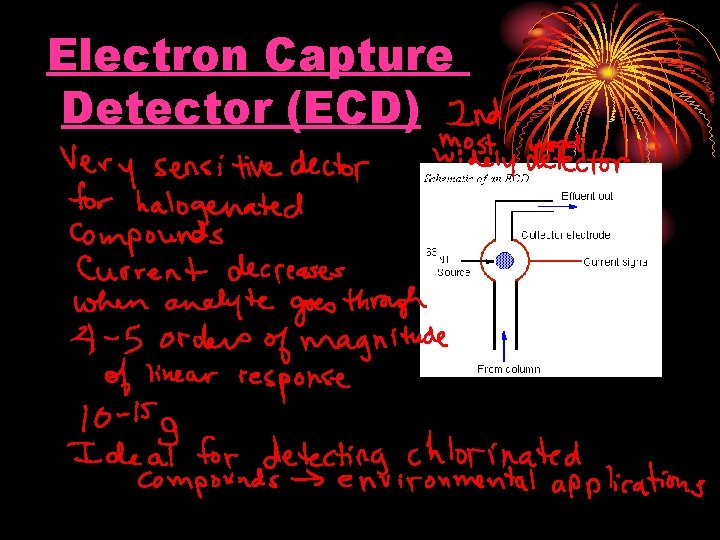
Electron Capture Detector (ECD)
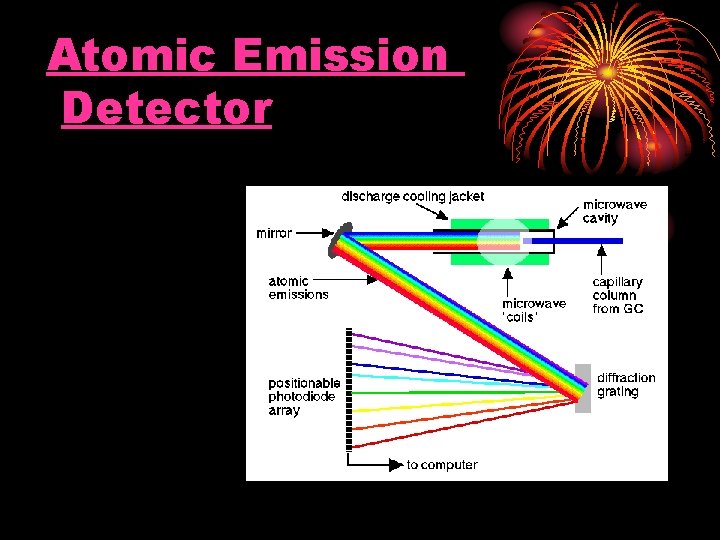
Atomic Emission Detector

Mass Spectrometer as a GC Detector
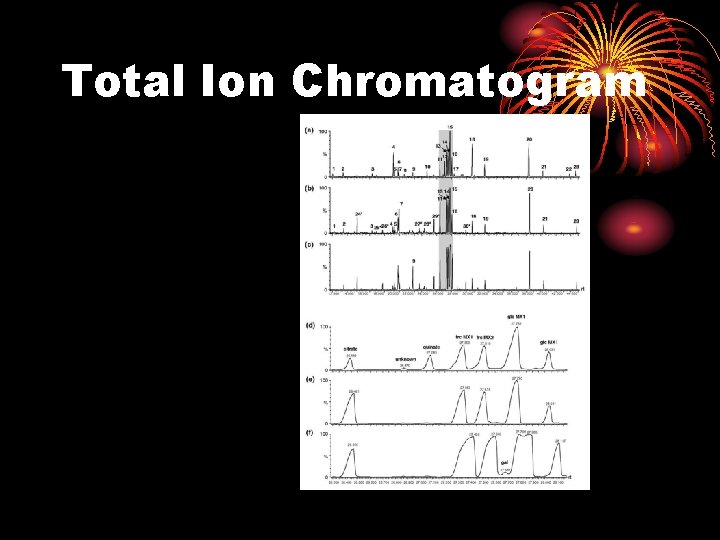
Total Ion Chromatogram
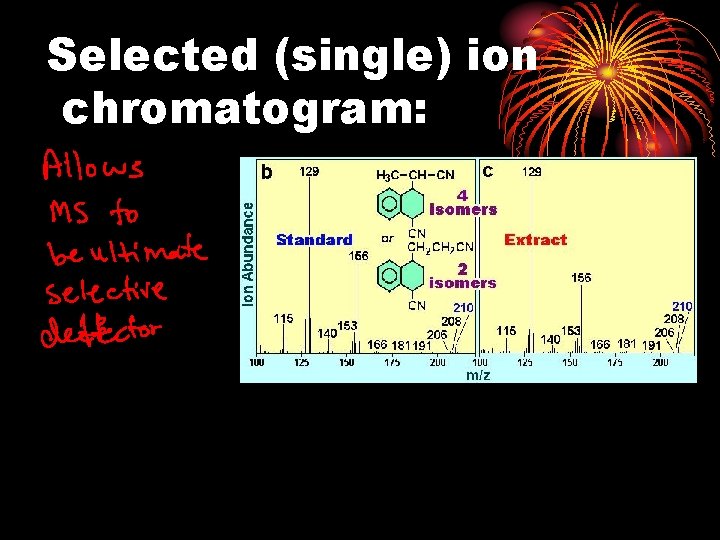
Selected (single) ion chromatogram:

Connecting the GC to the MS This is difficult! Why?

How is a GC interfaced to an MS? 1. Direct Connection
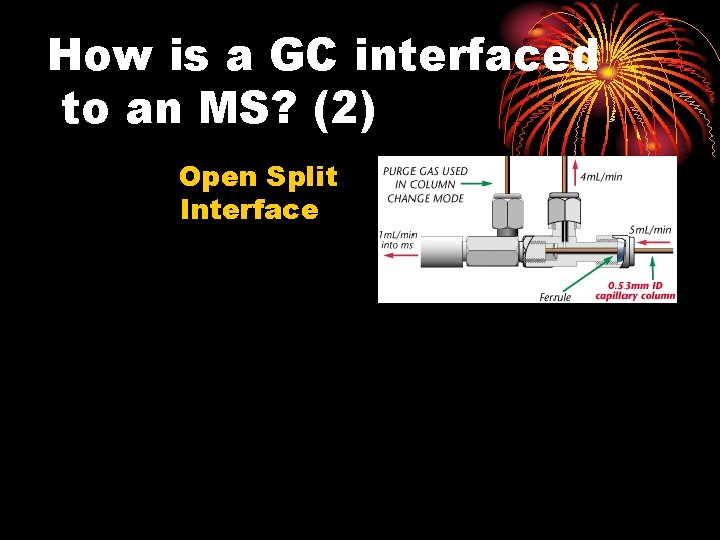
How is a GC interfaced to an MS? (2) Open Split Interface
Advantages of Open Split Design
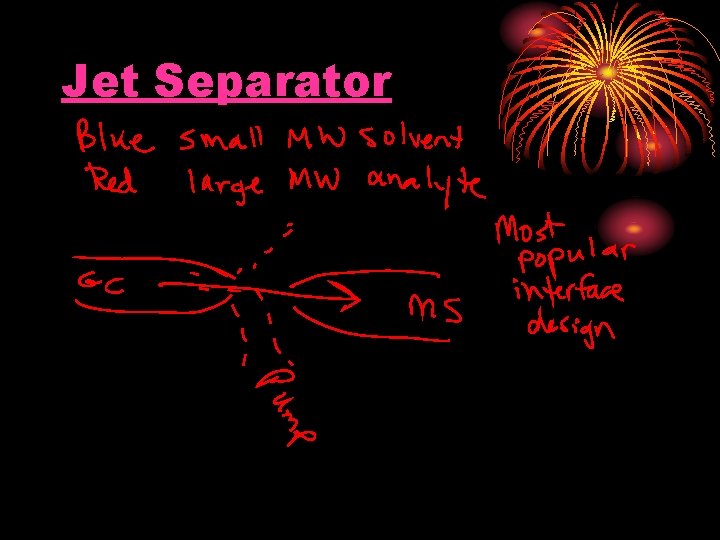
Jet Separator
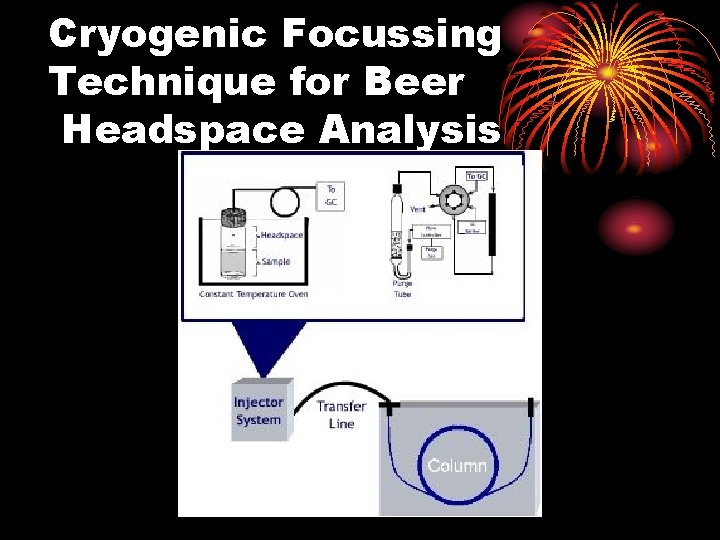
Cryogenic Focussing Technique for Beer Headspace Analysis

Kovat’s Retention Index



Gas Chromatography

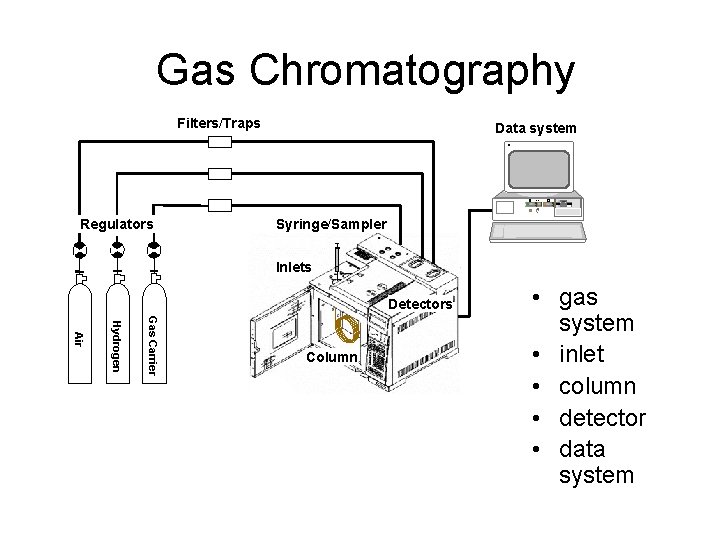
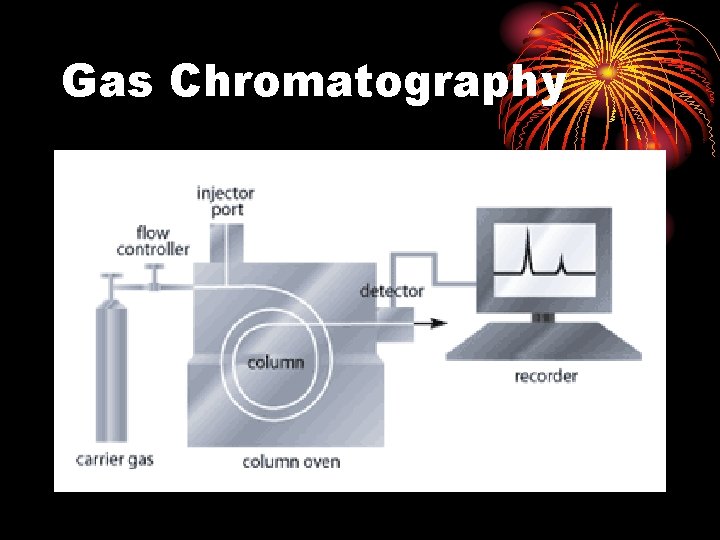
Gas Chromatography Filters/Traps Data system H RESET Regulators Syringe/Sampler Inlets Detectors Gas Carrier Hydrogen Air Column • gas system • inlet • column • detector • data system
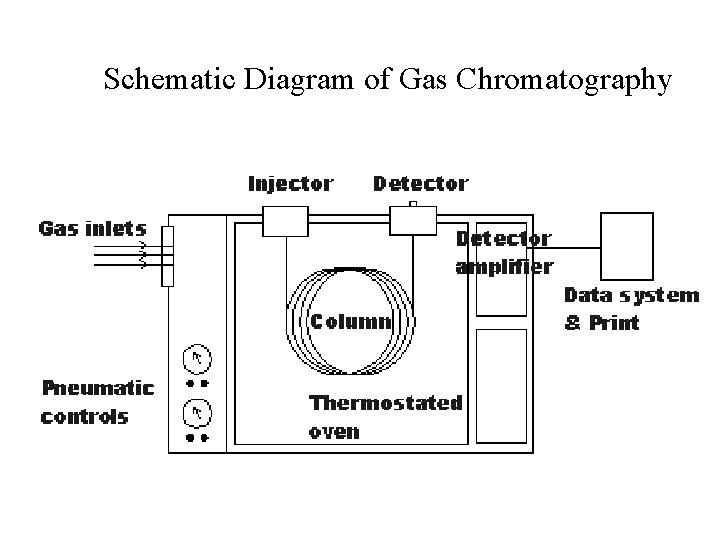
Schematic Diagram of Gas Chromatography
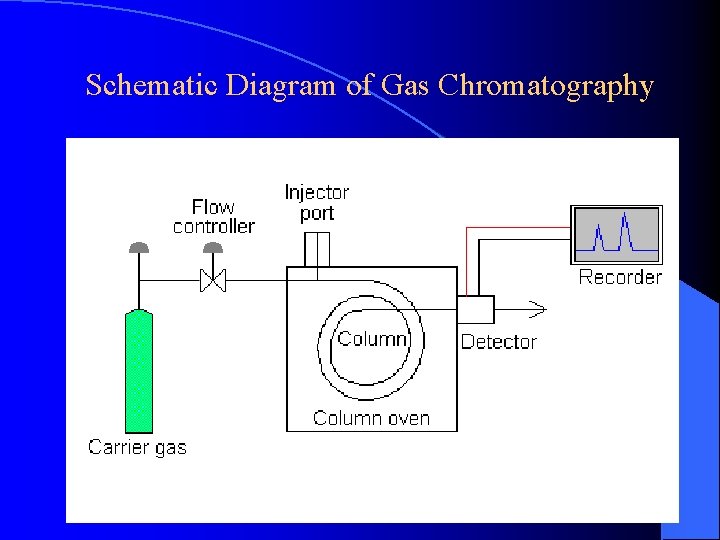
Schematic Diagram of Gas Chromatography

DETECTORS Flame Ionization Detector (Nanogram - ng) High temperature of hydrogen flame (H 2 +O 2 + N 2) ionizes compounds eluted from column into flame. The ions collected on collector or electrode and were recorded on recorder due to electric current.
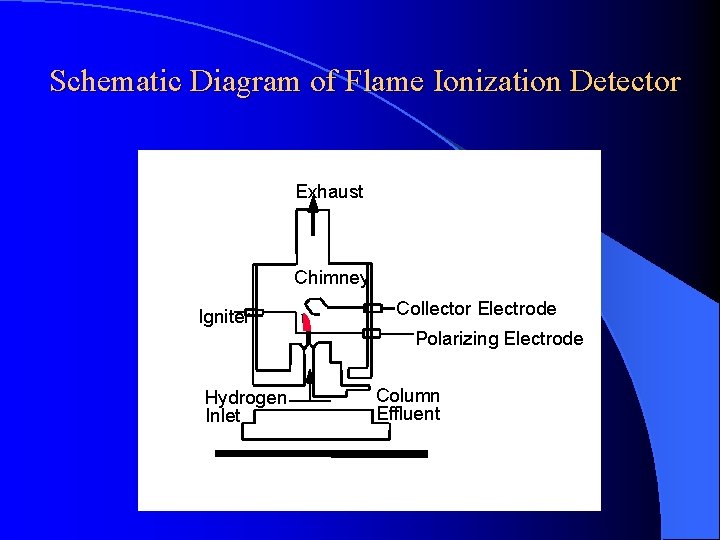
Schematic Diagram of Flame Ionization Detector Exhaust Chimney Igniter Collector Electrode Polarizing Electrode Hydrogen Inlet Column Effluent
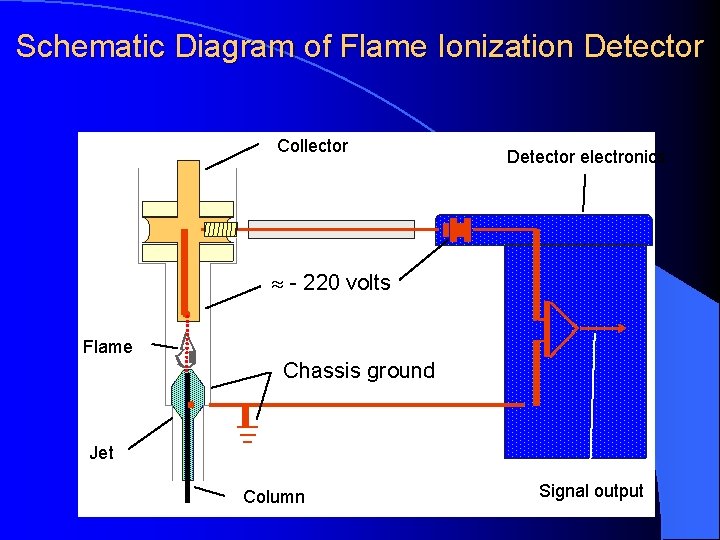
Schematic Diagram of Flame Ionization Detector Collector Detector electronics - 220 volts Flame Chassis ground Jet Column Signal output

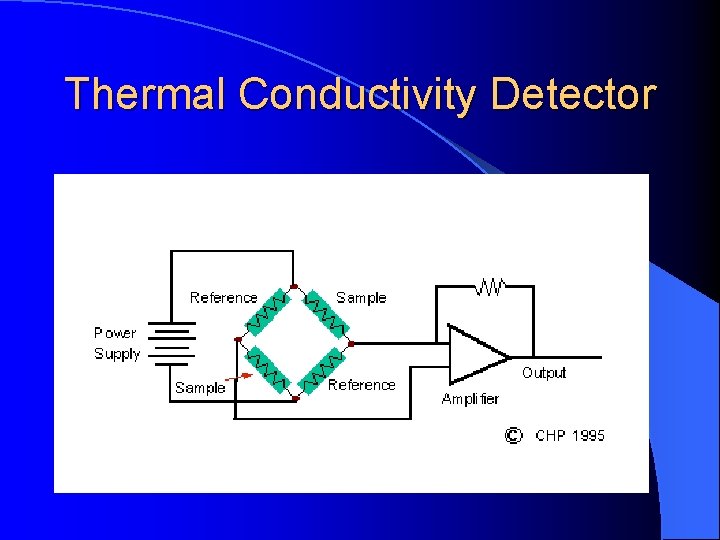
Thermal Conductivity Detector Measures the changes of thermal conductivity due to the sample (mg). Sample can be recovered.
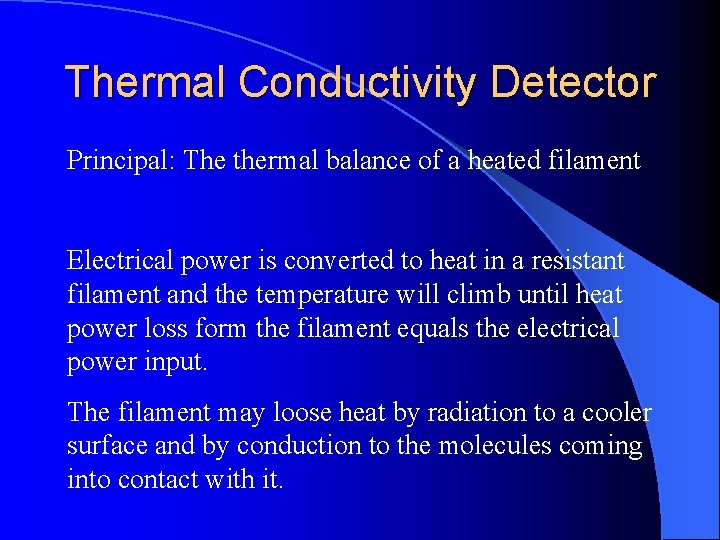
Thermal Conductivity Detector Principal: The thermal balance of a heated filament Electrical power is converted to heat in a resistant filament and the temperature will climb until heat power loss form the filament equals the electrical power input. The filament may loose heat by radiation to a cooler surface and by conduction to the molecules coming into contact with it.
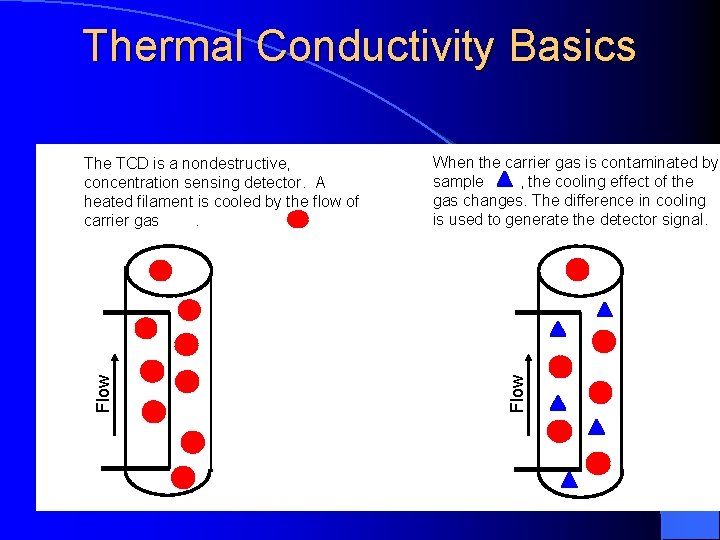
Thermal Conductivity Basics When the carrier gas is contaminated by sample , the cooling effect of the gas changes. The difference in cooling is used to generate the detector signal. Flow The TCD is a nondestructive, concentration sensing detector. A heated filament is cooled by the flow of carrier gas.
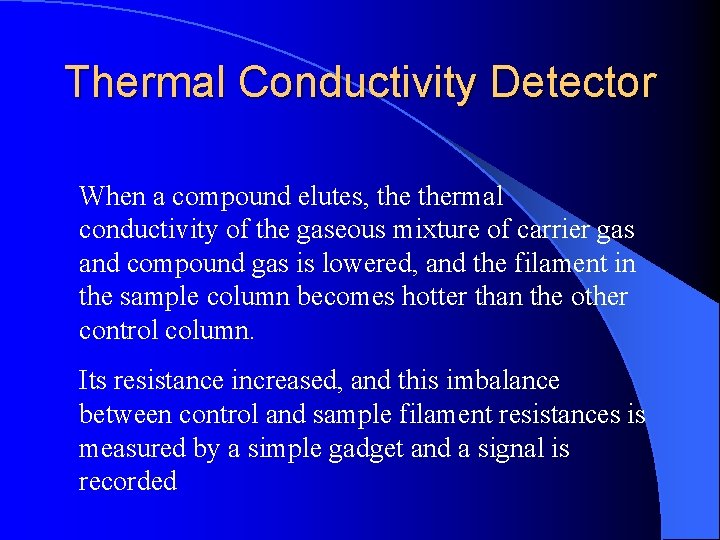
Thermal Conductivity Detector When a compound elutes, thermal conductivity of the gaseous mixture of carrier gas and compound gas is lowered, and the filament in the sample column becomes hotter than the other control column. Its resistance increased, and this imbalance between control and sample filament resistances is measured by a simple gadget and a signal is recorded
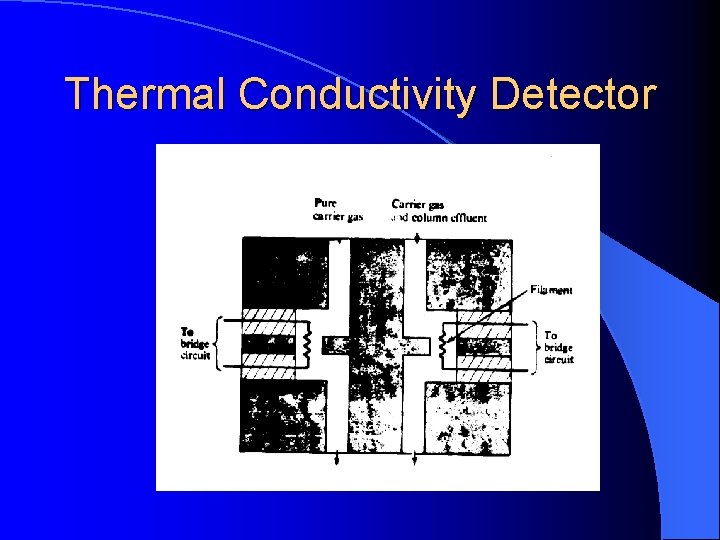
Thermal Conductivity Detector
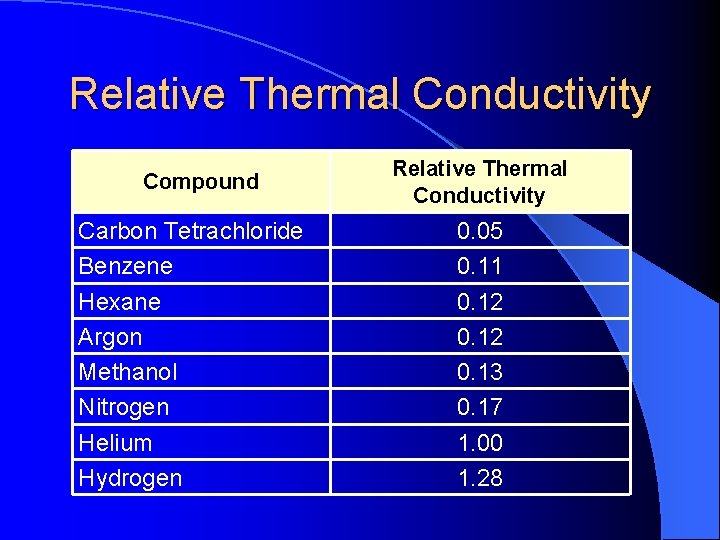
Relative Thermal Conductivity Compound Carbon Tetrachloride Benzene Hexane Argon Methanol Nitrogen Helium Hydrogen Relative Thermal Conductivity 0. 05 0. 11 0. 12 0. 13 0. 17 1. 00 1. 28
Thermal Conductivity Detector • Responds to all compounds • Adequate sensitivity for many compounds • Good linear range of signal • Simple construction • Signal quite stable provided carrier gas glow rate, block temperature, and filament power are controlled • Nondestructive detection

Electron Capture Detector For pesticide analysis (picogram). Accept electrons of carrier gas.
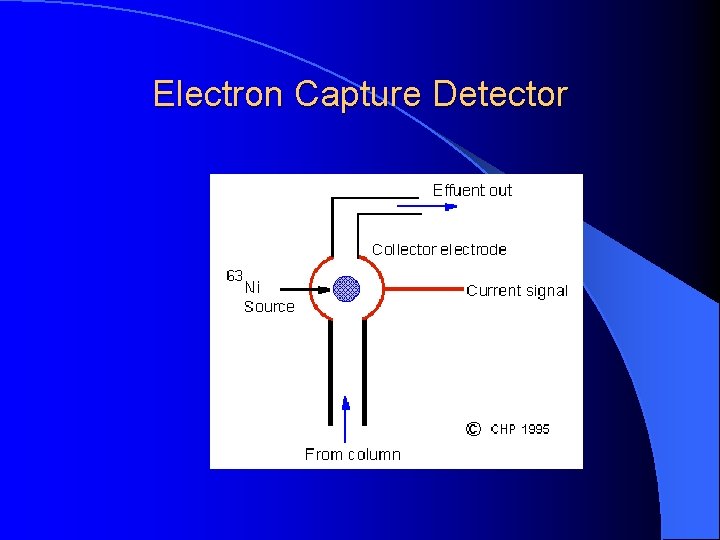
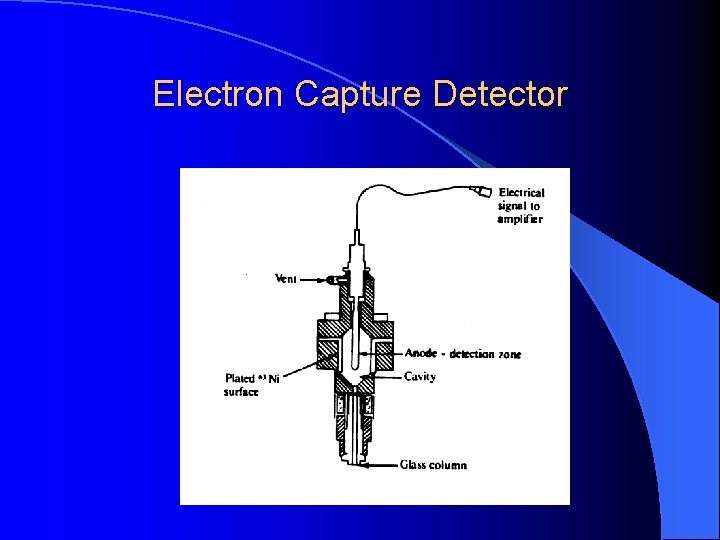
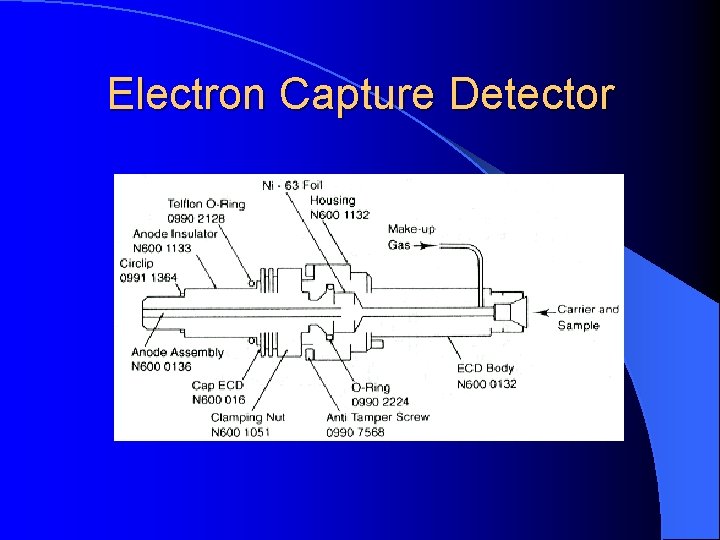
Electron Capture Detector ECD detects ions in the exiting from the gas chromatographic column by the anode electrode. 3 H or 63 Ni which emits particles. Ionization : N 2 (Nitrogen carrier gas) + (e) = N 2+ + 2 e These N 2+ establish a “base line” X (F, Cl and Br) containing sample + (e) XIon recombination : X- + N 2+ = X + N 2 The “base line” will decrease and this decrease constitutes the signal. Insecticides, pesticides, vinyl chloride, and fluorocarbons
Electron Capture Detector
Electron Capture Detector
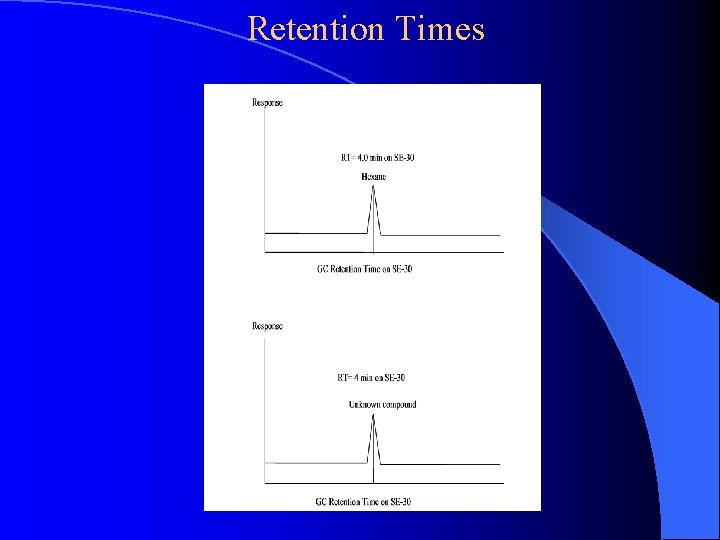
Retention Times

GLC ADVANTAGES 1. Very good separation 2. Time (analysis is short) 3. Small sample is needed - ml 4. Good detection system 5. Quantitatively analyzed

Thermal Conductivity Detector The TCD will respond to any substance different from the carrier gas as long as its concentration is sufficiently high enough.
DISADVANTAGES OF GAS CHROMATOGRAPHY Material has to be volatilized at 250 C without decomposition.
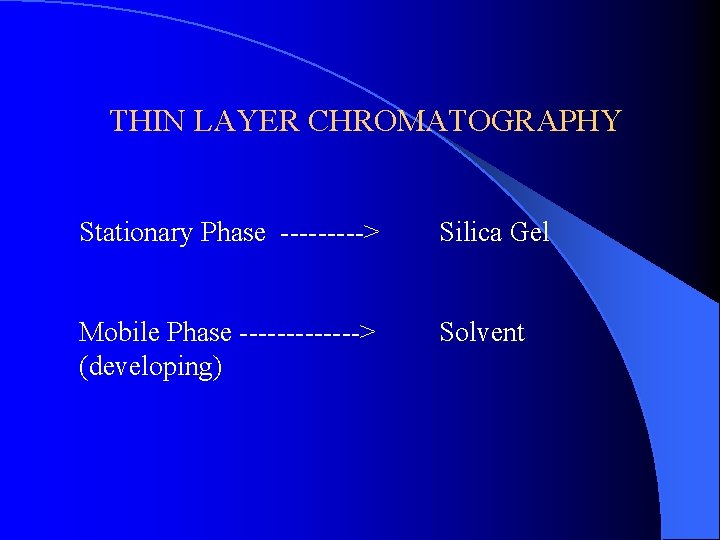
THIN LAYER CHROMATOGRAPHY Stationary Phase -----> Silica Gel Mobile Phase -------> (developing) Solvent

http: //www. chem. wits. ac. za/chem 212 -213 -280/0%20 Introduction%20 -%20 Lecture. ppt
Thermal Conductivity Detector The detector contains two filaments: one exposed only to carrier gas, while the other is exposed to the carrier gas for sample analysis. When the gas for the sample analysis is only carrier gas , the two filaments can be balanced. Instead of a direct measurement of filament temperature, the filament resistant, which is a function of temperature, is measured.

Thermal Conductivity Detector The ability of a colliding molecule to carry off heat depending on its thermal conductivity. Hydrogen and helium have high thermal conductivity and therefore will be more efficient at “cooling” a heated filament than other gases will
Thermal Conductivity Detector

Thermal Conductivity Detector
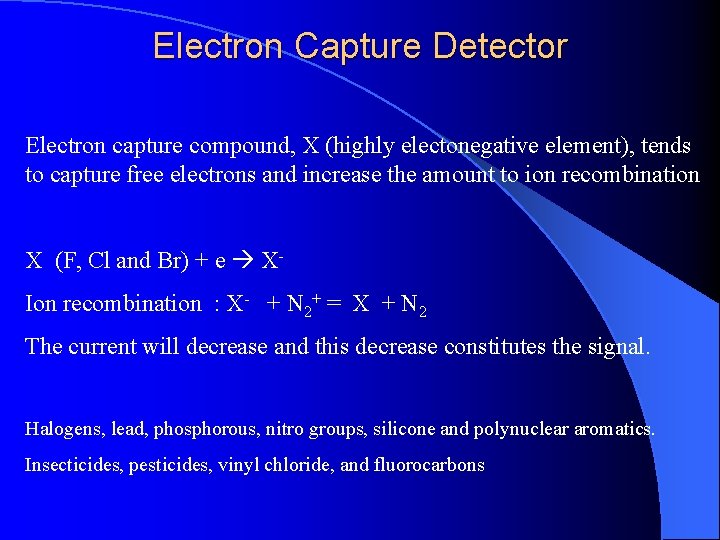
Electron Capture Detector Electron capture compound, X (highly electonegative element), tends to capture free electrons and increase the amount to ion recombination X (F, Cl and Br) + e XIon recombination : X- + N 2+ = X + N 2 The current will decrease and this decrease constitutes the signal. Halogens, lead, phosphorous, nitro groups, silicone and polynuclear aromatics. Insecticides, pesticides, vinyl chloride, and fluorocarbons
Electron Capture Detector

Gas Chromatography – Acetates Principals of Separation 1. Column is selected, packed with Liquid Phase, and installed. 2. Sample injected with microliter syringe into the injection port where it is vaporized and mixed into the Carrier Gas stream (helium, nitrogen, argon). 3. Sample vapor becomes partitioned between Moving Gas Phase and Stationary Liquid Phase. 4. The time the different compounds in the sample spend in the Vapor Phase is a function of their Vapor Pressure. 5. The more volatile (Low Boiling Point / Higher Vapor Pressure) compounds arrive at the end of the column first and pass into the detector.


The Beginning 4 concept of GC announced in 1941 by Martin and Synge (also did liquid partition chromatography) 4 10+ years later GC used experimentally 4 1955, first commercial apparatus for GC appeared on the market

Today 4 estimate : 200, 000 gas chromatographs are currently used through out the world. 4 30+ instrument manufactures 4 130 different models 4 cost 1, 500 to 40, 000 dollars 4 improvements: computers- automatic control open tubular columns-separate a multitude of analytes in relatively short times

- Slides: 69