An Introduction to Chromatography What Is chromatography Chromatography














- Slides: 77

An Introduction to Chromatography What Is chromatography? Chromatography is a physical method of separation in which the components to be separated are distributed between two phases one of which is stationary (stationary phase) while the other (the mobile phase) moves through it in a definite direction. The chromatographic process occurs due to differences in the distribution constant of the individual sample components.

Milestones in Chromatography • 1903 Tswett - plant pigments separated on chalk columns • 1931 Lederer & Kuhn - LC of carotenoids • 1938 TLC and ion exchange • 1950 reverse phase LC • 1954 Martin & Synge (Nobel Prize) • 1959 Gel permeation • 1965 instrumental LC (Waters)

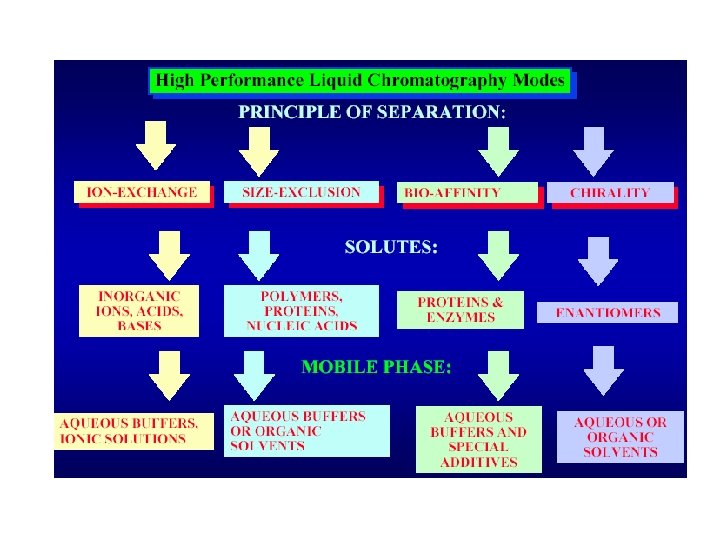
Different types of Chromatography Classification according to the force of separation: 1 - Adsorption chromatography. 2 - Partition chromatography. 3 - Ion exchange chromatography. 4 - Gel filtration chromatography. 5 - Affinity chromatography.

Classification according to the packing of the stationary phase: 1 - Thin layer chromatography (TLC): the stationary phase is a thin layer supported on glass, plastic or aluminium plates. 2 - Paper chromatography (PC): the stationary phase is a thin film of liquid supported on an inert support. 3 - Column chromatography (CC): stationary phase is packed in a glass column.

Classification of chromatography according to mobile phase: 1 - Liquid chromatography: mobile phase is a liquid. 2 - Gas chromatography : mobile phase is a gas.

Purpose of Chromatography • Analytical - determine chemical composition of a sample • Preparative - purify and collect one or more components of a sample

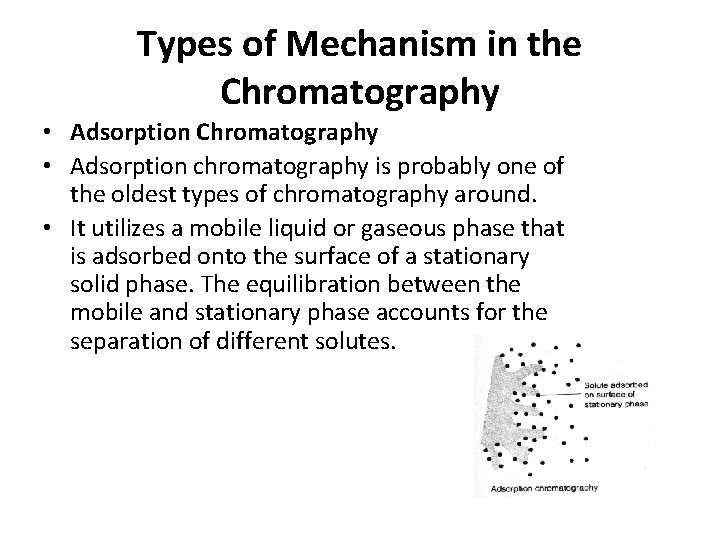
Types of Mechanism in the Chromatography • Adsorption Chromatography • Adsorption chromatography is probably one of the oldest types of chromatography around. • It utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibration between the mobile and stationary phase accounts for the separation of different solutes.

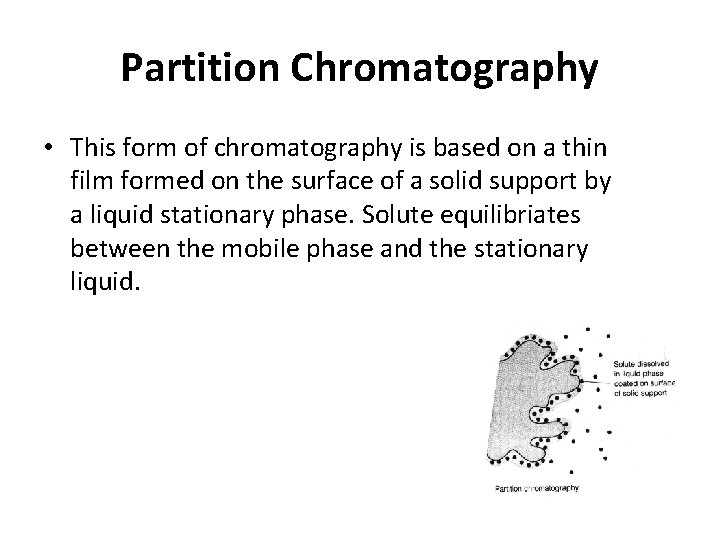
Partition Chromatography • This form of chromatography is based on a thin film formed on the surface of a solid support by a liquid stationary phase. Solute equilibriates between the mobile phase and the stationary liquid.

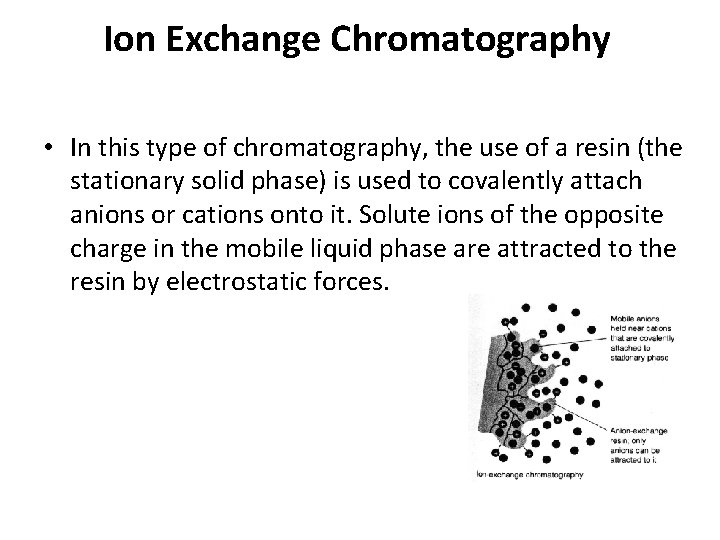
Ion Exchange Chromatography • In this type of chromatography, the use of a resin (the stationary solid phase) is used to covalently attach anions or cations onto it. Solute ions of the opposite charge in the mobile liquid phase are attracted to the resin by electrostatic forces.

Ion Exchange Chromatography Principle Process by which ions of an electrolyte solution are brought into contact with an ion exchange resin. The ion exchange resin is an insoluble polymer consisting of a "matrix" (Lattice or framework) that carries fixed charges (not exchangeable) and mobile active ions "counter ions" which are loosely attached to the matrix. In water, the counter-ions move more or less freely in the framework & can be replaced by ions of the same sign present in the surrounding solution. The "matrix" (framework) of a "cation exchanger" is considered as a crystalline non-ionized "polyanion" & the matrix of an "anion exchanger" as a non-ionized "polycation".

Cation Exchangers Active ions (counter ions) are cations. The polar groups attached to the matrix are acidic (sulphonic acids, carboxylic acids, phenols, phosphoric acids) e. g. a cation exchanger in the free carboxylic acid form: X-COO- H+ X = Frame work (matrix) -COO- = Fixed charge (anionic), Non-exchangeable H+ = Counter ion (cation), Exchangeable

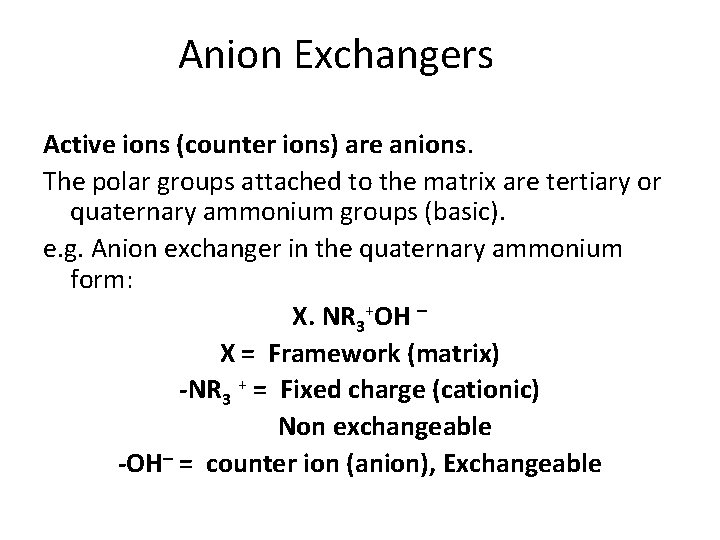
Anion Exchangers Active ions (counter ions) are anions. The polar groups attached to the matrix are tertiary or quaternary ammonium groups (basic). e. g. Anion exchanger in the quaternary ammonium form: X. NR 3+OH – X = Framework (matrix) -NR 3 + = Fixed charge (cationic) Non exchangeable -OH– = counter ion (anion), Exchangeable

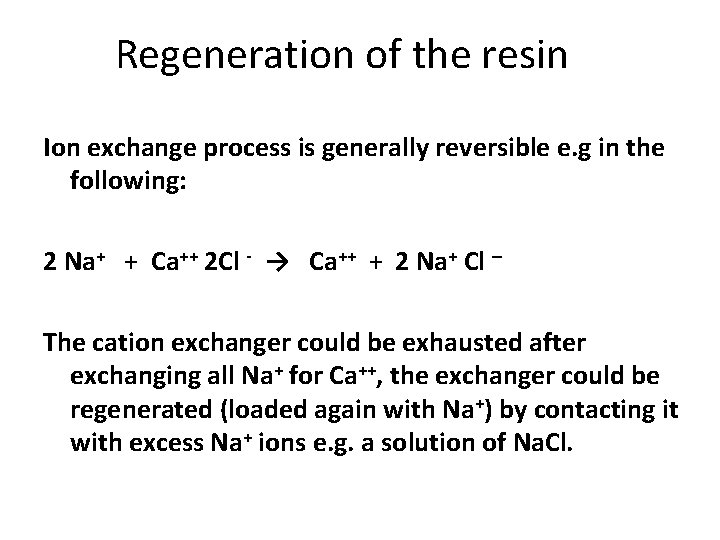
Regeneration of the resin Ion exchange process is generally reversible e. g in the following: 2 Na+ + Ca++ 2 Cl - → Ca++ + 2 Na+ Cl – The cation exchanger could be exhausted after exchanging all Na+ for Ca++, the exchanger could be regenerated (loaded again with Na+) by contacting it with excess Na+ ions e. g. a solution of Na. Cl.

Types of Exchangers Ion Exchangers These are either cation or anion exchangers of either organic or inorganic nature. A- Inorganic ion exchangers Common clays, soils, minerals e. g. zeolites used for "softening water". Disadvantage: low ion-exchange capacity. Advantages: Great resistance to high temperatures. High volume capacity. Great selectivity towards simple inorganic ions.

B- Organic exchangers They may be natural or synthetic. Preparation of organic synthetic ion exchangers : Polycondensation of phenols, sulpho- & carboxyderivatives with formaldehyde → cationic exchangers. Polycondensation of aromatic amines with formaldehyde → anionic exchangers. These techniques yield products linear in structure & relatively soluble in water which are now replaced by resin materials based on styrene divinyl benzene copolymers and polyacrylate.

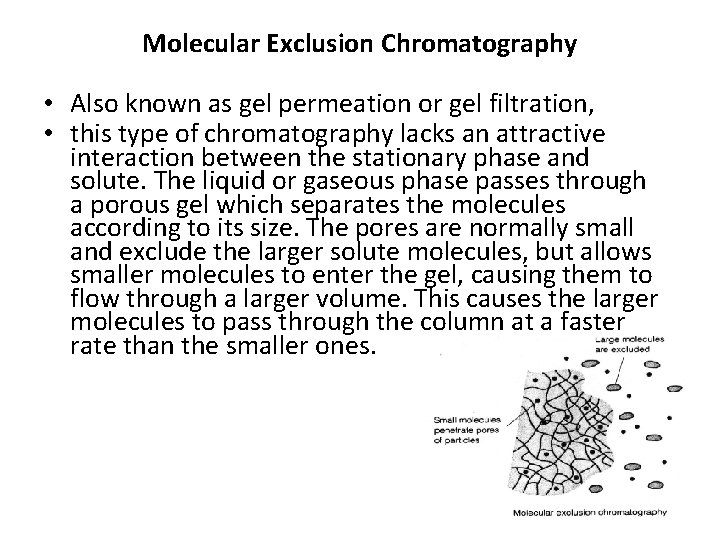
Molecular Exclusion Chromatography • Also known as gel permeation or gel filtration, • this type of chromatography lacks an attractive interaction between the stationary phase and solute. The liquid or gaseous phase passes through a porous gel which separates the molecules according to its size. The pores are normally small and exclude the larger solute molecules, but allows smaller molecules to enter the gel, causing them to flow through a larger volume. This causes the larger molecules to pass through the column at a faster rate than the smaller ones.

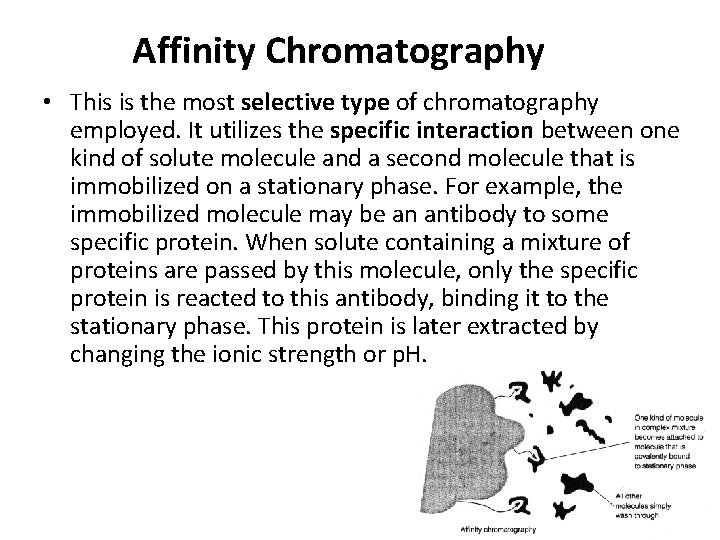
Affinity Chromatography • This is the most selective type of chromatography employed. It utilizes the specific interaction between one kind of solute molecule and a second molecule that is immobilized on a stationary phase. For example, the immobilized molecule may be an antibody to some specific protein. When solute containing a mixture of proteins are passed by this molecule, only the specific protein is reacted to this antibody, binding it to the stationary phase. This protein is later extracted by changing the ionic strength or p. H.

Thin layer chromatography (TLC) is a method for identifying substances and testing the purity of compounds. TLC is a useful technique because it is relatively quick and requires small quantities of material.

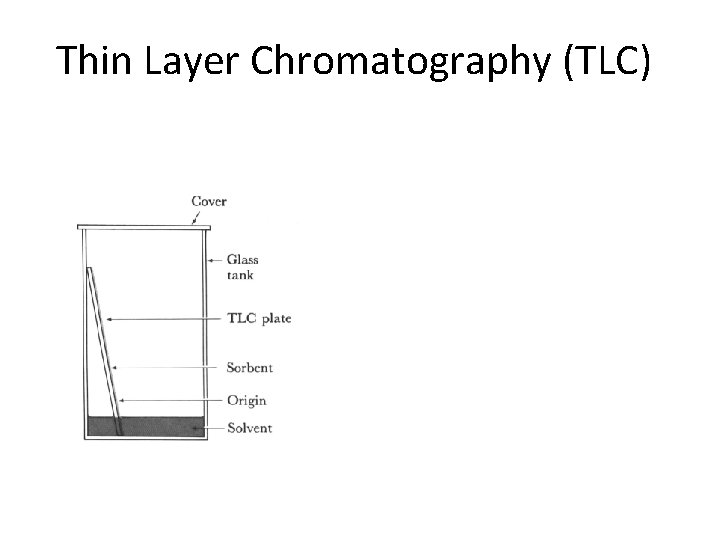
Separations in TLC involve distributing a mixture of two or more substances between a stationary phase and a mobile phase. The stationary phase: is a thin layer of adsorbent (usually silica gel or alumina) coated on a plate. The mobile phase: is a developing liquid which travels up the stationary phase, carrying the samples with it. Components of the samples will separate on the stationary phase according to how much they adsorb on the stationary phase versus how much they dissolve in the mobile phase.

Thin Layer Chromatography (TLC)

Preparing the Chamber To a jar with a tight-fitting lid add enough of the appropriate developing liquid so that it is 0. 5 to 1 cm deep in the bottom of the jar. Close the jar tightly, and let it stand for about 30 minutes so that the atmosphere in the jar becomes saturated with solvent.

Preparing the Plates for Development

Developing the Plates After preparing the development chamber and spotting the samples, the plates are ready for development. Be careful to handle the plates only by their edges, and try to leave the development chamber uncovered for as little time as possible. When the plates are removed from the chamber, quickly trace the solvent front (the highest solvent level on the plate) with a pencil.

Identifying the Spots (visualization) If the spots can be seen, outline them with a pencil. If no spots are obvious, the most common visualization technique is to hold the plate under a UV lamp. Many organic compounds can be seen using this technique, and many commercially made plates often contain a substance which aids in the visualization of compounds.

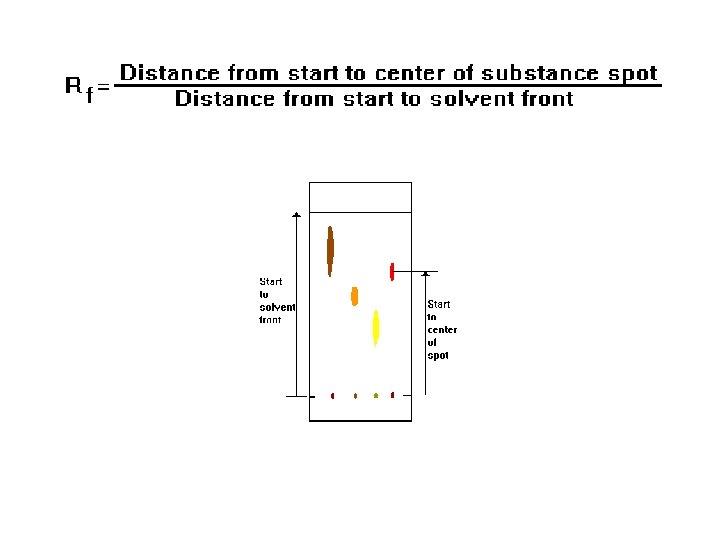
Interpreting the Data The Rf (retention factor) value for each spot should be calculated. It is characteristic for any given compound on the same stationary phase using the same mobile phase for development of the plates. Hence, known Rf values can be compared to those of unknown substances to aid in their identifications.


(Note: Rf values often depend on the temperature and the solvent used in the TLC experiment. the most effective way to identify a compound is to spot known substances – authentic - next to unknown substances on the same plate. ) In addition, the purity of a sample may be estimated from the chromatogram. An impure sample will often develop as two or more spots, while a pure sample will show only one spot

Summary A TLC plate is a sheet of glass, metal, or plastic which is coated with a thin layer of a solid adsorbent (usually silica or alumina). A small amount of the mixture to be analyzed is spotted near the bottom of this plate. The TLC plate is then placed in a shallow pool of a solvent in a developing chamber so that only the very bottom of the plate is in the liquid. This liquid, or the eluent, is the mobile phase, and it slowly rises up the TLC plate by capillary action. As the solvent moves past the spot that was applied, an equilibrium is established for each component of the mixture between the molecules of that component which are adsorbed on the solid and the molecules which are in solution.

In principle, the components will differ in solubility and in the strength of their adsorption to the adsorbent and some components will be carried farther up the plate than others. When the solvent has reached the top of the plate, the plate is removed from the developing chamber, dried, and the separated components of the mixture are visualized. If the compounds are colored, visualization is straightforward. Usually the compounds are not colored, so a UV lamp is used to visualize the plates.

Paper Chromatography A method of partition chromatography using filter paper strips as carrier or inert support. The factor governing separation of mixtures of solutes on filter paper is the partition between two immiscible phases. One is usually water adsorbed on cellulose fibres in the paper (stationary phase). The second is the organic solvent flows past the sample on the paper (Mobile phase).

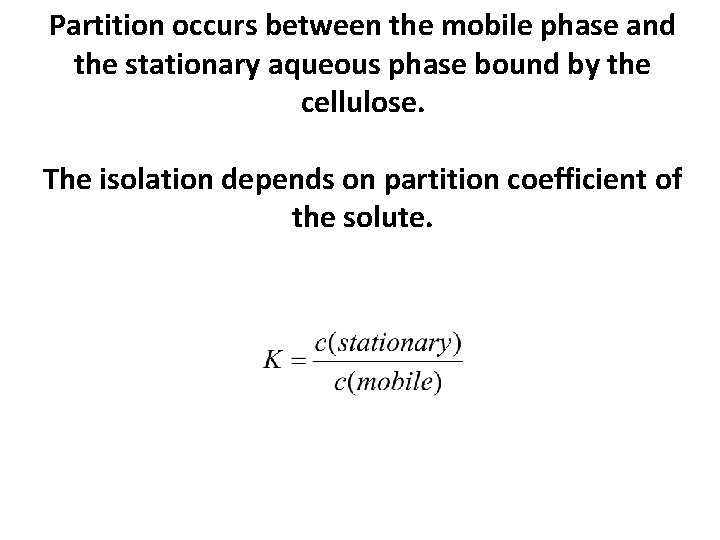
Partition occurs between the mobile phase and the stationary aqueous phase bound by the cellulose. The isolation depends on partition coefficient of the solute.

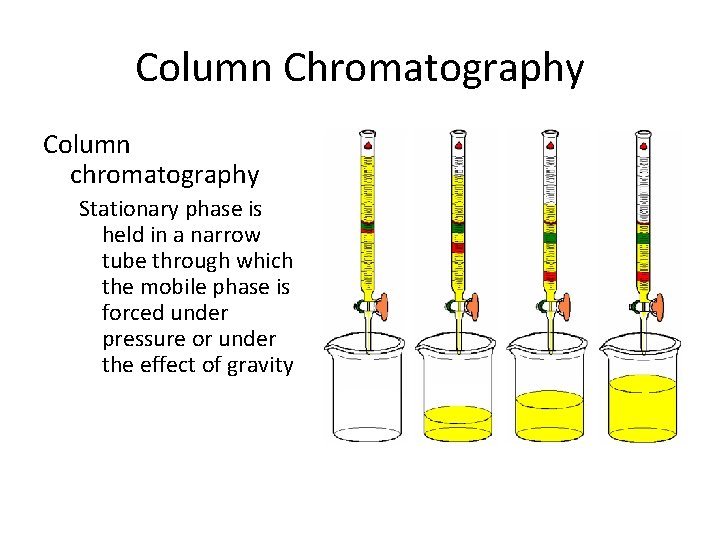
Column Chromatography (CC) This includes chromatographic methods in which: The stationary phase is packed into a column. The mobile phase is a moving liquid or gas. According to the mechanism of separation of solutes, five major types of CC are distinguished. Usually, one mechanism predominates but does not exclude the others

Column Chromatography Column chromatography Stationary phase is held in a narrow tube through which the mobile phase is forced under pressure or under the effect of gravity

Open Column Chromatography (Traditional column chromatography) • Traditional column chromatography is characterized by addition of mobile phase under atmospheric pressure and the stationary phase is packed in a glass column.

Packing & operating the column 1 - Packing The selection of the method of packing depends mainly on the density of the solid. Techniques used are the wet, dry & slurry methods. In all cases avoid inclusion of air bubbles

2 - Sample Application • Apply evenly & in a concentrated solution to the top of the column which is protected from disturbance (e. g. add glass wool or filter paper).

4 - Detection On-column detection for colored or fluorescent compounds directly after developing the chromatogram. Monitoring of eluted fractions (PC or TLC). Using special detectors connected to the column such as refractive index, UV detectors, etc…

Liquid Chromatography ØPreparative Column Chromatography: Conventional, can be performed in different ways v. Flash v VLC ØDepending on pressure used, can be classified as: v. LPLC v. MPLC v. HPLC

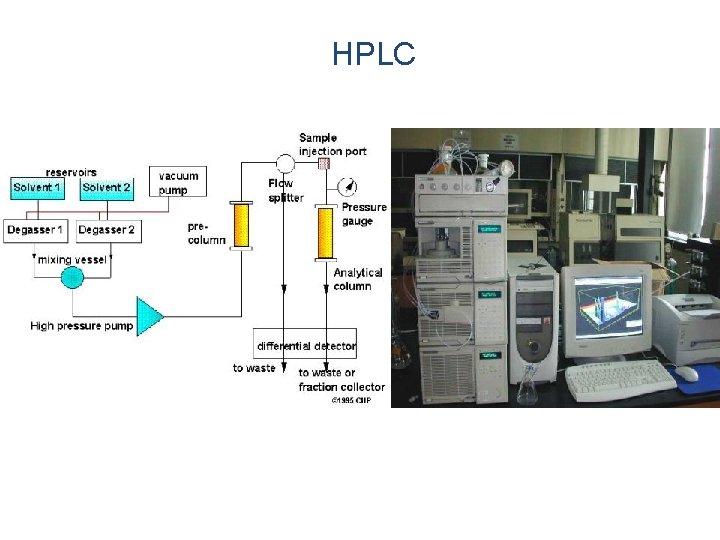
HPLC

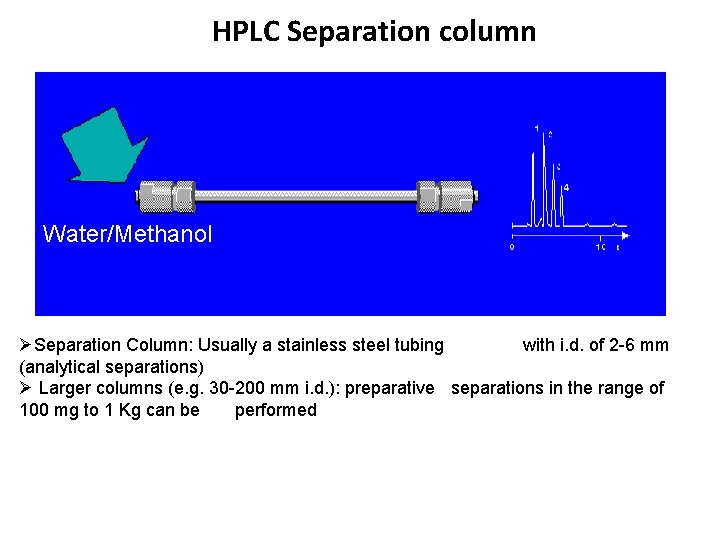
HPLC Separation column Water/Methanol ØSeparation Column: Usually a stainless steel tubing with i. d. of 2 -6 mm (analytical separations) Ø Larger columns (e. g. 30 -200 mm i. d. ): preparative separations in the range of 100 mg to 1 Kg can be performed

HPLC Separation column ØA fine grained chromatographic material (e. g. silica gel or RP-18), serves as stationary phase with particle size of 5 -10 m (analytical separations) and 10 -50 m (preparative separations). ØTo overcome the flow resistance of stationary phase, mobile phase must placed under a relatively high pressure up to 100 bar.

Twin Pistion pump ØThe twin piston pumps with short stroke are among the most commonly used pumps for HPLC. Ø Both pump heads are switched in series, whereby the piston in the first pump head delivers a specific volume per stroke. ØAn eccentric disk presses piston 1 to the right and displaces the solvent. The double ball saphire valves ensure that the solvent stream can flow in only one direction. Ø The second piston is used to produce a nearly complete pulsation damping. With the twin piston pump, a pressure of 40 MPa is achieved.

Detectors used with HPLC ØUV-Visible Detector ØPhotodiode Array (PDA) Detector Ø Fluorescence Detector ØRefractive Index (RI) Detector Ø Evaporative Light Scattering Dectector (ELSD) ØConductivity Detector ØMass Detector

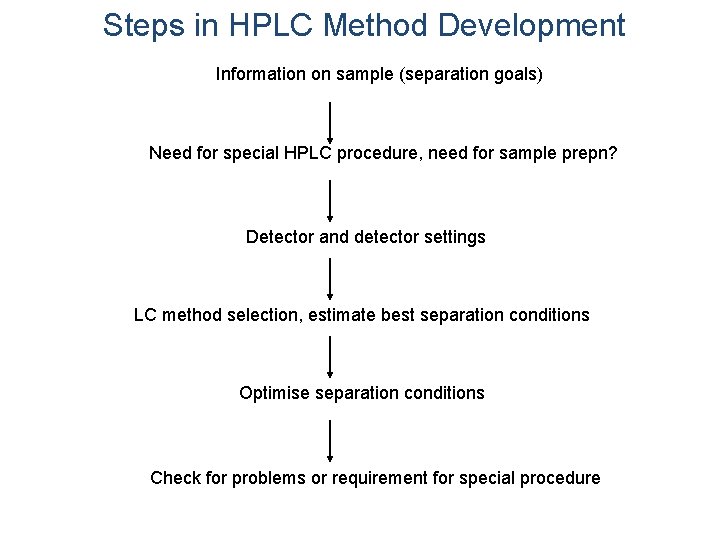
Steps in HPLC Method Development Information on sample (separation goals) Need for special HPLC procedure, need for sample prepn? Detector and detector settings LC method selection, estimate best separation conditions Optimise separation conditions Check for problems or requirement for special procedure

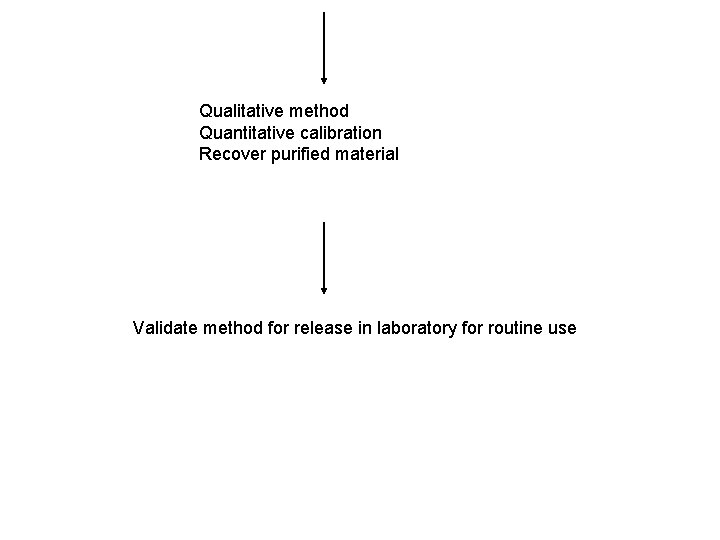
Qualitative method Quantitative calibration Recover purified material Validate method for release in laboratory for routine use

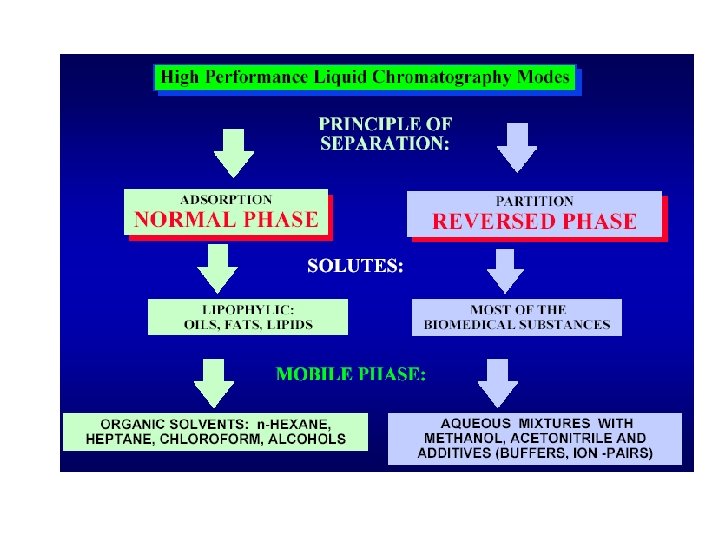
The most common modes of HPLC ØNormal Phase ØReverse Phase ØIon Exchange ØChiral ØSpecialty



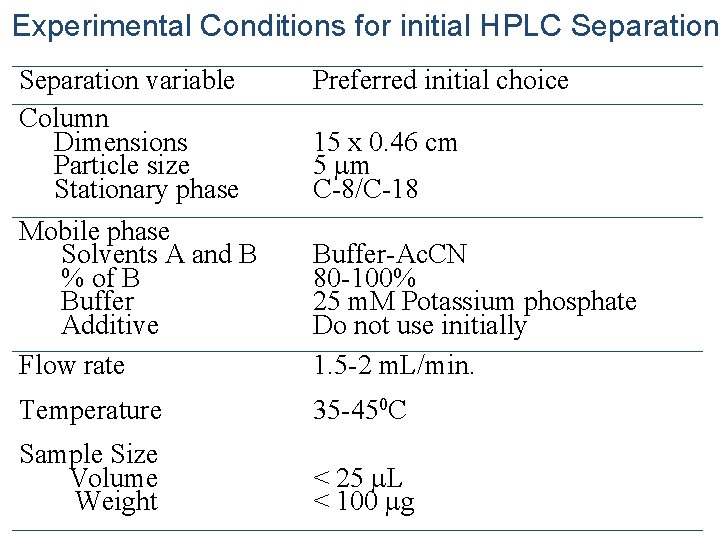
Experimental Conditions for initial HPLC Separation variable Column Dimensions Particle size Stationary phase Mobile phase Solvents A and B % of B Buffer Additive Flow rate Preferred initial choice Temperature 35 -450 C Sample Size Volume Weight < 25 L < 100 g 15 x 0. 46 cm 5 m C-8/C-18 Buffer-Ac. CN 80 -100% 25 m. M Potassium phosphate Do not use initially 1. 5 -2 m. L/min.

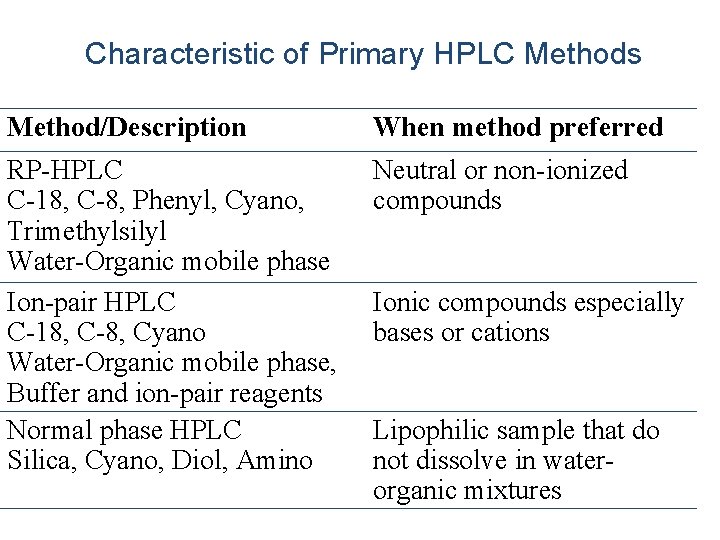
Characteristic of Primary HPLC Methods Method/Description When method preferred RP-HPLC C-18, C-8, Phenyl, Cyano, Trimethylsilyl Water-Organic mobile phase Ion-pair HPLC C-18, C-8, Cyano Water-Organic mobile phase, Buffer and ion-pair reagents Normal phase HPLC Silica, Cyano, Diol, Amino Neutral or non-ionized compounds Ionic compounds especially bases or cations Lipophilic sample that do not dissolve in waterorganic mixtures

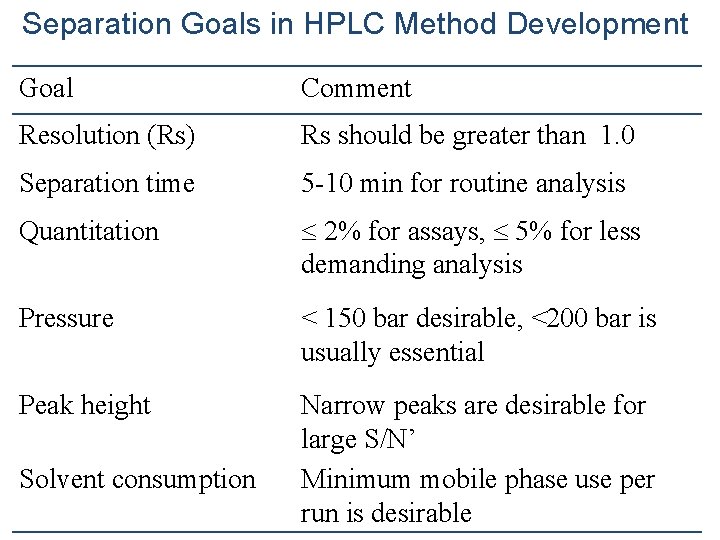
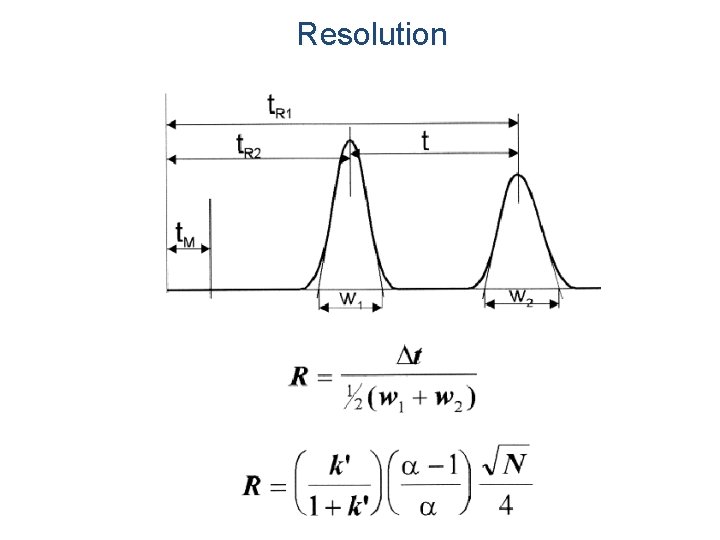
Separation Goals in HPLC Method Development Goal Comment Resolution (Rs) Rs should be greater than 1. 0 Separation time 5 -10 min for routine analysis Quantitation 2% for assays, 5% for less demanding analysis Pressure < 150 bar desirable, <200 bar is usually essential Peak height Narrow peaks are desirable for large S/N’ Minimum mobile phase use per run is desirable Solvent consumption

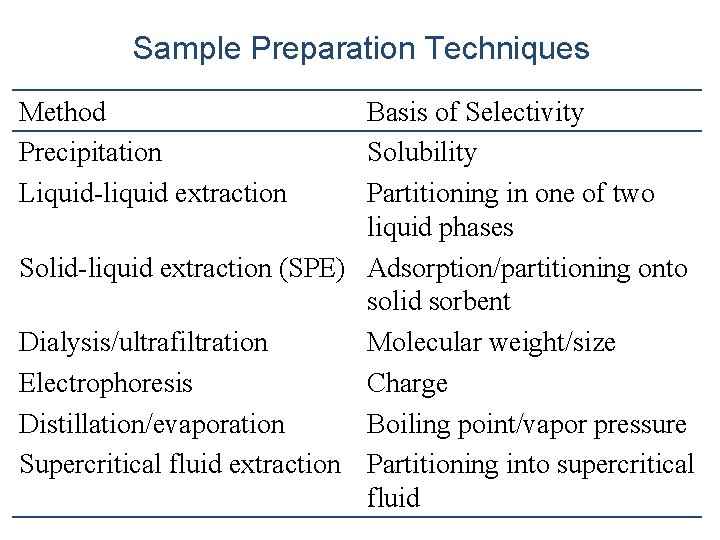
Sample Preparation Techniques Method Precipitation Liquid-liquid extraction Basis of Selectivity Solubility Partitioning in one of two liquid phases Solid-liquid extraction (SPE) Adsorption/partitioning onto solid sorbent Dialysis/ultrafiltration Molecular weight/size Electrophoresis Charge Distillation/evaporation Boiling point/vapor pressure Supercritical fluid extraction Partitioning into supercritical fluid

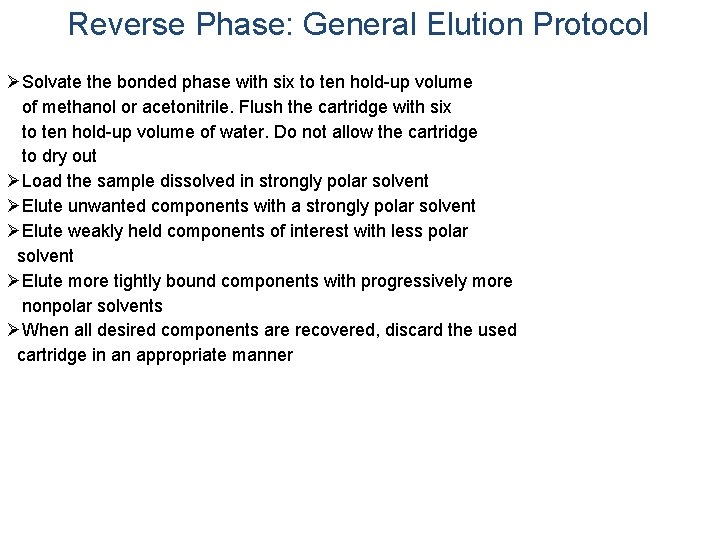
Reverse Phase: General Elution Protocol ØSolvate the bonded phase with six to ten hold-up volume of methanol or acetonitrile. Flush the cartridge with six to ten hold-up volume of water. Do not allow the cartridge to dry out ØLoad the sample dissolved in strongly polar solvent ØElute unwanted components with a strongly polar solvent ØElute weakly held components of interest with less polar solvent ØElute more tightly bound components with progressively more nonpolar solvents ØWhen all desired components are recovered, discard the used cartridge in an appropriate manner

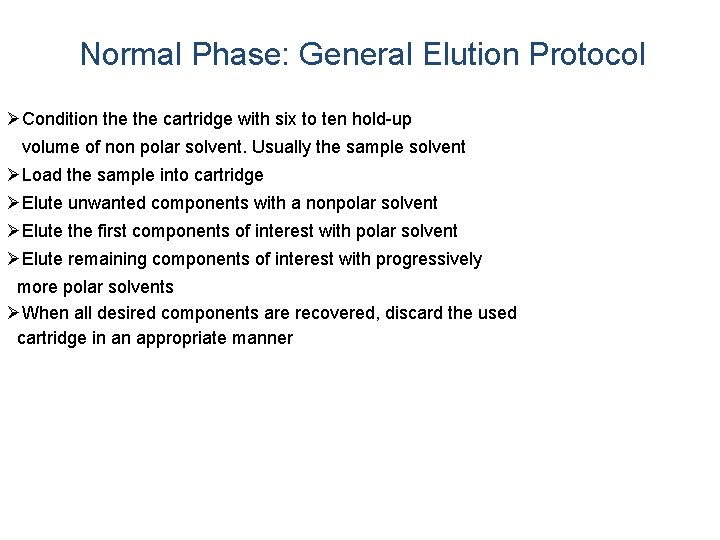
Normal Phase: General Elution Protocol ØCondition the cartridge with six to ten hold-up volume of non polar solvent. Usually the sample solvent ØLoad the sample into cartridge ØElute unwanted components with a nonpolar solvent ØElute the first components of interest with polar solvent ØElute remaining components of interest with progressively more polar solvents ØWhen all desired components are recovered, discard the used cartridge in an appropriate manner

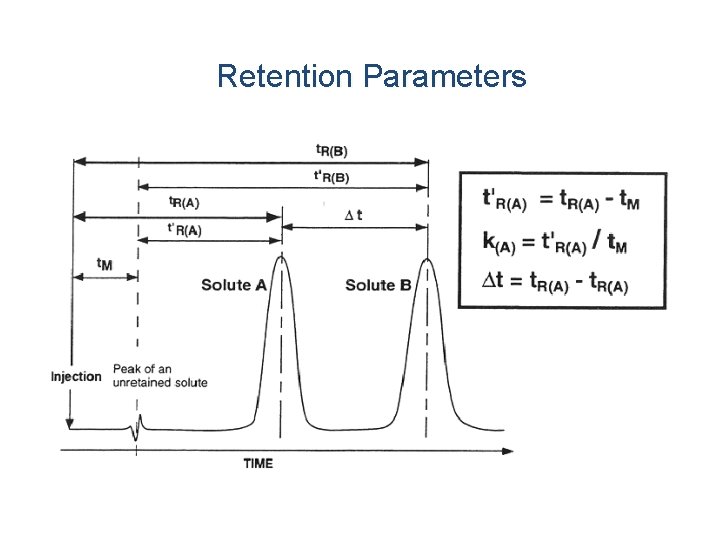
Retention Parameters

HPLC Retention v. Major parameters Ø VR is retention volume, depends on the column type, size, and on the instrument parameters Vo is dead volume, volume of the liquid phase inside the column Ø k’ is capacity factor, Ø

Resolution

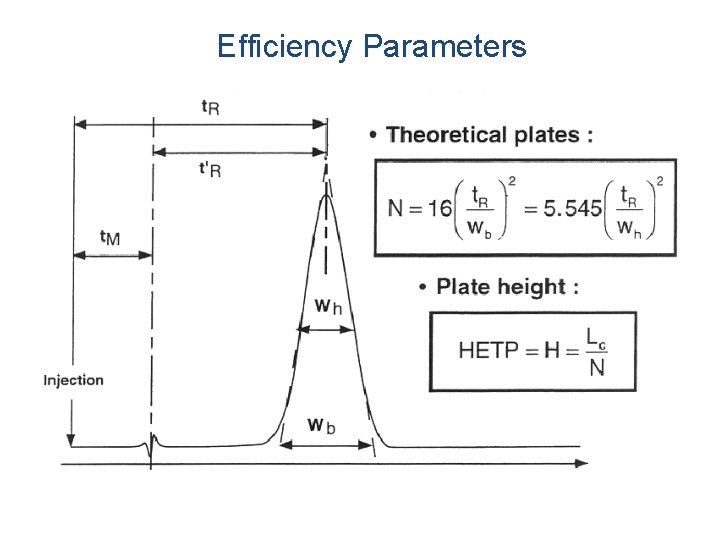
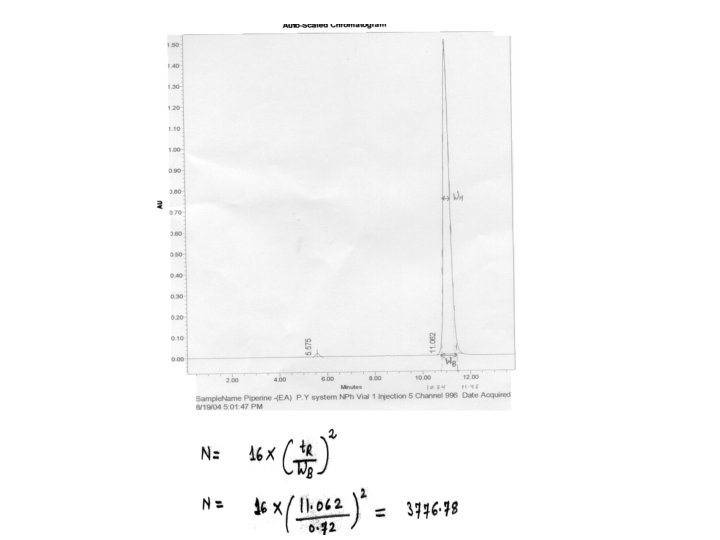
Efficiency Parameters


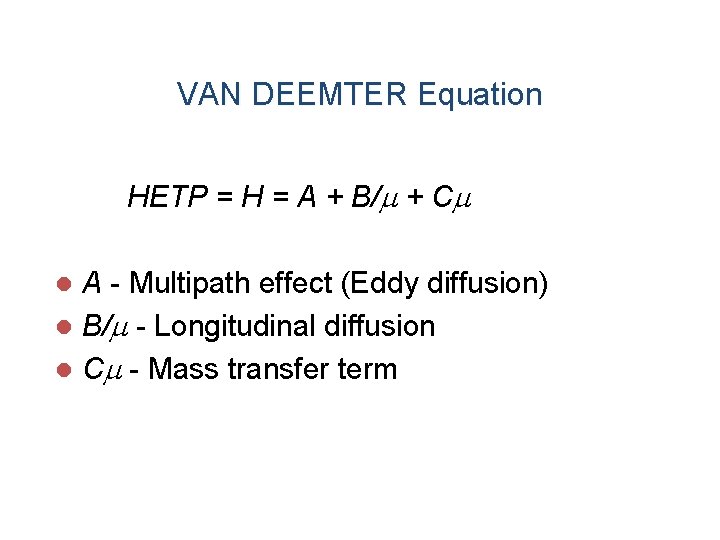
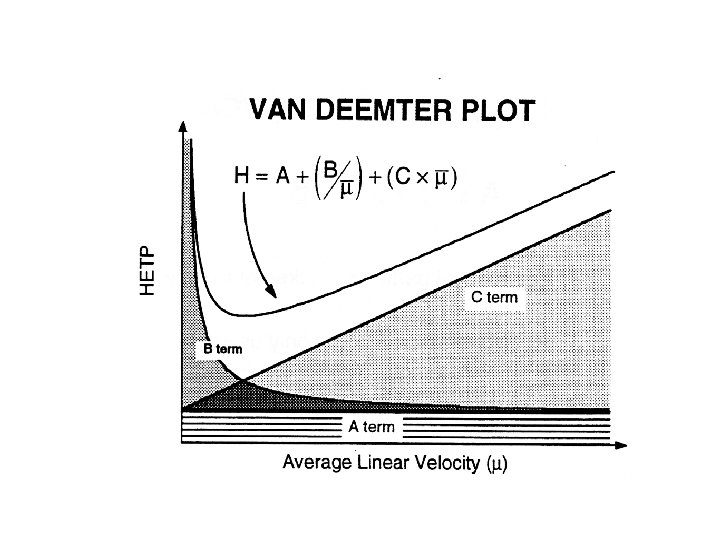
VAN DEEMTER Equation HETP = H = A + B/ + C A - Multipath effect (Eddy diffusion) l B/ - Longitudinal diffusion l C - Mass transfer term l

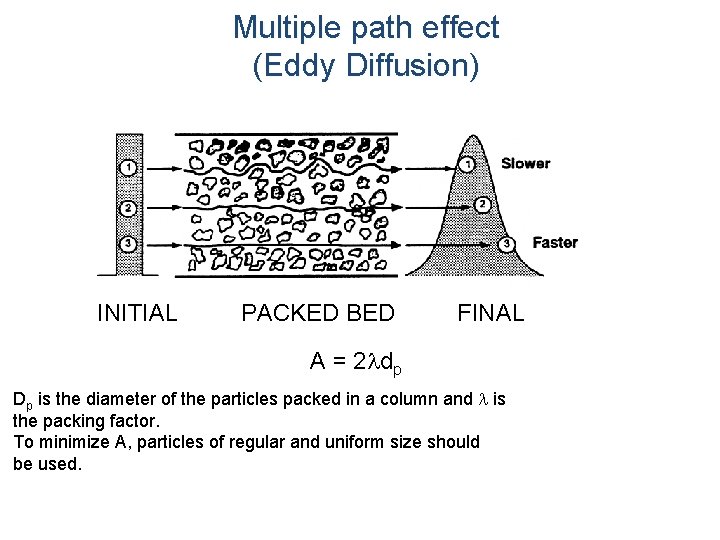
Multiple path effect (Eddy Diffusion) INITIAL PACKED BED FINAL A = 2 ldp Dp is the diameter of the particles packed in a column and l is the packing factor. To minimize A, particles of regular and uniform size should be used.

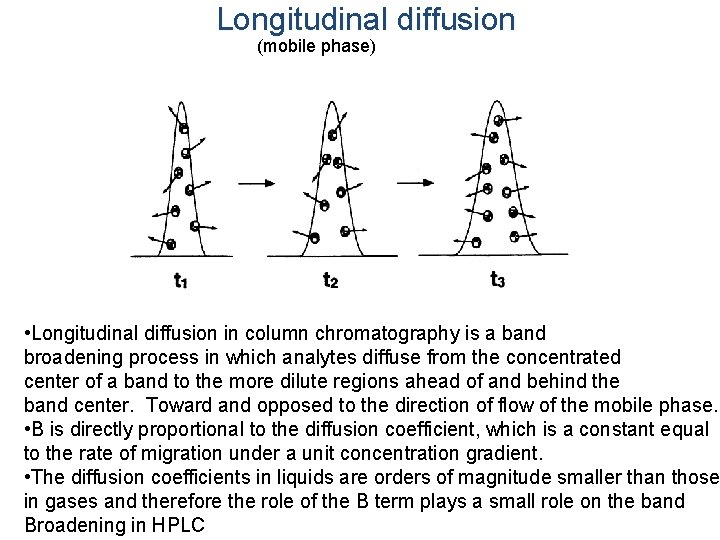
Longitudinal diffusion (mobile phase) • Longitudinal diffusion in column chromatography is a band broadening process in which analytes diffuse from the concentrated center of a band to the more dilute regions ahead of and behind the band center. Toward and opposed to the direction of flow of the mobile phase. • B is directly proportional to the diffusion coefficient, which is a constant equal to the rate of migration under a unit concentration gradient. • The diffusion coefficients in liquids are orders of magnitude smaller than those in gases and therefore the role of the B term plays a small role on the band Broadening in HPLC

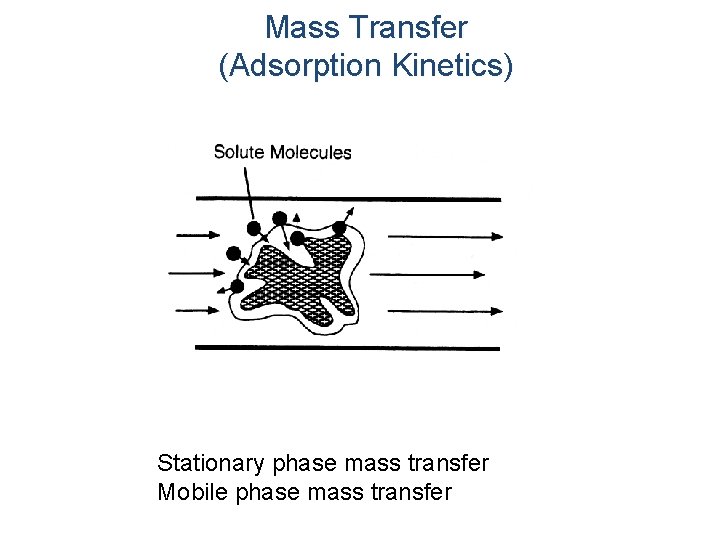
Mass Transfer (Adsorption Kinetics) Stationary phase mass transfer Mobile phase mass transfer


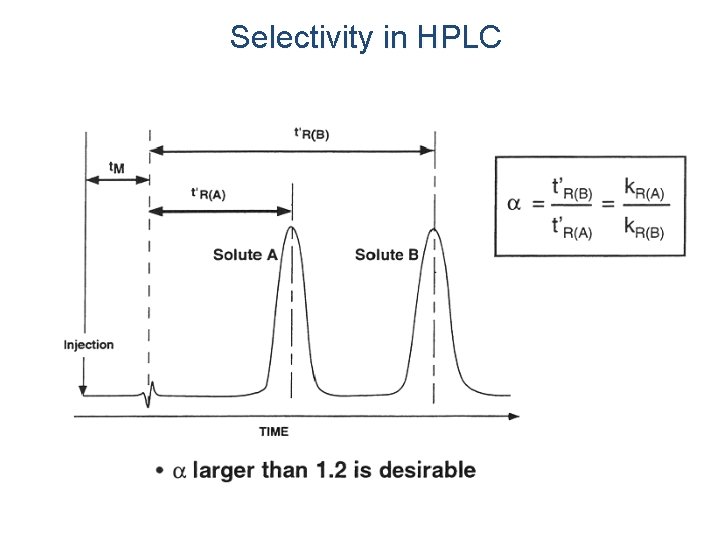
Selectivity in HPLC

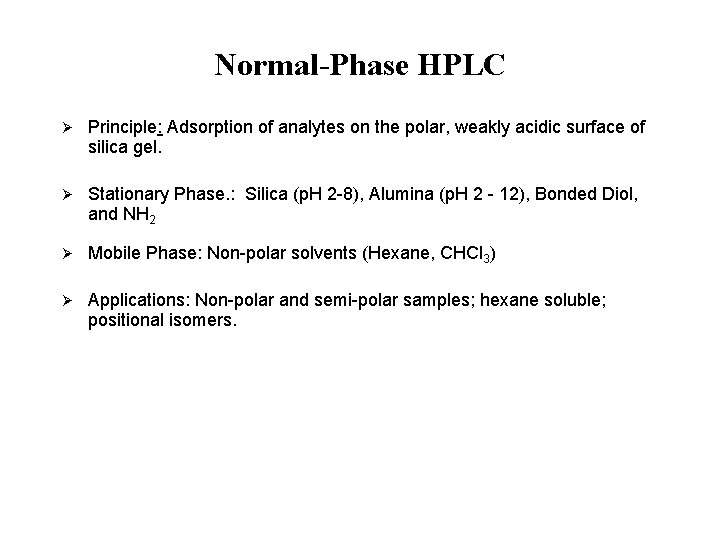
Normal-Phase HPLC Ø Principle: Adsorption of analytes on the polar, weakly acidic surface of silica gel. Ø Stationary Phase. : Silica (p. H 2 -8), Alumina (p. H 2 - 12), Bonded Diol, and NH 2 Ø Mobile Phase: Non-polar solvents (Hexane, CHCl 3) Ø Applications: Non-polar and semi-polar samples; hexane soluble; positional isomers.

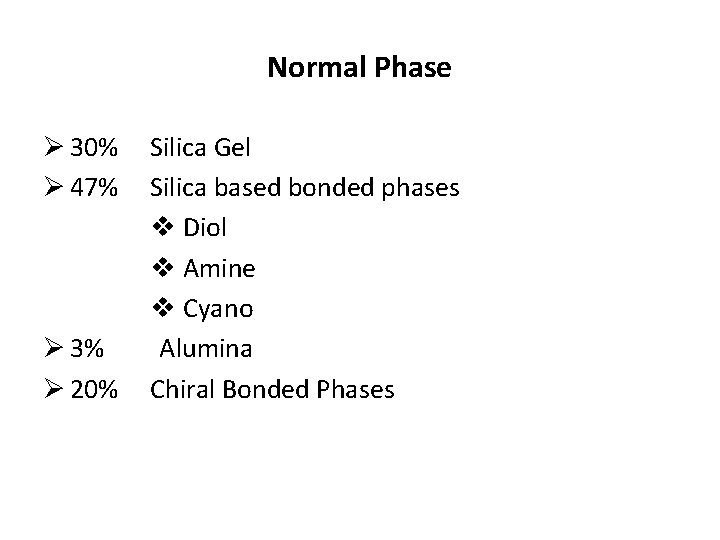
Normal Phase Ø 30% Ø 47% Ø 3% Ø 20% Silica Gel Silica based bonded phases v Diol v Amine v Cyano Alumina Chiral Bonded Phases

NP: Adsorption Mechanism Ø Competitive polar interactions v The more polar component, the more stronger it will be adsorbed and so retained longer. v Physical adsorption is a dynamic reversible process. Ø Polarity of the eluent plays major role in separation v. The more polar eluent, the more competitive it is for adsorption sites and hence analyte will be

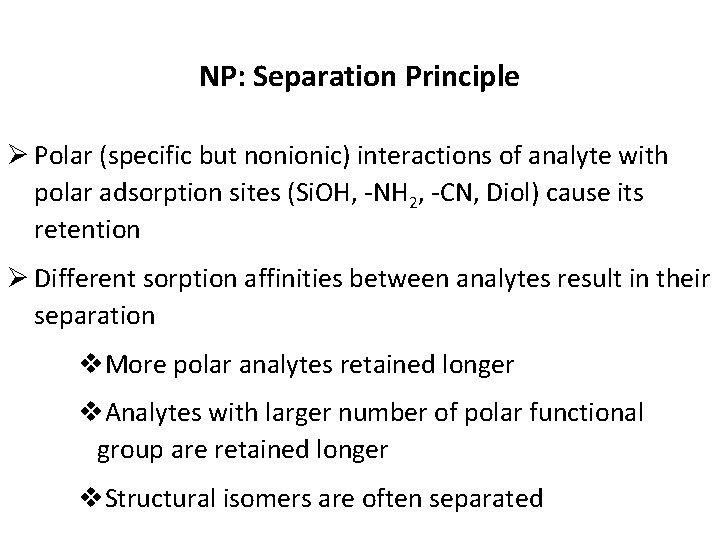
NP: Separation Principle Ø Polar (specific but nonionic) interactions of analyte with polar adsorption sites (Si. OH, -NH 2, -CN, Diol) cause its retention Ø Different sorption affinities between analytes result in their separation v. More polar analytes retained longer v. Analytes with larger number of polar functional group are retained longer v. Structural isomers are often separated

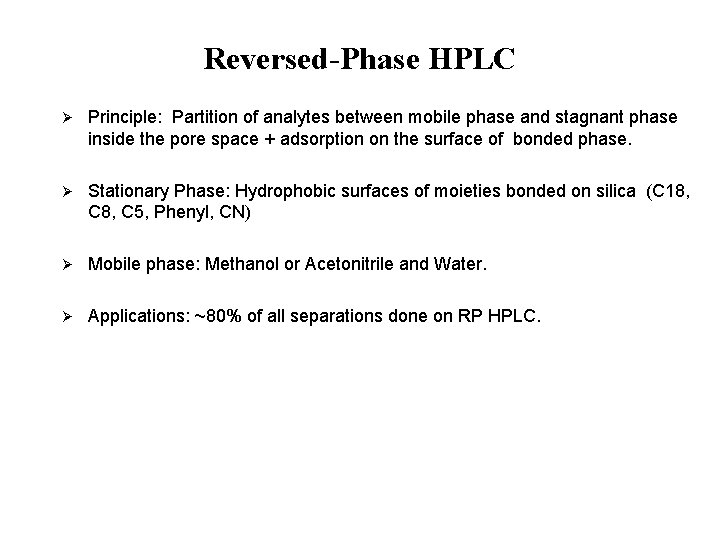
Reversed-Phase HPLC Ø Principle: Partition of analytes between mobile phase and stagnant phase inside the pore space + adsorption on the surface of bonded phase. Ø Stationary Phase: Hydrophobic surfaces of moieties bonded on silica (C 18, C 8, C 5, Phenyl, CN) Ø Mobile phase: Methanol or Acetonitrile and Water. Ø Applications: ~80% of all separations done on RP HPLC.

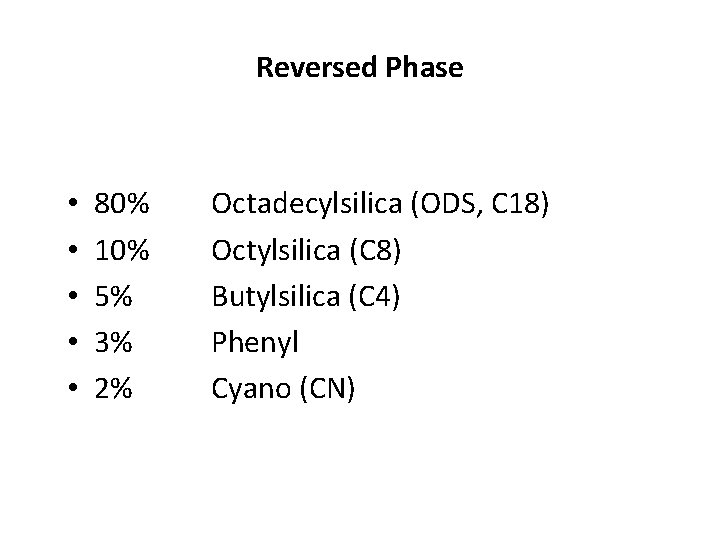
Reversed Phase • • • 80% 10% 5% 3% 2% Octadecylsilica (ODS, C 18) Octylsilica (C 8) Butylsilica (C 4) Phenyl Cyano (CN)

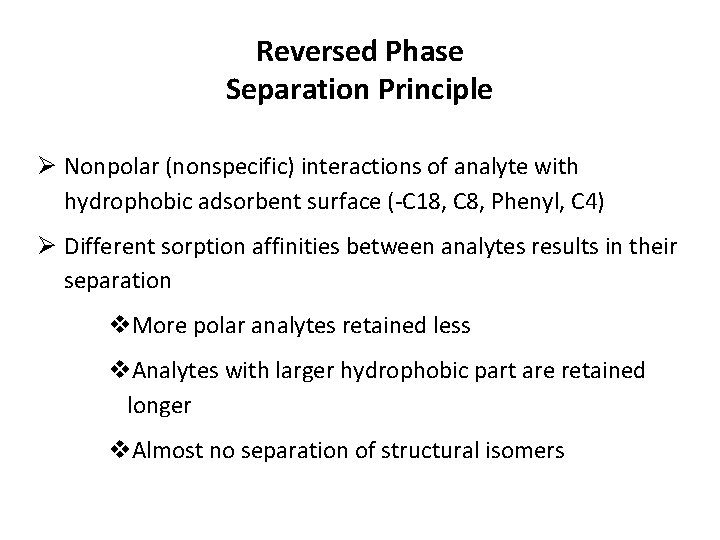
Reversed Phase Separation Principle Ø Nonpolar (nonspecific) interactions of analyte with hydrophobic adsorbent surface (-C 18, C 8, Phenyl, C 4) Ø Different sorption affinities between analytes results in their separation v. More polar analytes retained less v. Analytes with larger hydrophobic part are retained longer v. Almost no separation of structural isomers

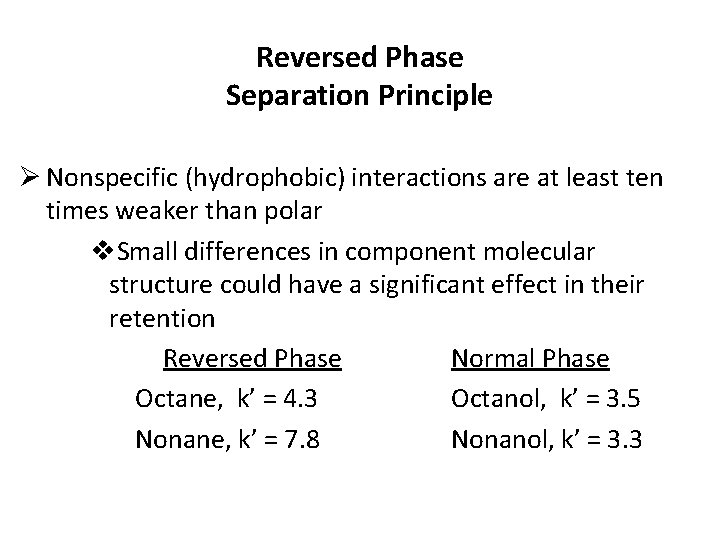
Reversed Phase Separation Principle Ø Nonspecific (hydrophobic) interactions are at least ten times weaker than polar v. Small differences in component molecular structure could have a significant effect in their retention Reversed Phase Normal Phase Octane, k’ = 4. 3 Octanol, k’ = 3. 5 Nonane, k’ = 7. 8 Nonanol, k’ = 3. 3

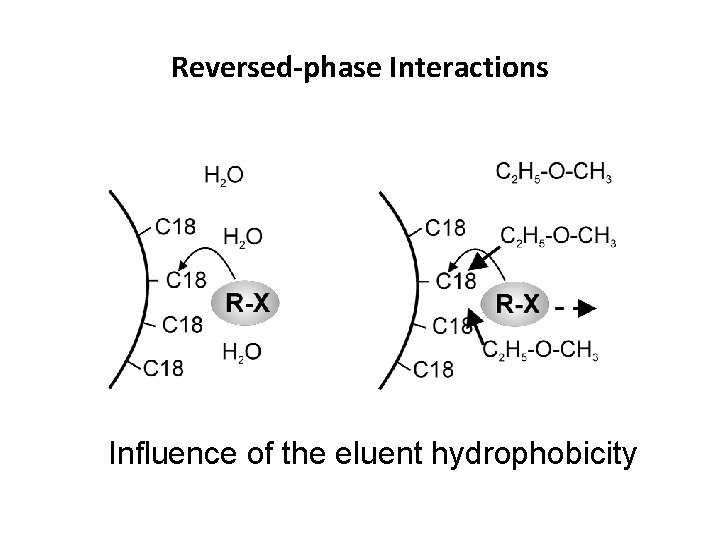
Reversed-phase Interactions Influence of the eluent hydrophobicity

Gas Chromatography v Good for volatile samples (up to about 250 o. C) v 0. 1 -1. 0 microliter of liquid or 1 -10 ml vapor v Can detect <1 ppm with certain detectors v Can be easily automated for injection and data analysis

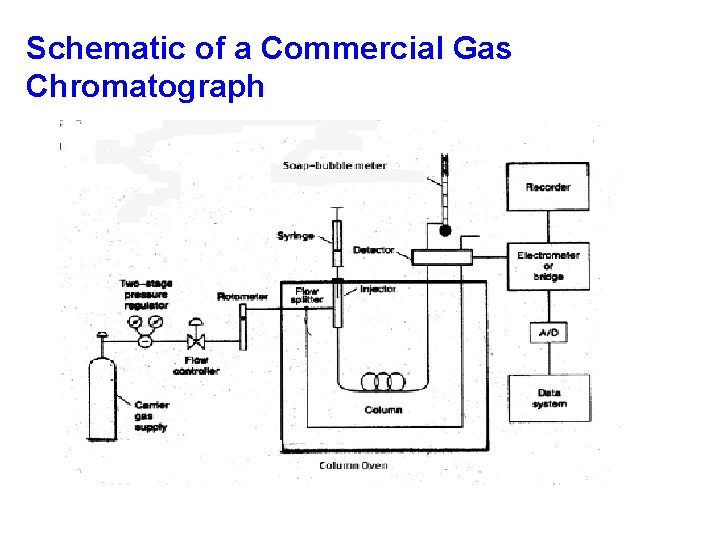
Components of a Gas Chromatograph Gas Supply: (usually N 2 or He) Sample Injector: (syringe / septum) Column: 1/8” or 1/4” x 6 -50’ tubing packed with small uniform size, inert support coated with thin film of nonvolatile liquid Detector: TC - thermal conductivity FID - flame ionization detector

Schematic of a Commercial Gas Chromatograph