GAS CHROMATOGRAPHY LECTURE 8 PRINCIPLES In Gas Chromatography









- Slides: 60

GAS CHROMATOGRAPHY LECTURE 8

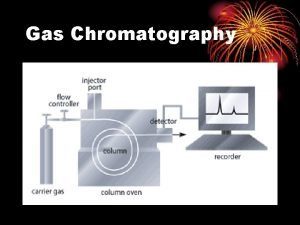
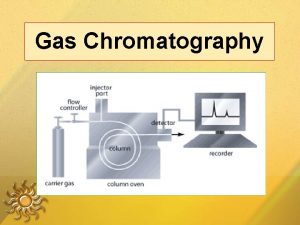
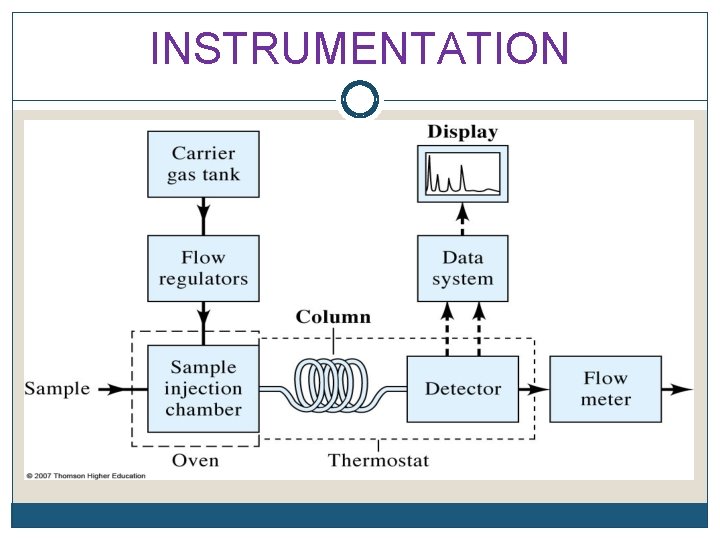
PRINCIPLES In Gas Chromatography, the components of a vaporized sample are separated as a result of being partitioned between a mobile gaseous phase and a liquid or a solid stationary phase held in the column.

PRINCIPLES A sample is being injected at the inlet/injector and vaporized into the chromatographic column. The sample is transported through the column by the flow of inert gaseous mobile phase. As the sample passes through the column, they are separated and detected electronically by detector.

Gas Chromatography Gas is called “carrier gas”. Typical carrier gas: helium or nitrogen. Pressure from a compressed gas cylinder containing the carrier gas is sufficient to create the flow through the column.

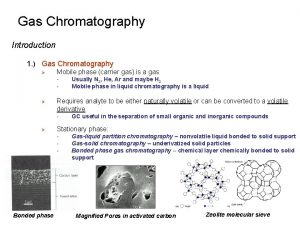
Gas Chromatography There are two types. q Gas-liquid chromatography (GLC) Shortened to Gas mobile phase – gas Chromatography stationary phase - liquid q Gas-solid chromatography (GSC) mobile phase – gas stationary phase - solid

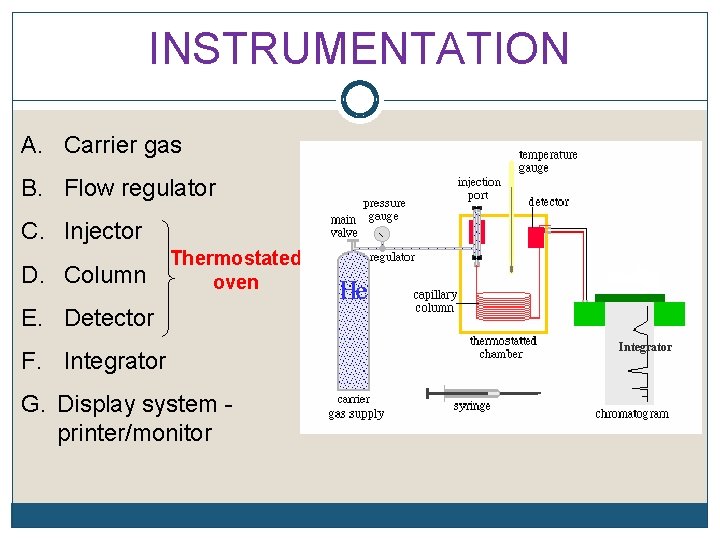
INSTRUMENTATION A. Carrier gas B. Flow regulator C. Injector D. Column Thermostated oven E. Detector F. Integrator G. Display system printer/monitor Integrator

INSTRUMENTATION

INSTRUMENTATION Injection port and detector must be kept warmer than the column, 1. To promote rapid vaporization of the injected sample. 2. To prevent sample condensation in the detector.

Samples must be…. . q q q Volatile Thermally stable. When injected onto the head of a chromatographic column and vaporized.

Mobile phase q q q Mobile phase transports the analytes (sample) through column. Mobile phase can not interact with the molecules of the analyte. Referred as carrier gas.

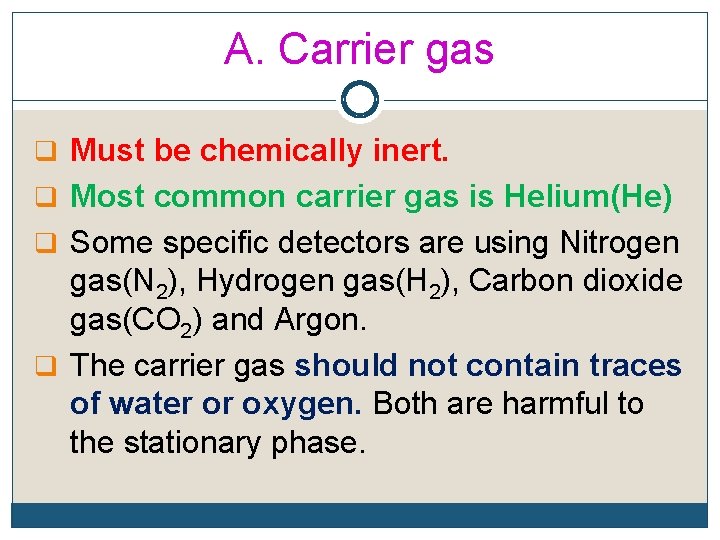
A. Carrier gas q Must be chemically inert. q Most common carrier gas is Helium(He) q Some specific detectors are using Nitrogen gas(N 2), Hydrogen gas(H 2), Carbon dioxide gas(CO 2) and Argon. q The carrier gas should not contain traces of water or oxygen. Both are harmful to the stationary phase.

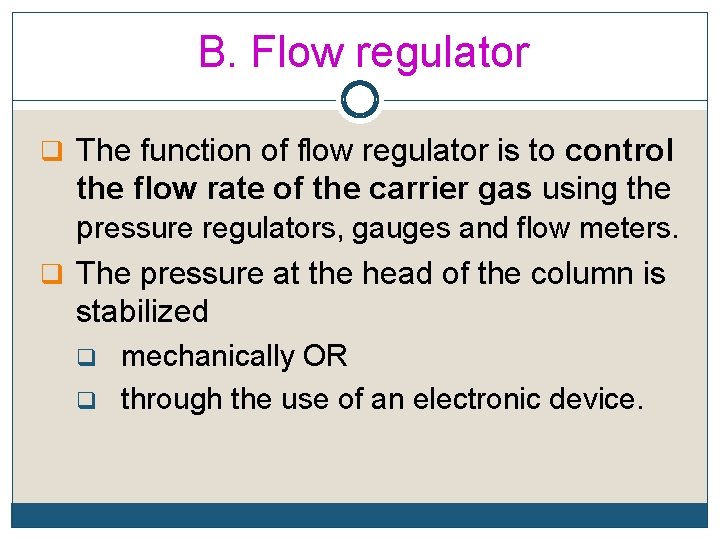
B. Flow regulator q The function of flow regulator is to control the flow rate of the carrier gas using the pressure regulators, gauges and flow meters. q The pressure at the head of the column is stabilized q q mechanically OR through the use of an electronic device.

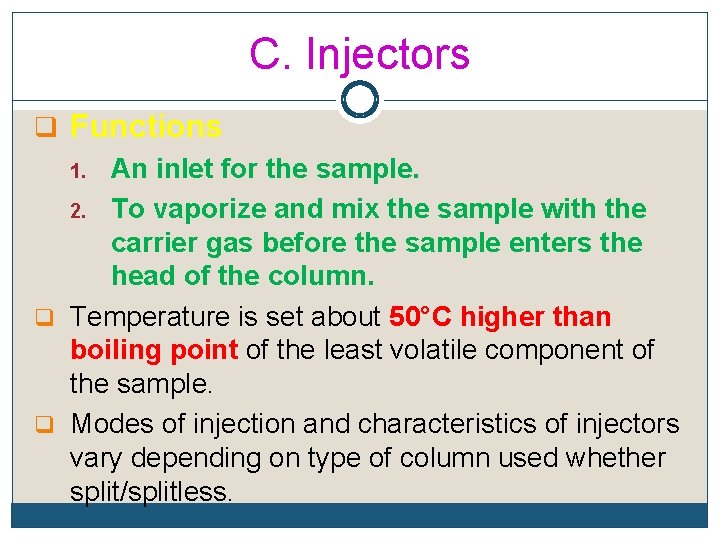
C. Injectors q Functions 1. An inlet for the sample. 2. To vaporize and mix the sample with the carrier gas before the sample enters the head of the column. q Temperature is set about 50°C higher than boiling point of the least volatile component of the sample. q Modes of injection and characteristics of injectors vary depending on type of column used whether split/splitless.

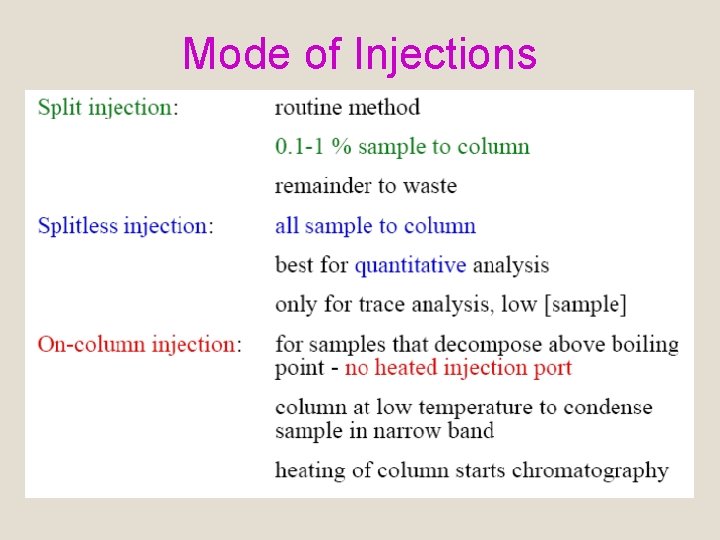
Mode of Injections

D. Sample Injection System q Column efficiency requires sample to be…… 1. Of a suitable size 2. Introduced as a “plug” of vapor q Band broadening and poor resolution are caused by……. 1. Slow injection. 2. Oversized sample.

D. Sample Injection System q Sample introduction usually…… 1. 2. In the form of neat liquid or solution. Introduced in a small volumes. a. 1 μL - 20 μL for packed column. b. 1 x 10 -3 μL for capillary column.

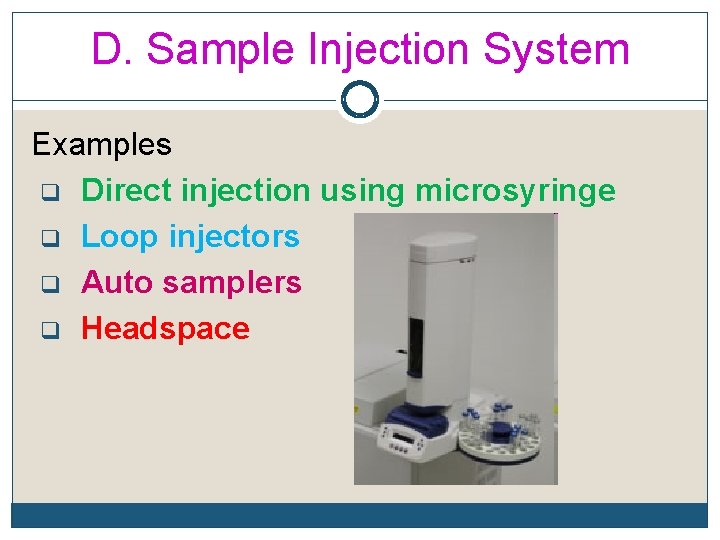
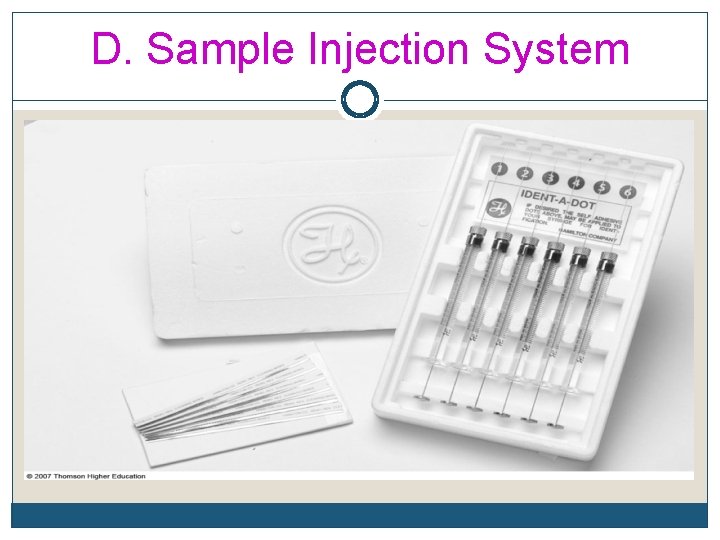
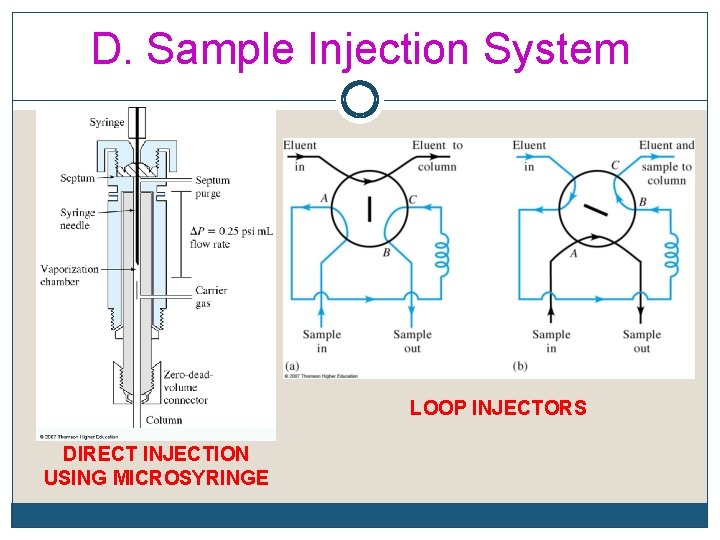
D. Sample Injection System Examples q Direct injection using microsyringe q Loop injectors q Auto samplers q Headspace

D. Sample Injection System

D. Sample Injection System LOOP INJECTORS DIRECT INJECTION USING MICROSYRINGE

D. Sample Injection System Headspace q A headspace sample is normally prepared in a vial containing the sample, the dilution solvent, a matrix modifier and the headspace. q Volatile components from complex sample mixtures can be extracted from non-volatile sample components and isolated in the headspace or gas portion of a sample vial. q A sample of the gas in the headspace is injected into a GC system for separation of all of the volatile components.

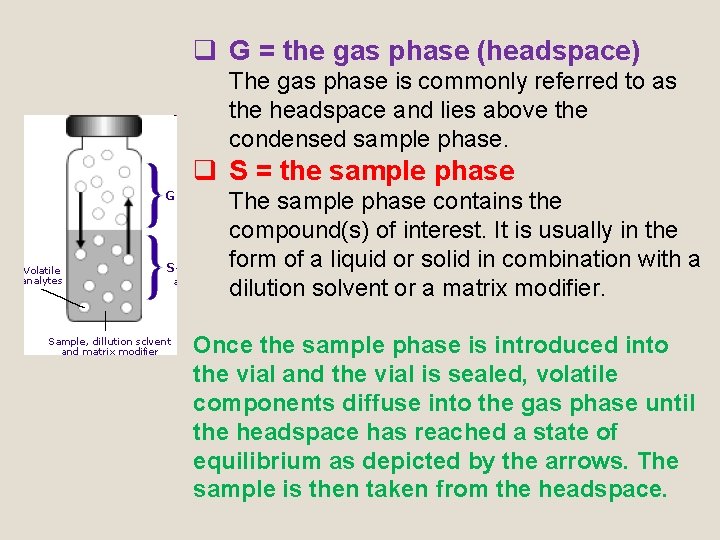
q G = the gas phase (headspace) The gas phase is commonly referred to as the headspace and lies above the condensed sample phase. q S = the sample phase The sample phase contains the compound(s) of interest. It is usually in the form of a liquid or solid in combination with a dilution solvent or a matrix modifier. Once the sample phase is introduced into the vial and the vial is sealed, volatile components diffuse into the gas phase until the headspace has reached a state of equilibrium as depicted by the arrows. The sample is then taken from the headspace.

E. Oven q Must have sufficient space to hold the column. q Can be heated to the desired temperature for analysis. q Atmosphere inside the oven is constantly agitated by forced ventilation which has small thermal inertia. q Reproducible of retention time, t. R which require control of the column temperature within a few tenths of a degree.

E. Oven q Optimum temperature depends on the boiling points of the sample components. q A temperature that is roughly ≥ the average boiling point of the sample results in a reasonable elution period. q Samples with broad boiling range, necessary to employ temperature programming.

Temperature Programming q Definition: A technique in which the column temperature is increased either continuously or in steps as the separation proceeds. q In general, optimum resolution is associated with minimal temperature. q Low temperature, result in longer elution times hence slower analysis.

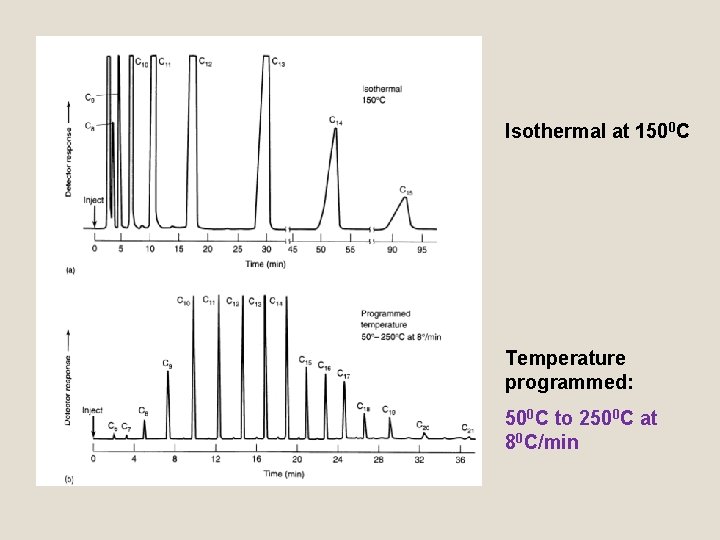
Temperature Programming q Using Temperature programming, low boiling point constituents are separated initially at temperatures that provide resolution. q As separation proceeds, column temperature is increased so that the higher boiling point constituents come off the column with good resolution and at reasonable lengths of time.

Isothermal Elution A technique in which the column temperature is constantly maintained throughout the separation.

Isothermal at 1500 C Temperature programmed: 500 C to 2500 C at 80 C/min

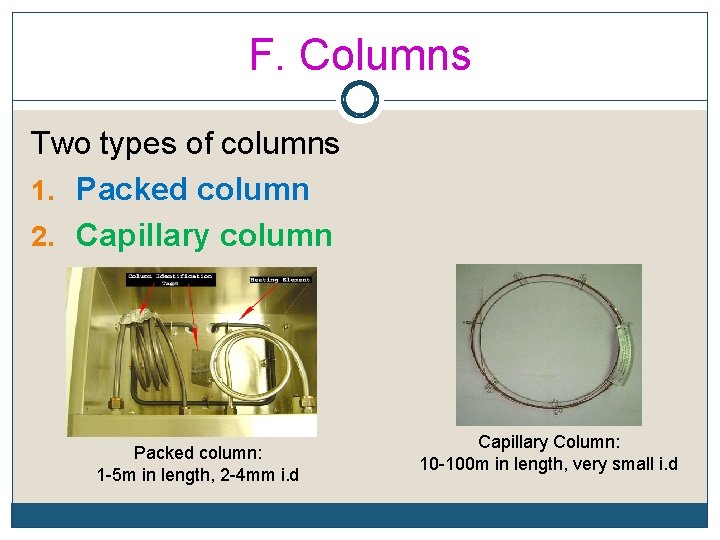
F. Columns Two types of columns 1. Packed column 2. Capillary column Packed column: 1 -5 m in length, 2 -4 mm i. d Capillary Column: 10 -100 m in length, very small i. d

1. Packed Column q Less commonly used q Made of glass or steel q Length: 1 to 5 m q Internal diameter: 2 to 4 mm Cross-sectional view of packed column q These column is densely packed with uniform, finely divided solid support, coated with thin layer (0. 05 to 1μm) of stationary liquid phase. q Accommodate larger samples.

1. Packed Column q Carrier gas flow between 10 – 40 m. L/min. q Not well adapted for trace analysis. q Contain an inert & stable porous support on which the stationary phase can be impregnated(coated) or bound. q Advantages: Large sample size 2. Ease & convenience of use 1.

2. Capillary Column q Widely used in GC analysis q Also known as open tubular column q Length: 10 – 100 m q Coiled around a light weight of metallic support. q Types of capillary column FSOT (Fused Silica Wall Coated) - i. d. 0. 1 0. 3 mm II. WCOT (Wall Coated) - i. d. 0. 25 – 0. 75 mm III. SCOT (Support Coated) - i. d. 0. 5 mm I.

2. Capillary Column q Advantages: High resolution 2. Short analysis time 3. High sensitivity 1.

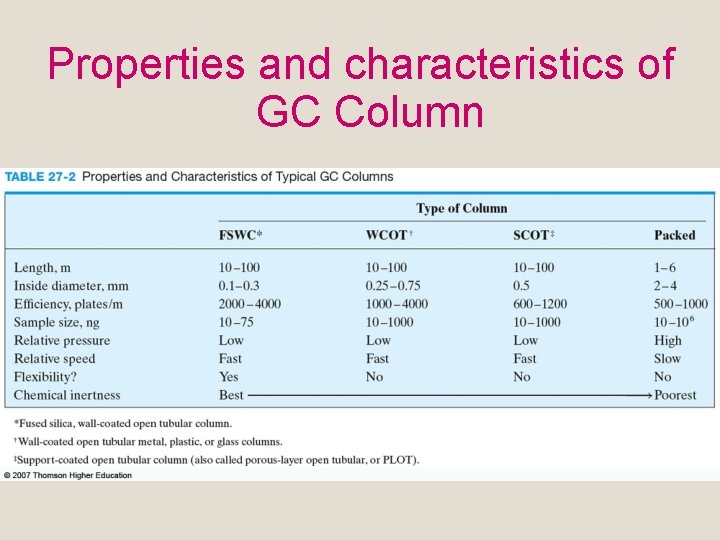
Properties and characteristics of GC Column

G. Stationary Phase Desirable properties for the immobilized liquid stationary phase: q Low volatility (ideally the boiling point of the liquid at least 1000 C higher than the maximum operating temperature for the column) q Thermal stability. q Chemical inertness. q Solvent characteristics such as k and α values for the solutes to be resolved fall within a suitable range.

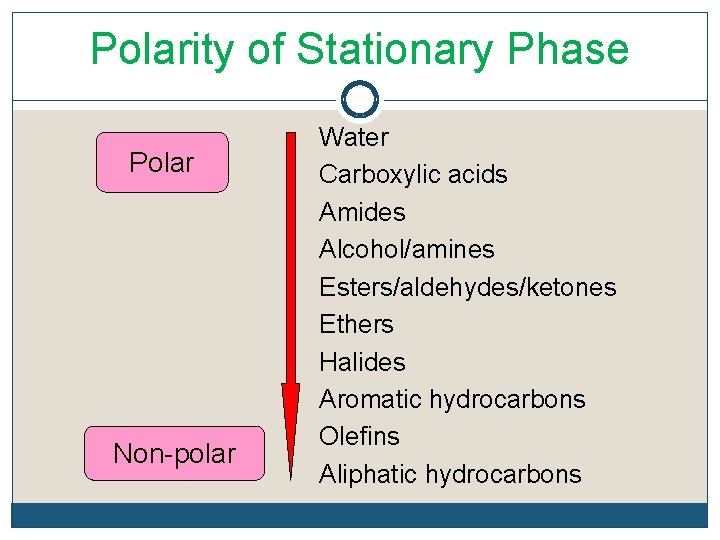
G. Stationary Phase q Separation principles q q Use the principle of “like dissolve like” where like refers to the polarity of the analyte and the immobilized liquid stationary phase. Polarity of organic functional group in increasing order q Aliphatic hydrocarbons<olefins<aromatic hydrocarbons<halides<ethers< esters/ aldehydes/ketones<alcohols/amines< amides<carboxylic acids<water

G. Stationary Phase q Polarity of the stationary phase should match that of sample components. q When the match is good, the order of elution is determined by the boiling point of the eluents.

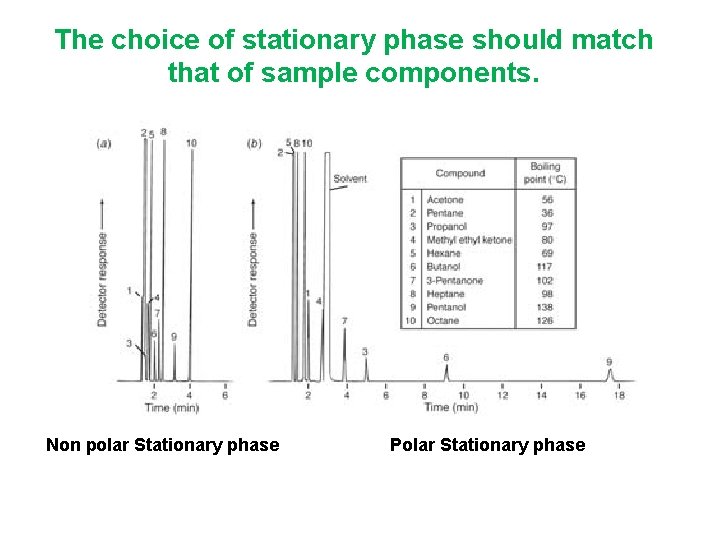
The choice of stationary phase should match that of sample components. Non polar Stationary phase Polar Stationary phase

Polarity of Stationary Phase Polar Non-polar Water Carboxylic acids Amides Alcohol/amines Esters/aldehydes/ketones Ethers Halides Aromatic hydrocarbons Olefins Aliphatic hydrocarbons

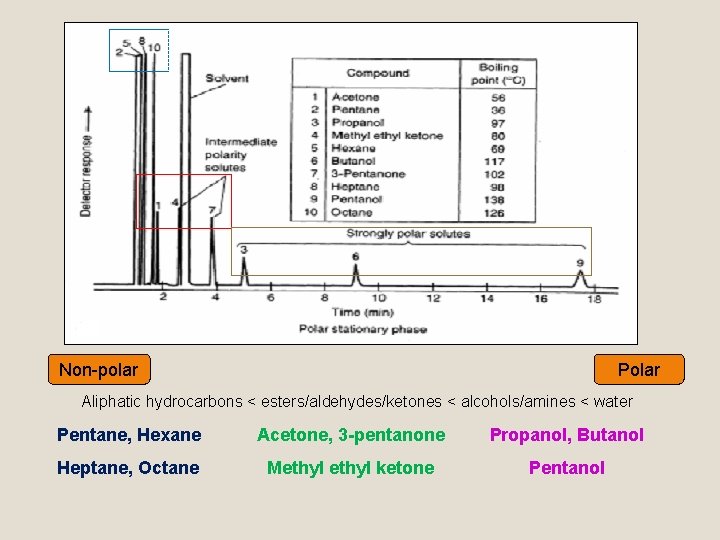
Non-polar Polar Aliphatic hydrocarbons < esters/aldehydes/ketones < alcohols/amines < water Pentane, Hexane Acetone, 3 -pentanone Propanol, Butanol Heptane, Octane Methyl ketone Pentanol

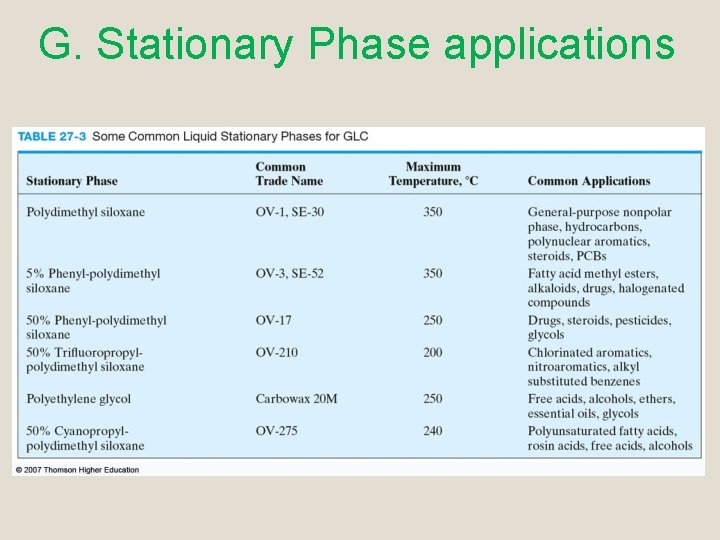
G. Stationary Phase applications


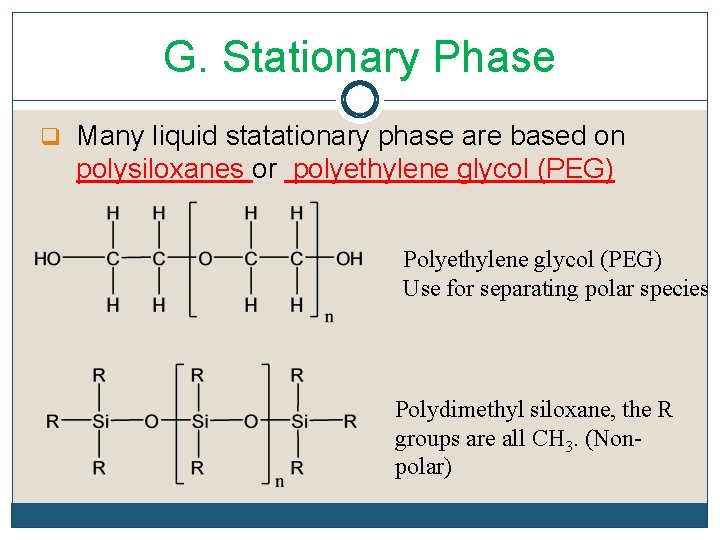
G. Stationary Phase q Many liquid statationary phase are based on polysiloxanes or polyethylene glycol (PEG) Polyethylene glycol (PEG) Use for separating polar species Polydimethyl siloxane, the R groups are all CH 3. (Nonpolar)

H. Detectors q Some detectors are universal. They are sensitive to almost every compound that elutes from the column. q Most detectors are selective. q They are sensitive to a particular type of compound. Give response that is dependent on the concentration of analyte in the carrier gas. q Yield(produce) simple chromatogram. q

H. Detectors q Characteristics of ideal detector 1. 2. 3. High reliability & ease to use. Similarity response toward all solutes or alternatively a high predictable & selective response toward one or more classes of solute. Detector should be nondestructive.

H. Detectors q Characteristics of ideal detector 4. 5. 6. 7. Adequate sensitivity. Good stability and reproducibility. Linear response to solutes that extends over several orders of magnitude. Temperature range (from room temperature to at least 400 0 C)

H. Detectors q Several types of detectors. 1. 2. 3. Flame Ionization Detector (FID) Thermal Conductivity Detector (TCD) Electron Captured Detector (ECD)

1. Flame Ionization Detector (FID)

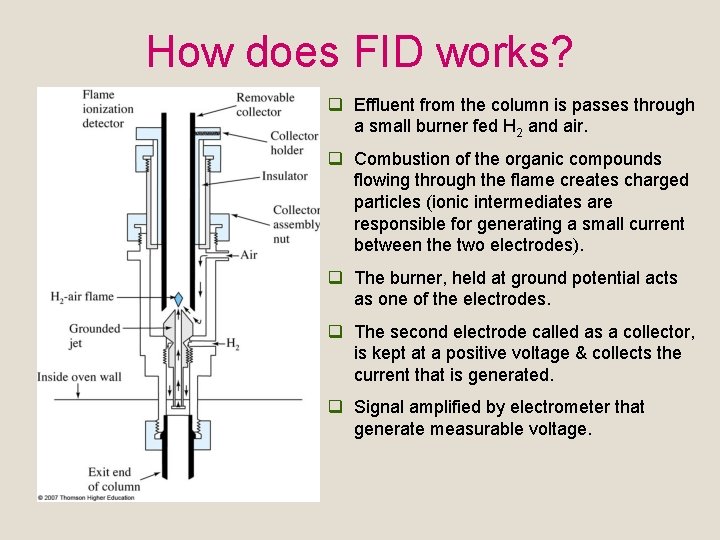
How does FID works? q Effluent from the column is passes through a small burner fed H 2 and air. q Combustion of the organic compounds flowing through the flame creates charged particles (ionic intermediates are responsible for generating a small current between the two electrodes). q The burner, held at ground potential acts as one of the electrodes. q The second electrode called as a collector, is kept at a positive voltage & collects the current that is generated. q Signal amplified by electrometer that generate measurable voltage.

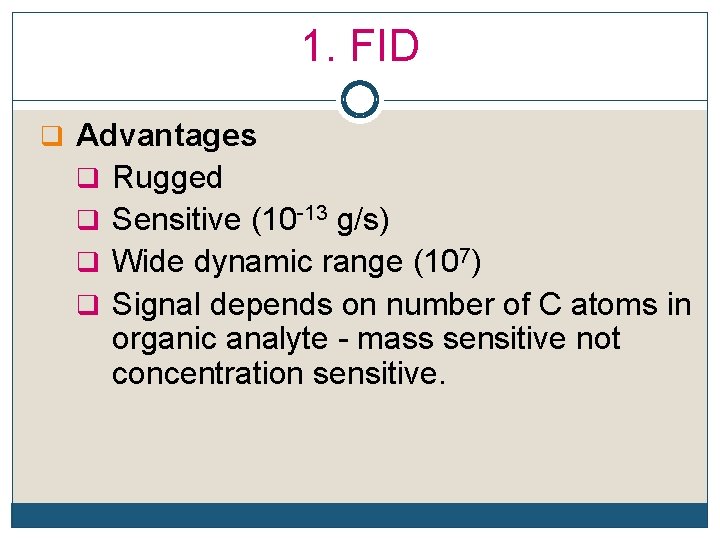
1. FID q Advantages q Rugged q Sensitive (10 -13 g/s) q Wide dynamic range (107) q Signal depends on number of C atoms in organic analyte - mass sensitive not concentration sensitive.

1. FID q Disadvantages q Weakly sensitive to carbonyl, amine, alcohol & amine groups. q Not sensitive to non-combustibles analyte such as H 2 O, CO 2, SO 2, NOx. q Destructive method.

2. Thermal Conductivity Detector (TCD)

2. TCD q A universal detector. q Has a moderate sensitivity. q Less satisfactory with carrier gas whose conductivities closely resemble those of most sample components.

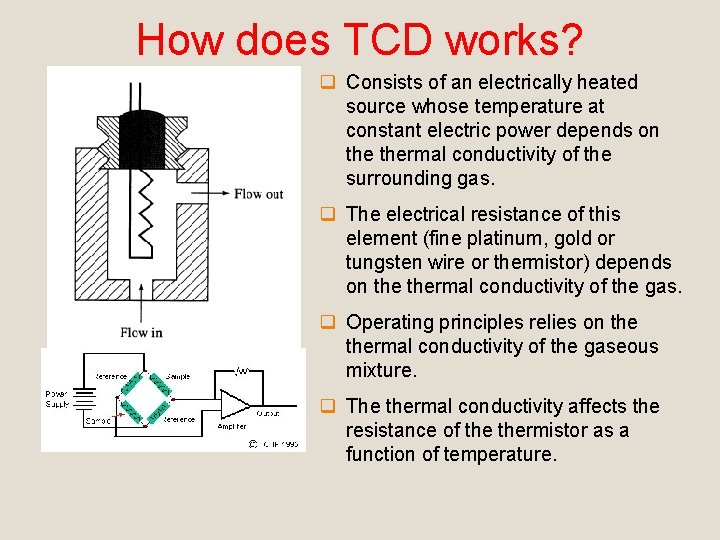
How does TCD works? q Consists of an electrically heated source whose temperature at constant electric power depends on thermal conductivity of the surrounding gas. q The electrical resistance of this element (fine platinum, gold or tungsten wire or thermistor) depends on thermal conductivity of the gas. q Operating principles relies on thermal conductivity of the gaseous mixture. q The thermal conductivity affects the resistance of thermistor as a function of temperature.

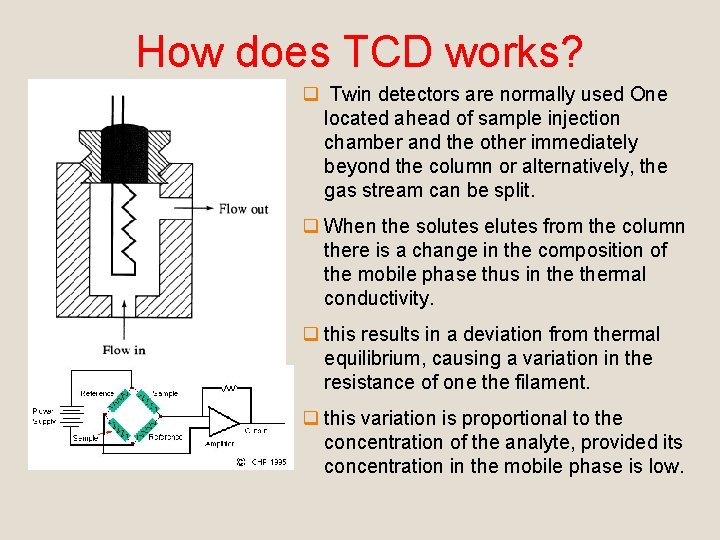
How does TCD works? q Twin detectors are normally used One located ahead of sample injection chamber and the other immediately beyond the column or alternatively, the gas stream can be split. q When the solutes elutes from the column there is a change in the composition of the mobile phase thus in thermal conductivity. q this results in a deviation from thermal equilibrium, causing a variation in the resistance of one the filament. q this variation is proportional to the concentration of the analyte, provided its concentration in the mobile phase is low.

2. TCD q Advantages q q Simple Large linear dynamic range Responds to both organic and inorganic species Nondestructive; permits collection of solutes after detection. q Disadvantage q Relatively low sensitivity.

3. Electron Capture Detector (ECD)

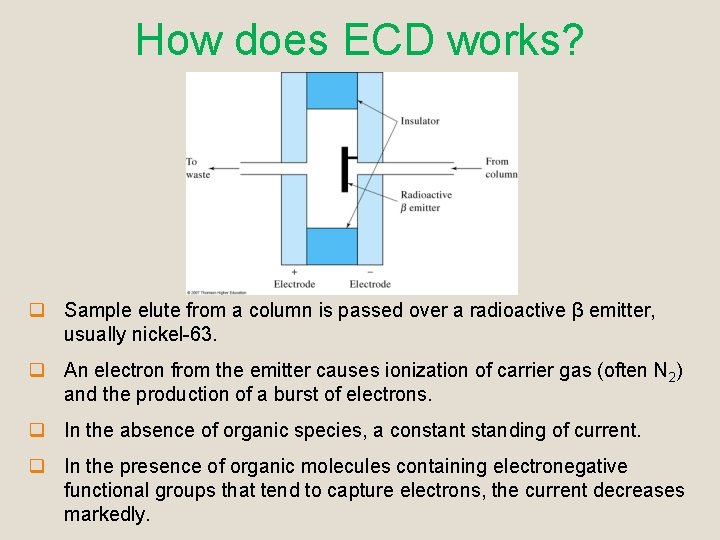
How does ECD works? q Sample elute from a column is passed over a radioactive β emitter, usually nickel-63. q An electron from the emitter causes ionization of carrier gas (often N 2) and the production of a burst of electrons. q In the absence of organic species, a constant standing of current. q In the presence of organic molecules containing electronegative functional groups that tend to capture electrons, the current decreases markedly.

3. ECD q Most widely used for environmental samples q Advantages q q Selectively responds to halogen-containing organic compounds such as pesticides and polychlorinated biphenyls. Highly sensitive towards halogens, peroxides, quinones and nitro groups. q Disadvantages q Insensitive to functional groups such as amines, alcohols and hydrocarbons.

H. Detectors q Other detectors q q q Nitrogen-Phosphorous Detector (NPD) Flame Photometry Detector (FPD) Mass spectrometer (GC-MS)

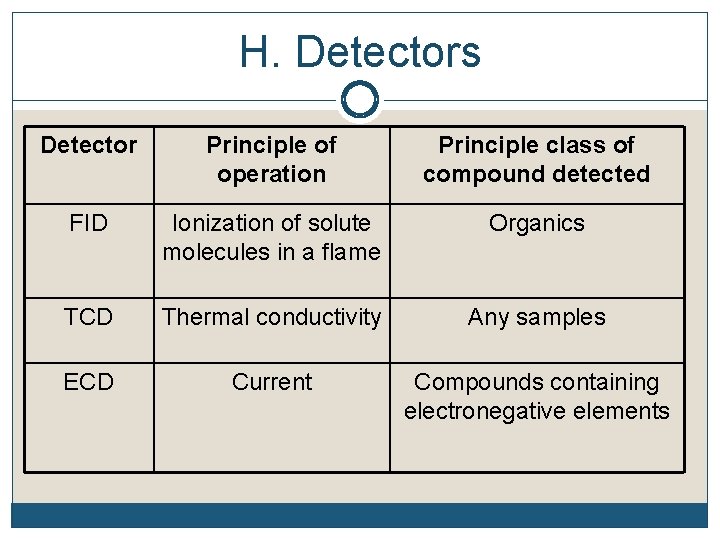
H. Detectors Detector Principle of operation Principle class of compound detected FID Ionization of solute molecules in a flame Organics TCD Thermal conductivity Any samples ECD Current Compounds containing electronegative elements
 Principles of gc
Principles of gc Basic principle of gas chromatography
Basic principle of gas chromatography 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Chromatography lecture
Chromatography lecture Chromatography was discovered by
Chromatography was discovered by Principles of economics powerpoint lecture slides
Principles of economics powerpoint lecture slides Comparative education
Comparative education Mikhail tswett
Mikhail tswett Hplc principle
Hplc principle Van deemter equation for open tubular column
Van deemter equation for open tubular column Gradient vs isocratic elution
Gradient vs isocratic elution Gas liquid chromatography
Gas liquid chromatography Percent composition gas chromatography
Percent composition gas chromatography Mobile phase in chromatography
Mobile phase in chromatography Basic gas chromatography
Basic gas chromatography Gas chromatography history
Gas chromatography history Column chromatography youtube
Column chromatography youtube Gas chromatography
Gas chromatography Pemisahan campuran
Pemisahan campuran Gas chromatography
Gas chromatography Gas chromatography
Gas chromatography Application of glc
Application of glc Gas chromatography theory
Gas chromatography theory Ideal gas vs perfect gas
Ideal gas vs perfect gas An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Gas law
Gas law Sutherland's law
Sutherland's law Bhopal gas tragedy reason
Bhopal gas tragedy reason Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Gas reale e gas ideale
Gas reale e gas ideale Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Contoh soal kinetika kimia orde 1
Contoh soal kinetika kimia orde 1 Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Project procurement management lecture notes
Project procurement management lecture notes Lecture about sport
Lecture about sport Lecture on healthy lifestyle
Lecture on healthy lifestyle Existential nihilism
Existential nihilism Meaning of this
Meaning of this Randy pausch last lecture summary
Randy pausch last lecture summary Tensorflow lecture
Tensorflow lecture Theology proper lecture notes
Theology proper lecture notes Strategic management lecture
Strategic management lecture Geology lecture series
Geology lecture series Social psychology lecture
Social psychology lecture In text citation for a lecture
In text citation for a lecture Definition of public sector accounting
Definition of public sector accounting 4ps of project management
4ps of project management Practical design to eurocode 2 lecture 3
Practical design to eurocode 2 lecture 3 Magnetism
Magnetism Classical mechanics
Classical mechanics Physics 101 lecture notes pdf
Physics 101 lecture notes pdf Physical science lecture notes
Physical science lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Natural language processing nlp - theory lecture
Natural language processing nlp - theory lecture Microbial physiology and metabolism lecture notes
Microbial physiology and metabolism lecture notes Introduction to mechatronics ppt
Introduction to mechatronics ppt Ternology
Ternology Les objectifs d'apprentissage de la lecture
Les objectifs d'apprentissage de la lecture Instruction de lecture
Instruction de lecture