CHROMATOGRAPHY CHROMATOGRAPHY Chromatography is used to separate and







- Slides: 27

CHROMATOGRAPHY

CHROMATOGRAPHY Chromatography is used to separate and analyse small amounts of mixtures Methods involve a stationary phase and a mobile phase. There are several forms of chromatography

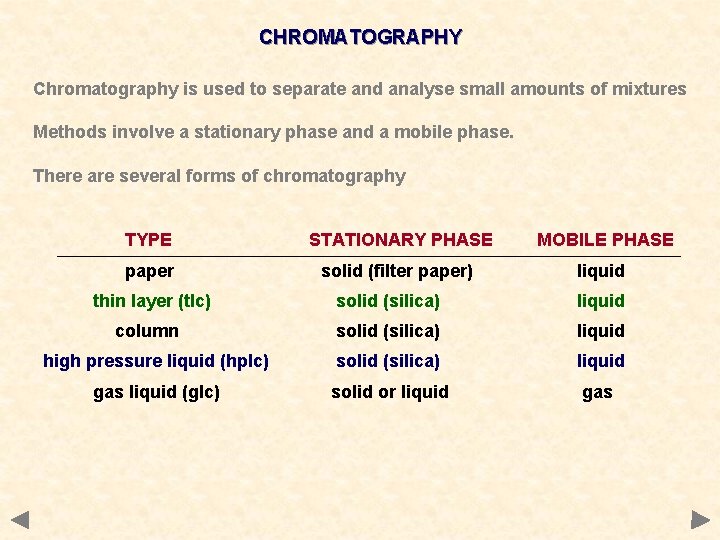
CHROMATOGRAPHY Chromatography is used to separate and analyse small amounts of mixtures Methods involve a stationary phase and a mobile phase. There are several forms of chromatography TYPE STATIONARY PHASE MOBILE PHASE paper solid (filter paper) liquid thin layer (tlc) solid (silica) liquid column solid (silica) liquid high pressure liquid (hplc) solid (silica) liquid gas liquid (glc) solid or liquid gas

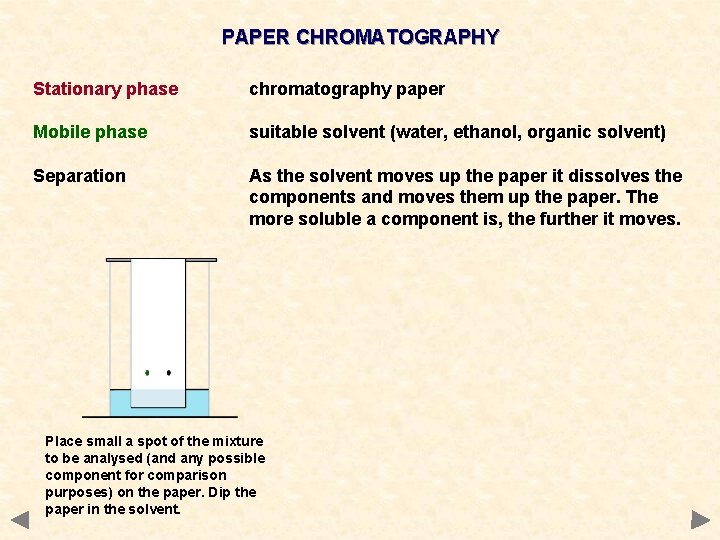
PAPER CHROMATOGRAPHY Stationary phase chromatography paper Mobile phase suitable solvent (water, ethanol, organic solvent) Separation As the solvent moves up the paper it dissolves the components and moves them up the paper. The more soluble a component is, the further it moves. Place small a spot of the mixture to be analysed (and any possible component for comparison purposes) on the paper. Dip the paper in the solvent.

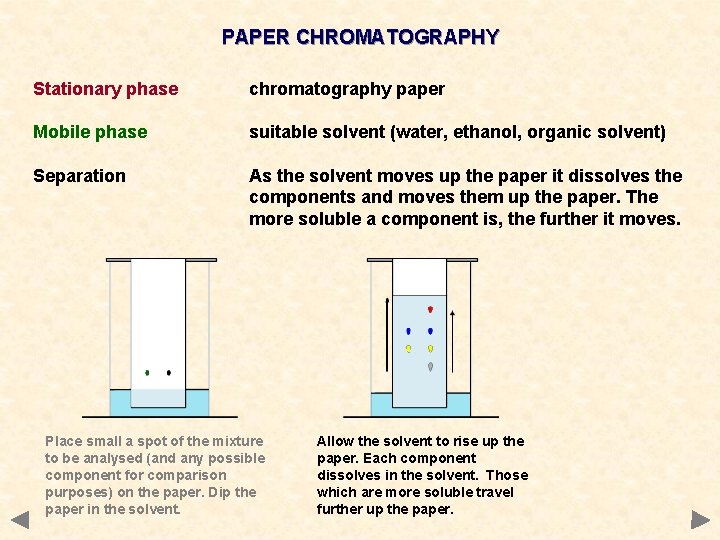
PAPER CHROMATOGRAPHY Stationary phase chromatography paper Mobile phase suitable solvent (water, ethanol, organic solvent) Separation As the solvent moves up the paper it dissolves the components and moves them up the paper. The more soluble a component is, the further it moves. Place small a spot of the mixture to be analysed (and any possible component for comparison purposes) on the paper. Dip the paper in the solvent. Allow the solvent to rise up the paper. Each component dissolves in the solvent. Those which are more soluble travel further up the paper.

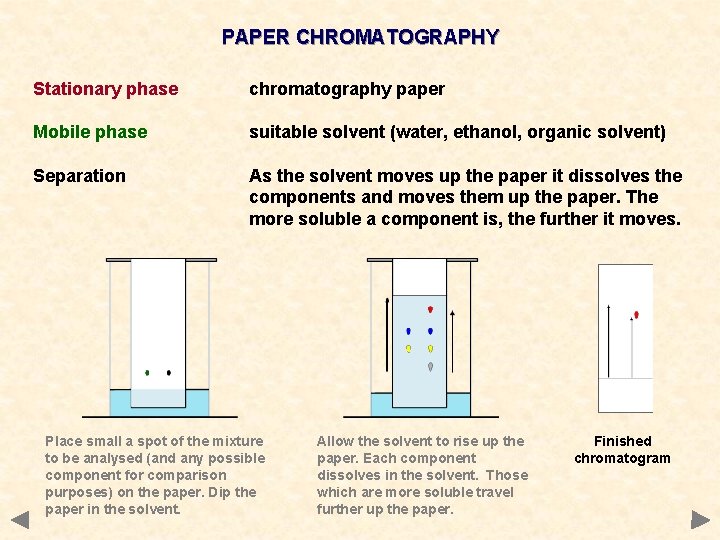
PAPER CHROMATOGRAPHY Stationary phase chromatography paper Mobile phase suitable solvent (water, ethanol, organic solvent) Separation As the solvent moves up the paper it dissolves the components and moves them up the paper. The more soluble a component is, the further it moves. Place small a spot of the mixture to be analysed (and any possible component for comparison purposes) on the paper. Dip the paper in the solvent. Allow the solvent to rise up the paper. Each component dissolves in the solvent. Those which are more soluble travel further up the paper. Finished chromatogram

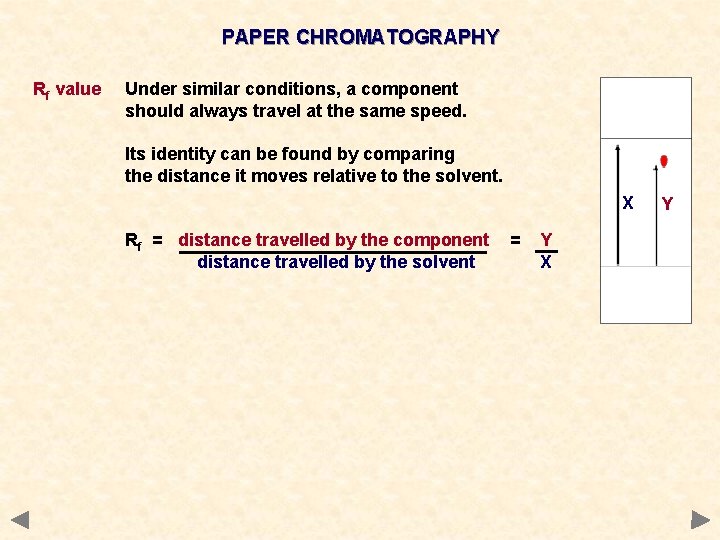
PAPER CHROMATOGRAPHY Rf value Under similar conditions, a component should always travel at the same speed. Its identity can be found by comparing the distance it moves relative to the solvent. X Rf = distance travelled by the component distance travelled by the solvent = Y X Y

PAPER CHROMATOGRAPHY Rf value Under similar conditions, a component should always travel at the same speed. Its identity can be found by comparing the distance it moves relative to the solvent. X Rf = distance travelled by the component distance travelled by the solvent Comparison can be a problem if… a) components have similar Rf values b) the unknown substance is new and there is no previous chemical to compare it with = Y X Y

THIN LAYER CHROMATOGRAPHY Stationary phase silica mounted on a glass plate Mobile phase suitable organic solvent Separation similar technique to paper chromatography Limitations similar to paper chromatography

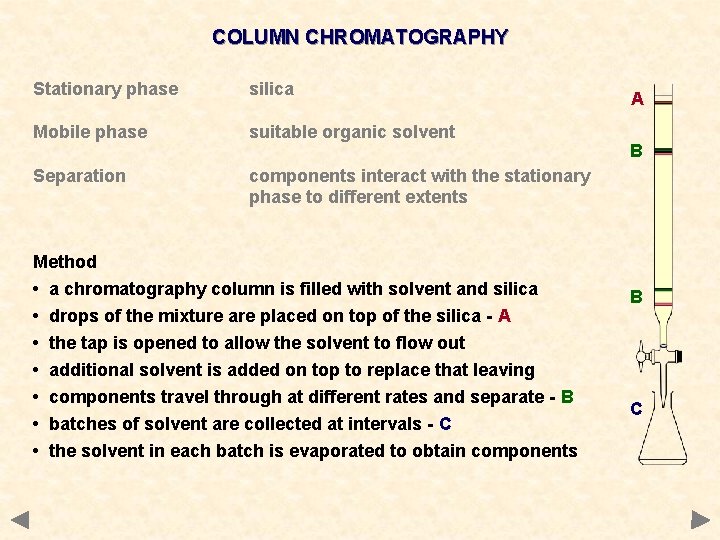
COLUMN CHROMATOGRAPHY Stationary phase silica Mobile phase suitable organic solvent Separation components interact with the stationary phase to different extents A B B C

COLUMN CHROMATOGRAPHY Stationary phase silica Mobile phase suitable organic solvent Separation components interact with the stationary phase to different extents Method • a chromatography column is filled with solvent and silica • drops of the mixture are placed on top of the silica - A • • • the tap is opened to allow the solvent to flow out additional solvent is added on top to replace that leaving components travel through at different rates and separate - B batches of solvent are collected at intervals - C the solvent in each batch is evaporated to obtain components A B B C

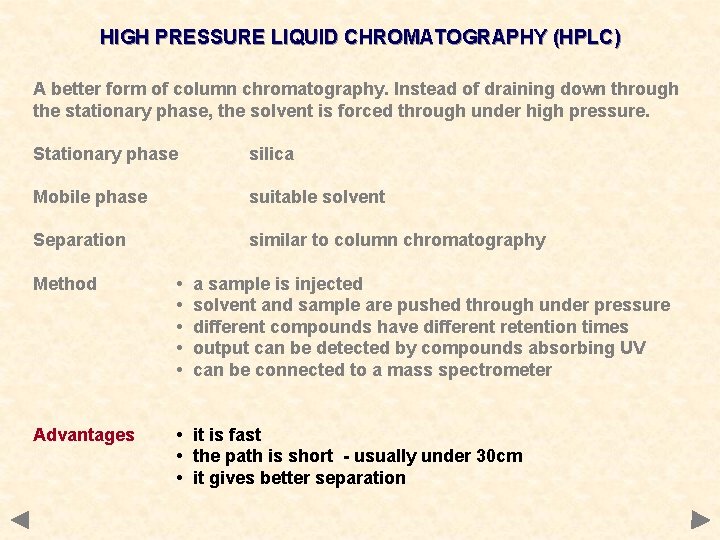
HIGH PRESSURE LIQUID CHROMATOGRAPHY (HPLC) A better form of column chromatography. Instead of draining down through the stationary phase, the solvent is forced through under high pressure. Stationary phase silica Mobile phase suitable solvent Separation similar to column chromatography

HIGH PRESSURE LIQUID CHROMATOGRAPHY (HPLC) A better form of column chromatography. Instead of draining down through the stationary phase, the solvent is forced through under high pressure. Stationary phase silica Mobile phase suitable solvent Separation similar to column chromatography Method • • • a sample is injected solvent and sample are pushed through under pressure different compounds have different retention times output can be detected by compounds absorbing UV can be connected to a mass spectrometer

HIGH PRESSURE LIQUID CHROMATOGRAPHY (HPLC) A better form of column chromatography. Instead of draining down through the stationary phase, the solvent is forced through under high pressure. Stationary phase silica Mobile phase suitable solvent Separation similar to column chromatography Method • • • a sample is injected solvent and sample are pushed through under pressure different compounds have different retention times output can be detected by compounds absorbing UV can be connected to a mass spectrometer Advantages • it is fast • the path is short - usually under 30 cm • it gives better separation

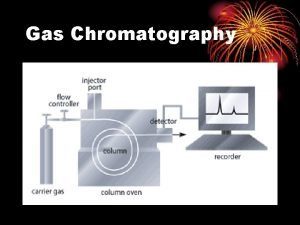
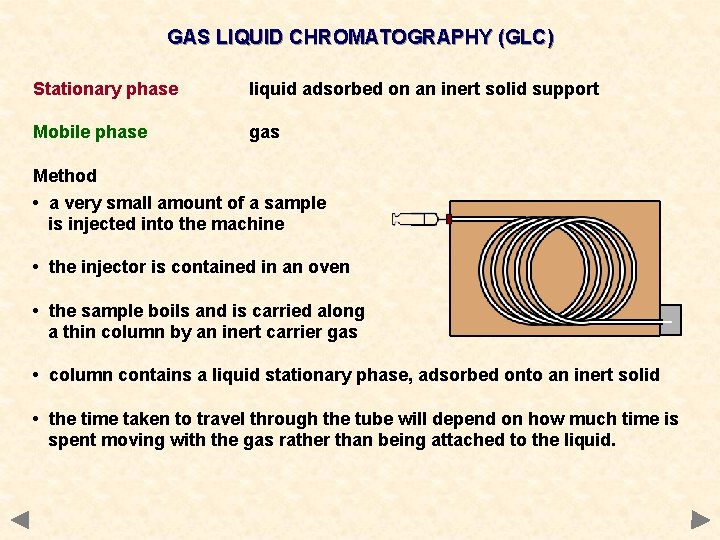
GAS LIQUID CHROMATOGRAPHY (GLC) Stationary phase liquid adsorbed on an inert solid support Mobile phase gas Method • a very small amount of a sample is injected into the machine • the injector is contained in an oven • the sample boils and is carried along a thin column by an inert carrier gas • column contains a liquid stationary phase, adsorbed onto an inert solid • the time taken to travel through the tube will depend on how much time is spent moving with the gas rather than being attached to the liquid.

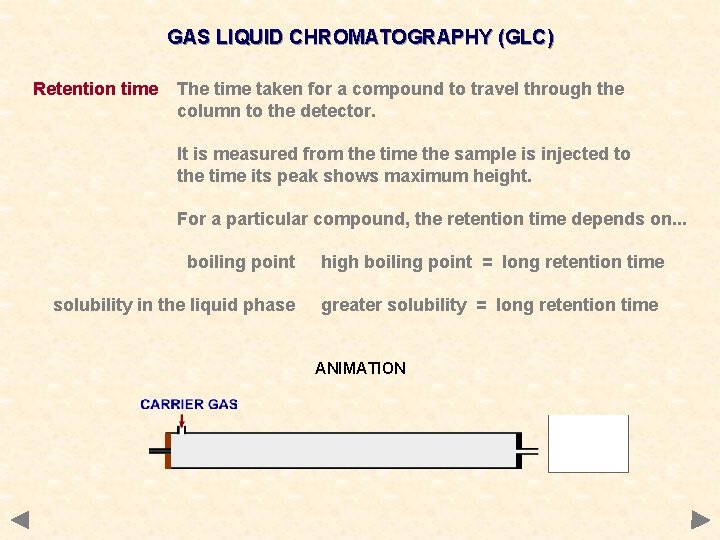
GAS LIQUID CHROMATOGRAPHY (GLC) Retention time The time taken for a compound to travel through the column to the detector. It is measured from the time the sample is injected to the time its peak shows maximum height.

GAS LIQUID CHROMATOGRAPHY (GLC) Retention time The time taken for a compound to travel through the column to the detector. It is measured from the time the sample is injected to the time its peak shows maximum height. For a particular compound, the retention time depends on. . . boiling point high boiling point = long retention time

GAS LIQUID CHROMATOGRAPHY (GLC) Retention time The time taken for a compound to travel through the column to the detector. It is measured from the time the sample is injected to the time its peak shows maximum height. For a particular compound, the retention time depends on. . . boiling point solubility in the liquid phase high boiling point = long retention time greater solubility = long retention time

GAS LIQUID CHROMATOGRAPHY (GLC) Retention time The time taken for a compound to travel through the column to the detector. It is measured from the time the sample is injected to the time its peak shows maximum height. For a particular compound, the retention time depends on. . . boiling point solubility in the liquid phase high boiling point = long retention time greater solubility = long retention time ANIMATION

GAS LIQUID CHROMATOGRAPHY (GLC) Detection • there are several ways to detect components • most involve destruction of the sample • one method is an FID - flame ionisation detector The FID • • • as a component exits, it is burned in a hydrogen flame ions are produced in the flame a detector produces an electric current greater the amount of a component = larger current the current can be represented by a chromatogram as the component is destroyed, GCMS doesn’t use FID

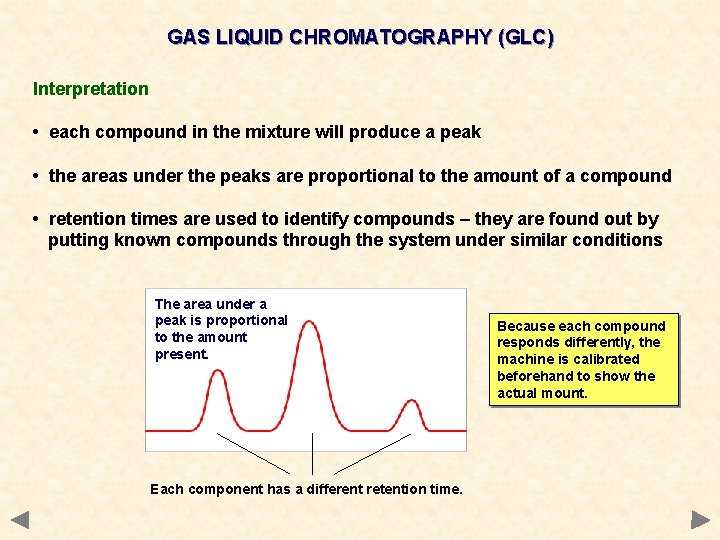
GAS LIQUID CHROMATOGRAPHY (GLC) Interpretation • each compound in the mixture will produce a peak • the areas under the peaks are proportional to the amount of a compound • retention times are used to identify compounds – they are found out by putting known compounds through the system under similar conditions The area under a peak is proportional to the amount present. Each component has a different retention time. Because each compound responds differently, the machine is calibrated beforehand to show the actual mount.

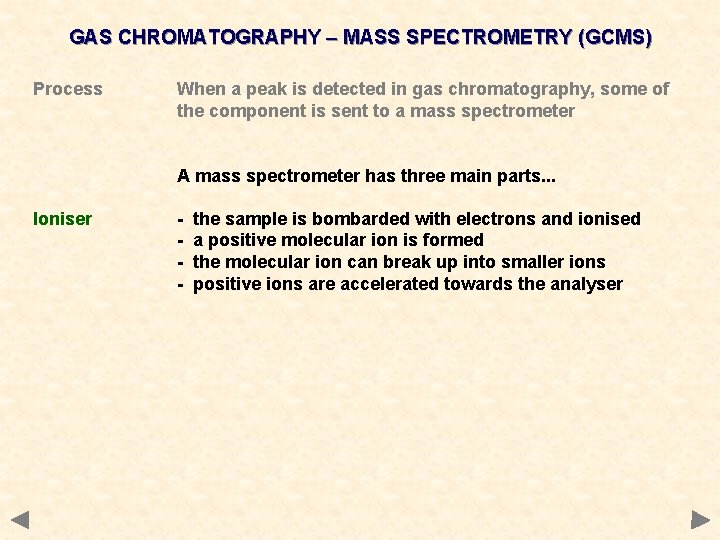
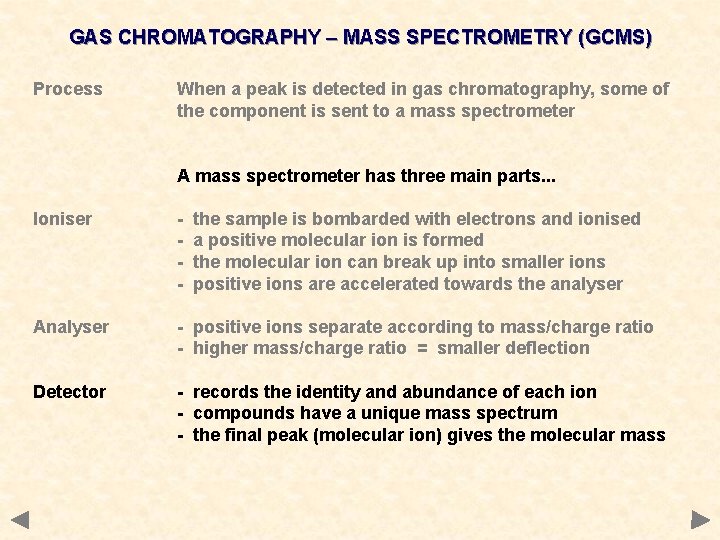
GAS CHROMATOGRAPHY – MASS SPECTROMETRY (GCMS) Process When a peak is detected in gas chromatography, some of the component is sent to a mass spectrometer A mass spectrometer has three main parts. . .

GAS CHROMATOGRAPHY – MASS SPECTROMETRY (GCMS) Process When a peak is detected in gas chromatography, some of the component is sent to a mass spectrometer A mass spectrometer has three main parts. . . Ioniser - the sample is bombarded with electrons and ionised a positive molecular ion is formed the molecular ion can break up into smaller ions positive ions are accelerated towards the analyser

GAS CHROMATOGRAPHY – MASS SPECTROMETRY (GCMS) Process When a peak is detected in gas chromatography, some of the component is sent to a mass spectrometer A mass spectrometer has three main parts. . . Ioniser - the sample is bombarded with electrons and ionised a positive molecular ion is formed the molecular ion can break up into smaller ions positive ions are accelerated towards the analyser Analyser - positive ions separate according to mass/charge ratio - higher mass/charge ratio = smaller deflection

GAS CHROMATOGRAPHY – MASS SPECTROMETRY (GCMS) Process When a peak is detected in gas chromatography, some of the component is sent to a mass spectrometer A mass spectrometer has three main parts. . . Ioniser - the sample is bombarded with electrons and ionised a positive molecular ion is formed the molecular ion can break up into smaller ions positive ions are accelerated towards the analyser Analyser - positive ions separate according to mass/charge ratio - higher mass/charge ratio = smaller deflection Detector - records the identity and abundance of each ion - compounds have a unique mass spectrum - the final peak (molecular ion) gives the molecular mass

GAS CHROMATOGRAPHY – MASS SPECTROMETRY (GCMS) Process When a peak is detected in gas chromatography, some of the component is sent to a mass spectrometer A mass spectrometer has three main parts. . . Ioniser - the sample is bombarded with electrons and ionised a positive molecular ion is formed the molecular ion can break up into smaller ions positive ions are accelerated towards the analyser Analyser - positive ions separate according to mass/charge ratio - higher mass/charge ratio = smaller deflection Detector - records the identity and abundance of each ion - compounds have a unique mass spectrum - the final peak (molecular ion) gives the molecular mass

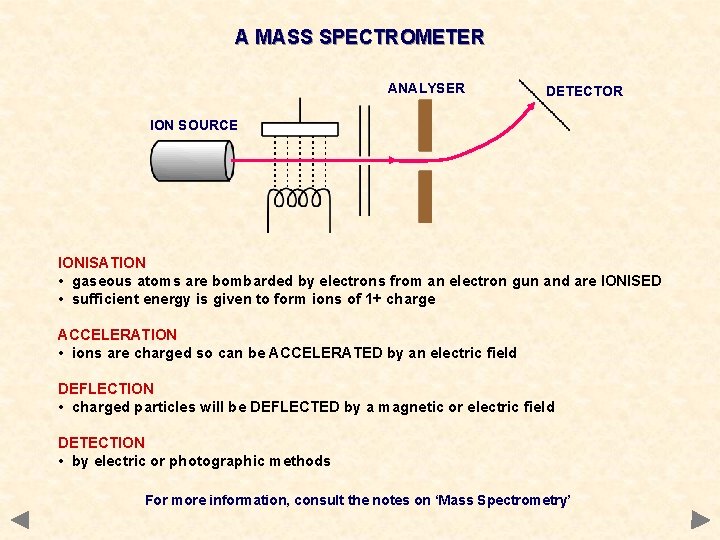
A MASS SPECTROMETER ANALYSER DETECTOR ION SOURCE IONISATION • gaseous atoms are bombarded by electrons from an electron gun and are IONISED • sufficient energy is given to form ions of 1+ charge ACCELERATION • ions are charged so can be ACCELERATED by an electric field DEFLECTION • charged particles will be DEFLECTED by a magnetic or electric field DETECTION • by electric or photographic methods For more information, consult the notes on ‘Mass Spectrometry’
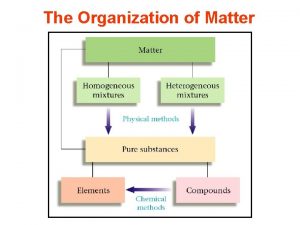
 Chromatography is a technique used to separate
Chromatography is a technique used to separate Centerlines are used to separate the title block and notes.
Centerlines are used to separate the title block and notes. Commas are used to separate
Commas are used to separate Filtration examples in everyday life
Filtration examples in everyday life Separating funnel is used to separate
Separating funnel is used to separate Commas are used to separate
Commas are used to separate Chromatography definition
Chromatography definition Paper chromatography is a method used in regents
Paper chromatography is a method used in regents Solid mixtures
Solid mixtures How to separate raisins and flour
How to separate raisins and flour How to separate salt and water
How to separate salt and water Is english lit and language separate gcses
Is english lit and language separate gcses How to separate salt and sand
How to separate salt and sand How to separate sand and finely ground polystyrene foam
How to separate sand and finely ground polystyrene foam What is magnetism in separating mixtures
What is magnetism in separating mixtures Sapratibandha daya and apratibandha daya
Sapratibandha daya and apratibandha daya What is an introductory clause
What is an introductory clause Separate result unknown
Separate result unknown Accompaniment salad definition
Accompaniment salad definition Guest bank adalah
Guest bank adalah Separate result unknown
Separate result unknown Horizontal lines that separate east from west
Horizontal lines that separate east from west Genre of a separate peace
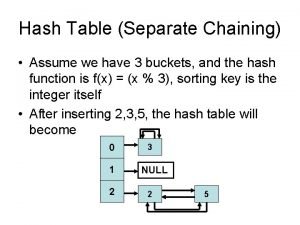
Genre of a separate peace Double hashing
Double hashing Hash function code
Hash function code Living things grow images
Living things grow images Using commas to separate coordinate adjectives
Using commas to separate coordinate adjectives Commas adjectives
Commas adjectives